Abstract
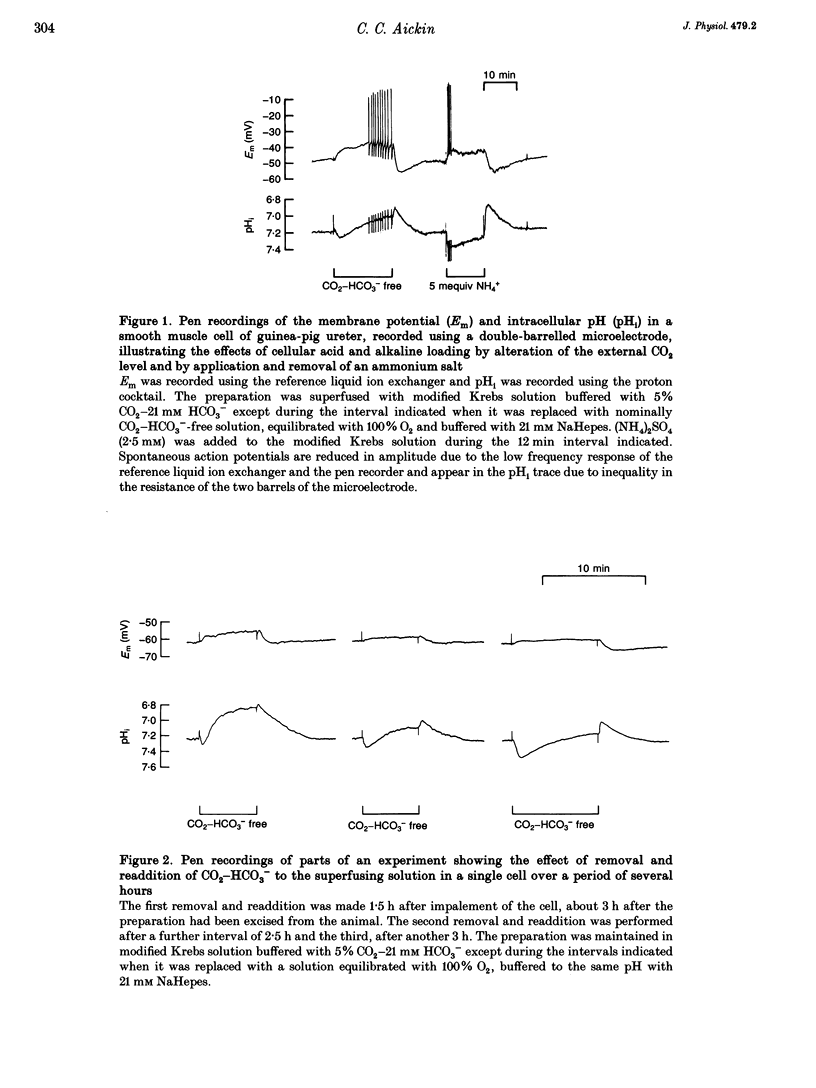
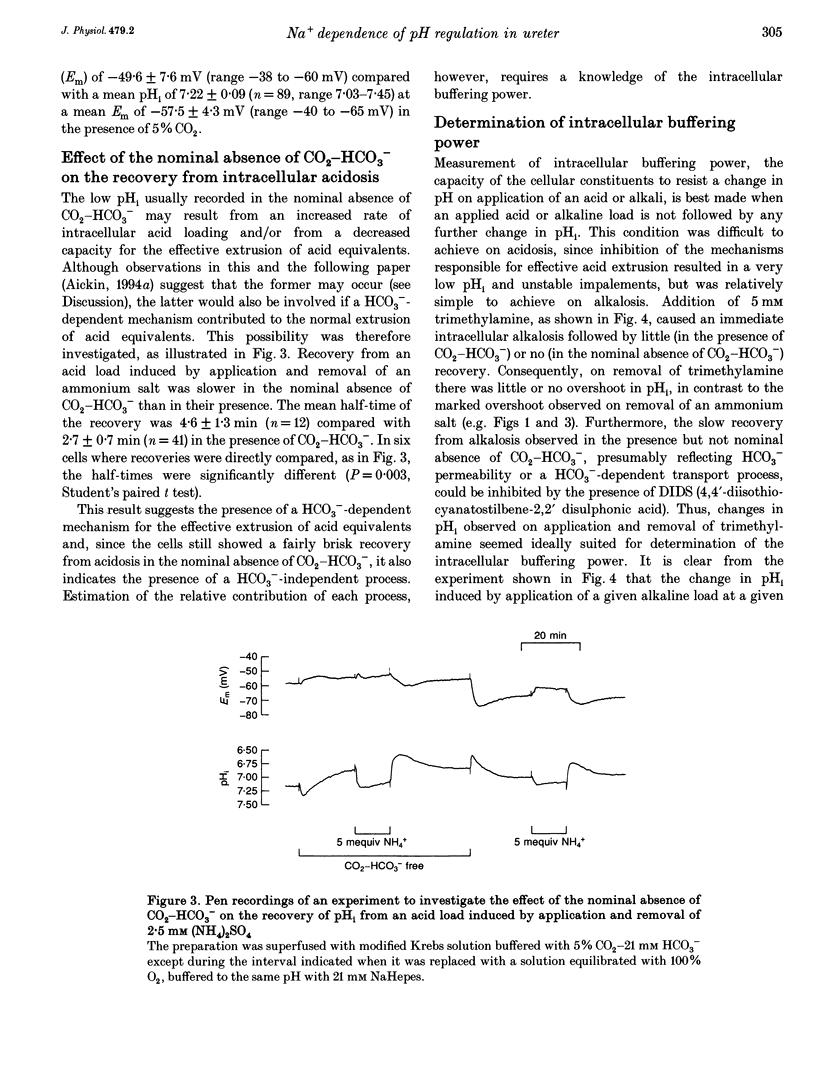
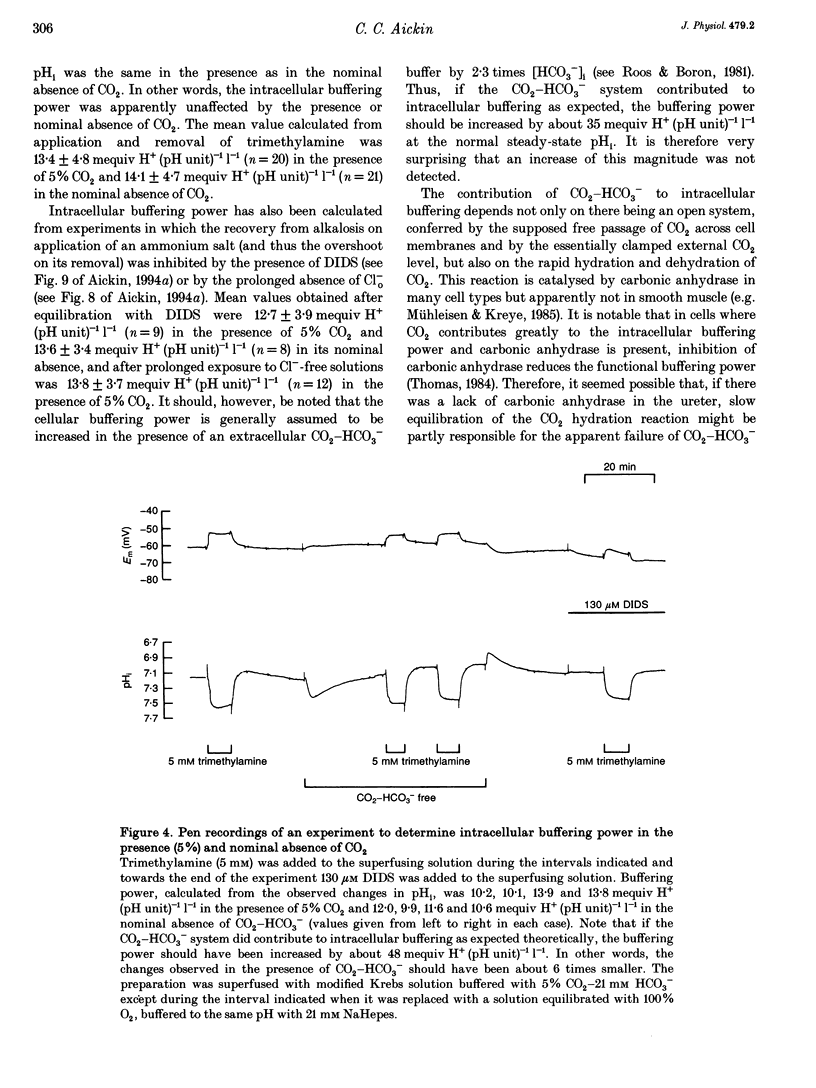
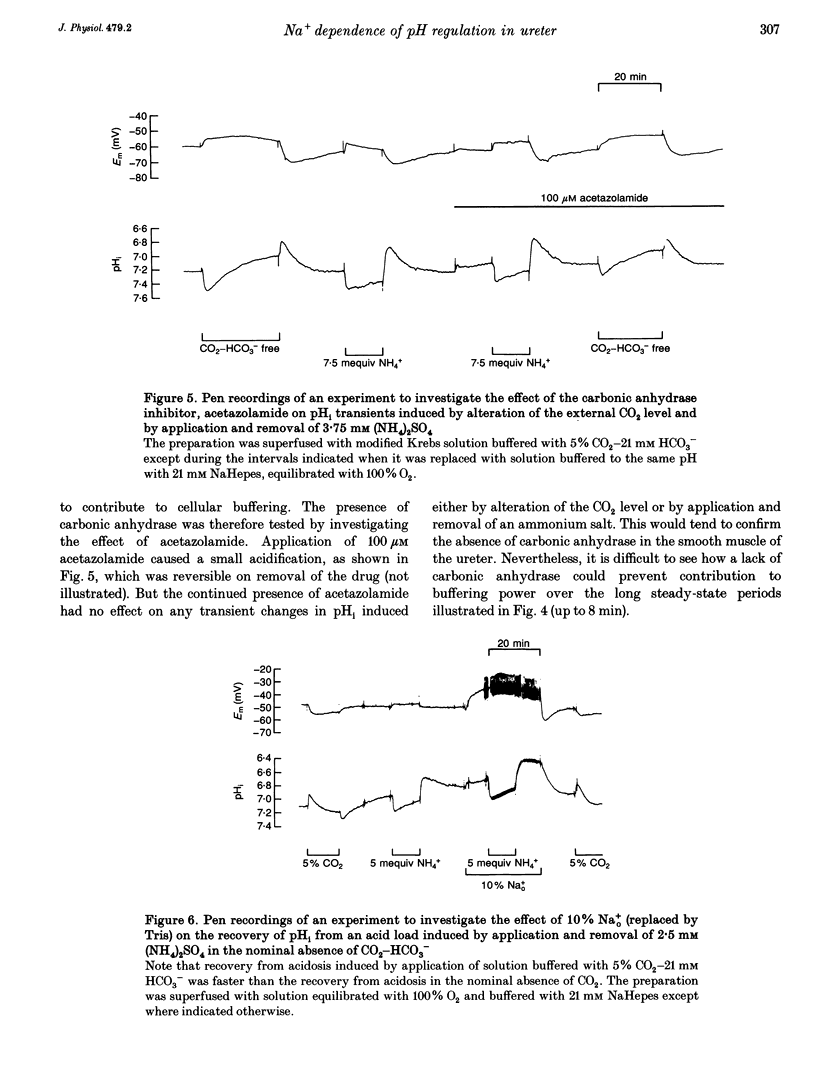
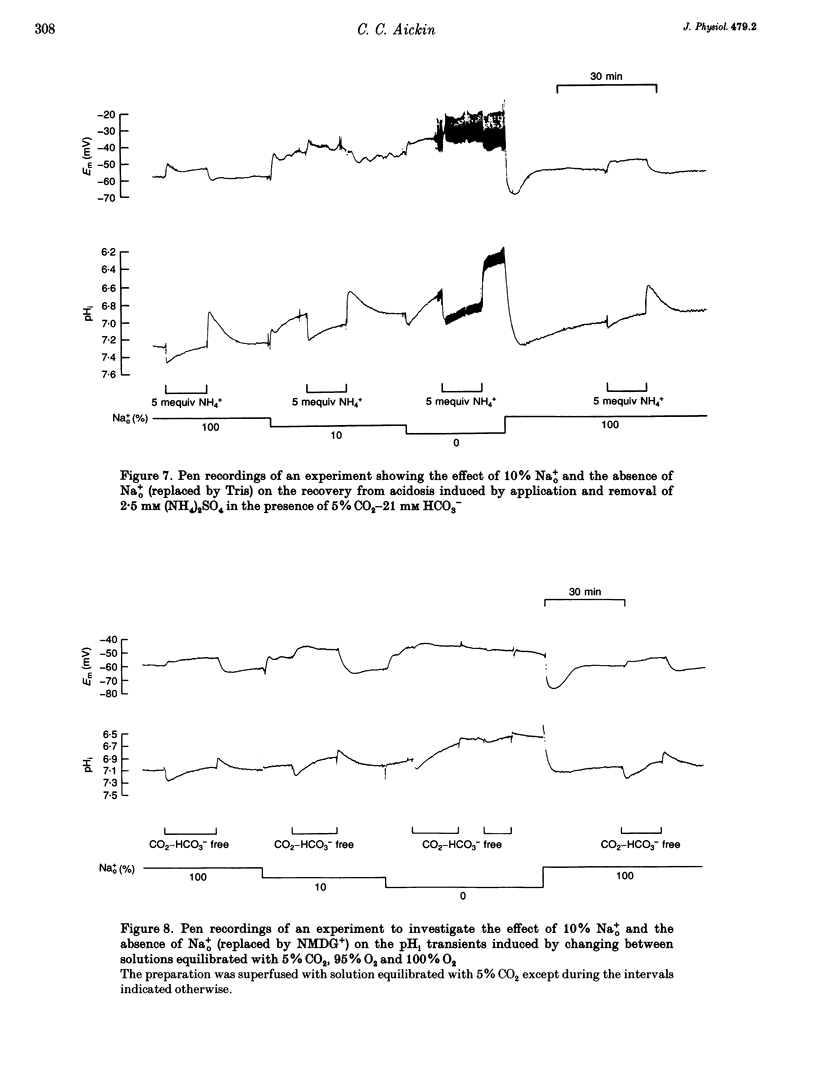
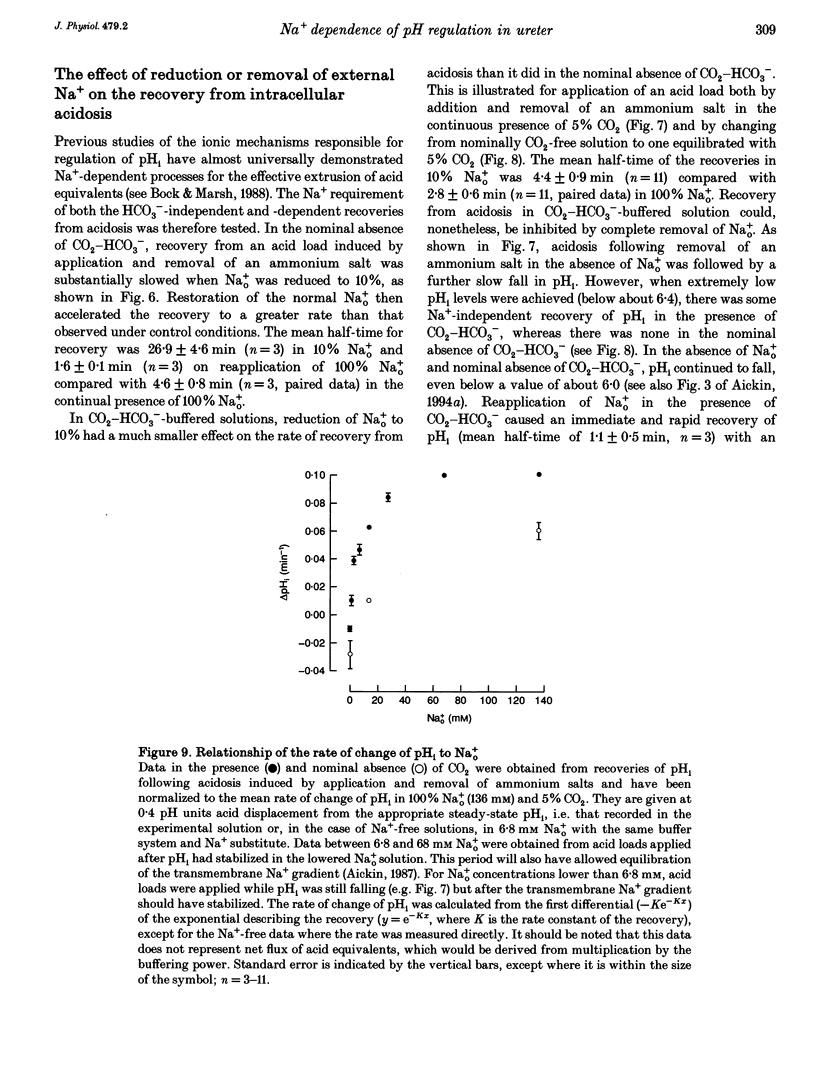
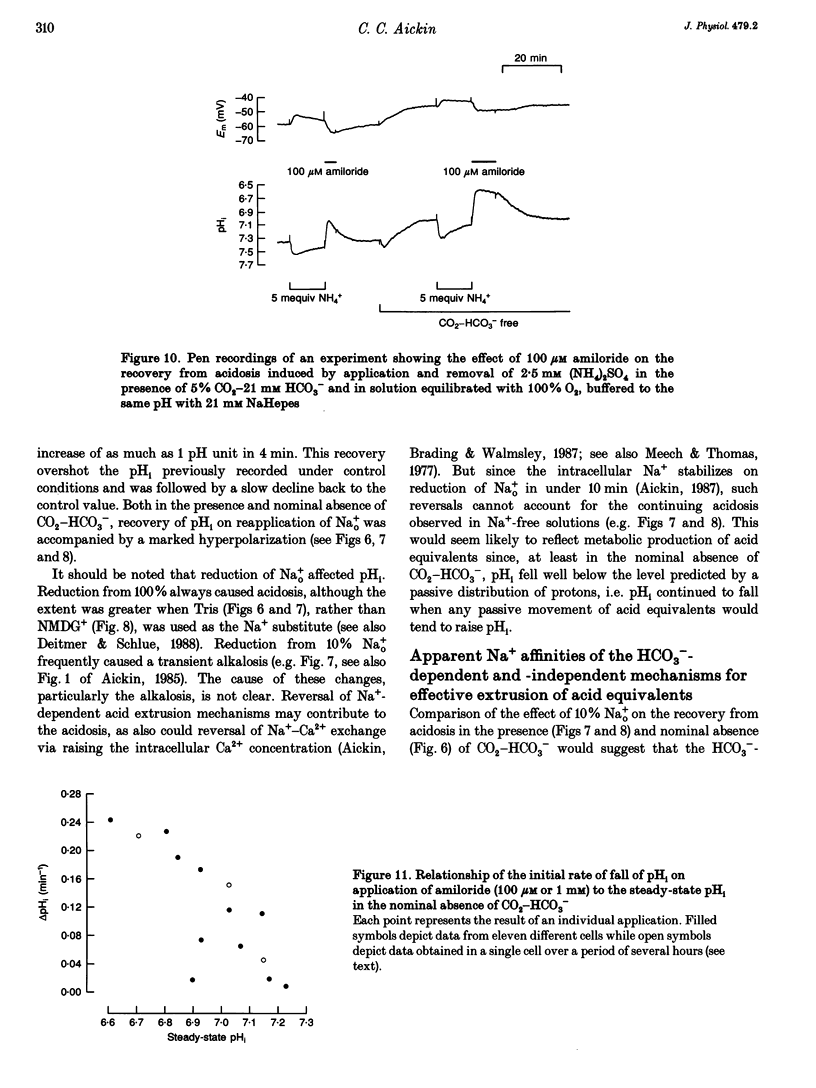
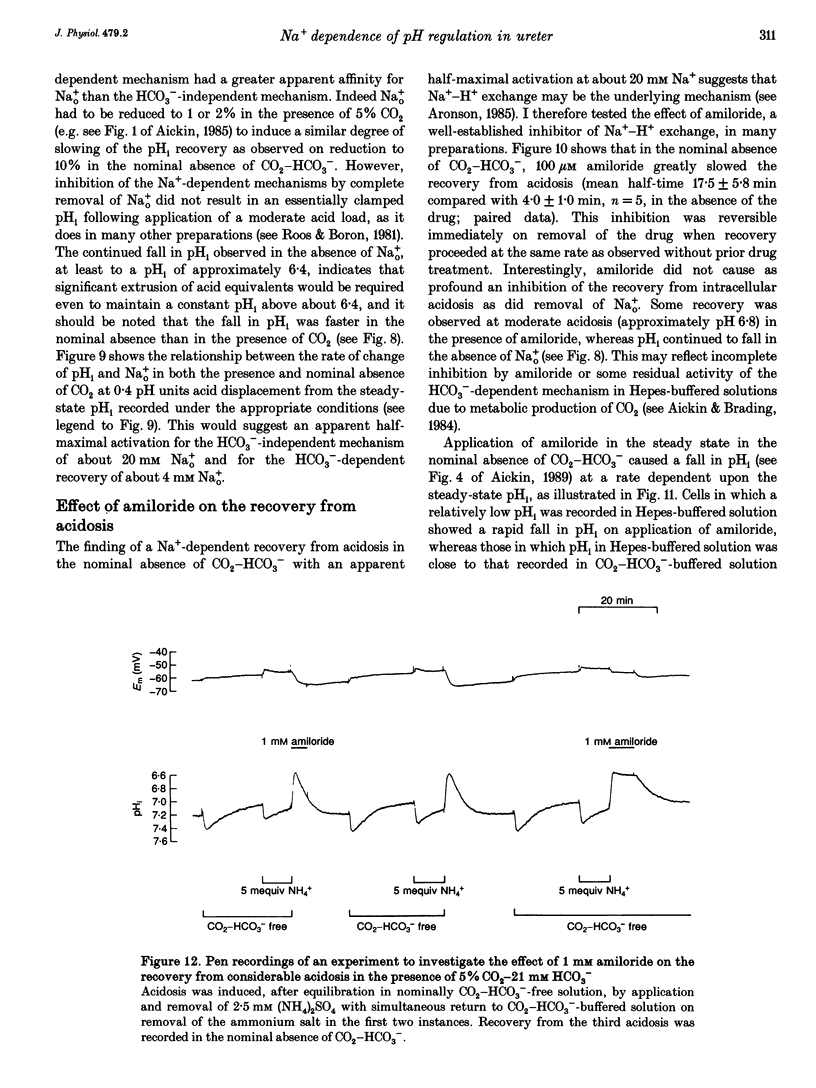
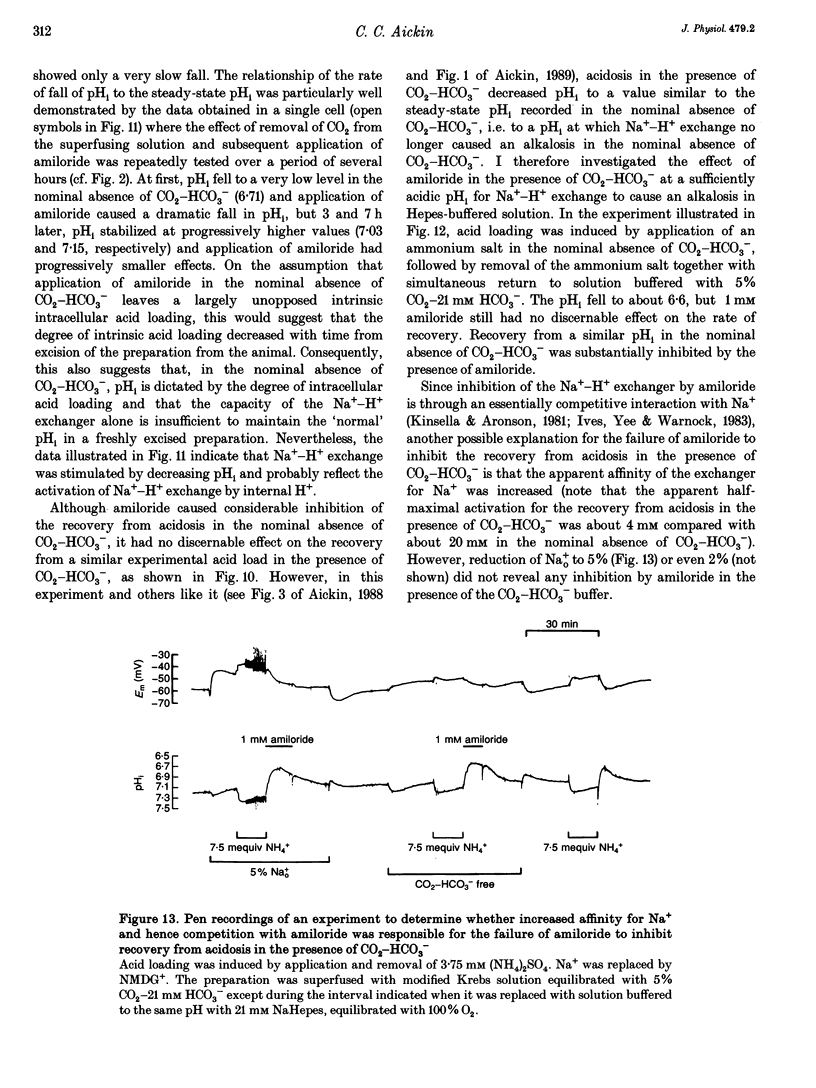
1. Mechanisms involved in the regulation of intracellular pH (pHi) in smooth muscle cells of guinea-pig ureter have been investigated using double-barrelled pH-sensitive microelectrodes in isolated strips of tissue. 2. Removal of CO2-HCO3- from the superfusing solution caused a fall in the steady-state pHi except in a few cells which had been excised from the animal for many hours (usually > 24 h). The pHi value was 7.22 +/- 0.09 (n = 89; mean +/- S.D. of an observation) in solution buffered with 5% CO2-21 mM HCO3-, compared with 6.92 +/- 0.24 (n = 67) in the nominal absence of CO2-HCO3-. Recovery from experimentally induced acidosis was faster in the presence, rather than nominal absence, of CO2-HCO3- (mean half-times of 2.7 +/- 0.7 min, n = 41, and 4.6 +/- 1.3 min, n = 12, respectively). These results suggest the presence of both HCO(3-)-dependent and -independent mechanisms for the effective extrusion of acid equivalents. 3. Recovery from acidosis was dependent on external Na+ (Na+o) in both the presence and nominal absence of CO2-HCO3-, with an apparent half-maximal activation at approximately 4 and 20 mM Na+o, respectively. Removal of Na+o in the steady state caused a fall in pHi which proceeded at a faster rate in the presence rather than in the nominal absence of CO2-HCO3-. 4. Amiloride (100 microM-1 mM) reversibly inhibited the recovery from acidosis and caused a fall in the steady-state pHi when applied in the nominal absence of CO2-HCO3-, but had no measurable effect on either the recovery from acidosis or steady-state pHi in the presence of CO2-HCO3-. These results suggest that Na(+)-H+ exchange was responsible for extrusion of acid equivalents in the nominal absence of CO2 and HCO3-, but that it played little part under more physiological conditions. 5. Although Na(+)-H+ exchange appeared to be activated below a pHi of about 7.2, it was incapable of maintaining a 'normal' pHi in the nominal absence of CO2-HCO3- in freshly excised cells, where values between 6.06 and 6.89 were recorded. Only in aged preparations, in which the intrinsic intracellular acid loading was substantially reduced (as judged from the rate of acidification on application of amiloride in the nominal absence of CO2-HCO3-) did the steady-state value approximate to that observed in the presence of CO2-HCO3-, at about 7.2.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Cragoe E. J., Jr Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol. 1988 Aug;402:391–410. doi: 10.1113/jphysiol.1988.sp017211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalkjaer C., Hughes A. Chloride and bicarbonate transport in rat resistance arteries. J Physiol. 1991 May;436:57–73. doi: 10.1113/jphysiol.1991.sp018539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Effect of Na+ and K+ on Cl- distribution in guinea-pig vas deferens smooth muscle: evidence for Na+, K+, Cl- co-transport. J Physiol. 1990 Feb;421:13–32. doi: 10.1113/jphysiol.1990.sp017931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F., Walmsley D. An investigation of sodium-calcium exchange in the smooth muscle of guinea-pig ureter. J Physiol. 1987 Oct;391:325–346. doi: 10.1113/jphysiol.1987.sp016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Direct measurement of intracellular pH and buffering power in smooth muscle cells of guinea-pig vas deferens. J Physiol. 1984 Apr;349:571–585. doi: 10.1113/jphysiol.1984.sp015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Intracellular pH regulation by vertebrate muscle. Annu Rev Physiol. 1986;48:349–361. doi: 10.1146/annurev.ph.48.030186.002025. [DOI] [PubMed] [Google Scholar]
- Aickin C. C. Investigation of factors affecting the intracellular sodium activity in the smooth muscle of guinea-pig ureter. J Physiol. 1987 Apr;385:483–505. doi: 10.1113/jphysiol.1987.sp016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Movement of acid equivalents across the mammalian smooth muscle cell membrane. Ciba Found Symp. 1988;139:3–22. doi: 10.1002/9780470513699.ch2. [DOI] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Boron W. F., McCormick W. C., Roos A. pH regulation in barnacle muscle fibers: dependence on intracellular and extracellular pH. Am J Physiol. 1979 Sep;237(3):C185–C193. doi: 10.1152/ajpcell.1979.237.3.C185. [DOI] [PubMed] [Google Scholar]
- Bountra C., Powell T., Vaughan-Jones R. D. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. J Physiol. 1990 May;424:343–365. doi: 10.1113/jphysiol.1990.sp018071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Wray S. The effects of pregnancy and parturition on phosphorus metabolites in rat uterus studied by 31P nuclear magnetic resonance. J Physiol. 1985 Nov;368:19–31. doi: 10.1113/jphysiol.1985.sp015844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989 Apr;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. Intracellular acidosis of identified leech neurones produced by substitution of external sodium. Brain Res. 1988 Oct 18;462(2):233–241. doi: 10.1016/0006-8993(88)90551-3. [DOI] [PubMed] [Google Scholar]
- Gerstheimer F. P., Mühleisen M., Nehring D., Kreye V. A. A chloride-bicarbonate exchanging anion carrier in vascular smooth muscle of the rabbit. Pflugers Arch. 1987 Jun;409(1-2):60–66. doi: 10.1007/BF00584750. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Rothstein A. Cytoplasmic pH regulation in thymic lymphocytes by an amiloride-sensitive Na+/H+ antiport. J Gen Physiol. 1984 Mar;83(3):341–369. doi: 10.1085/jgp.83.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R. Effect of H+ and elevated PCO2 on membrane electrical properties of rat cerebral arteries. Pflugers Arch. 1982 Aug;394(2):182–185. doi: 10.1007/BF00582922. [DOI] [PubMed] [Google Scholar]
- Hatori N., Fine B. P., Nakamura A., Cragoe E., Jr, Aviv A. Angiotensin II effect on cytosolic pH in cultured rat vascular smooth muscle cells. J Biol Chem. 1987 Apr 15;262(11):5073–5078. [PubMed] [Google Scholar]
- Ives H. E., Yee V. J., Warnock D. G. Mixed type inhibition of the renal Na+/H+ antiporter by Li+ and amiloride. Evidence for a modifier site. J Biol Chem. 1983 Aug 25;258(16):9710–9716. [PubMed] [Google Scholar]
- Kaila K., Vaughan-Jones R. D. Influence of sodium-hydrogen exchange on intracellular pH, sodium and tension in sheep cardiac Purkinje fibres. J Physiol. 1987 Sep;390:93–118. doi: 10.1113/jphysiol.1987.sp016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H., Schlue W. R. Intracellular pH regulation in cultured mouse oligodendrocytes. J Physiol. 1988 Dec;406:147–162. doi: 10.1113/jphysiol.1988.sp017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Korbmacher C., Helbig H., Stahl F., Wiederholt M. Evidence for Na/H exchange and Cl/HCO3 exchange in A10 vascular smooth muscle cells. Pflugers Arch. 1988 Jul;412(1-2):29–36. doi: 10.1007/BF00583728. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühleisen M., Kreye V. A. Lack of soluble carbonic anhydrase in aortic smooth muscle of the rabbit. Pflugers Arch. 1985 Oct;405(3):234–236. doi: 10.1007/BF00582566. [DOI] [PubMed] [Google Scholar]
- Owen N. E. Effect of catecholamines on Na/H exchange in vascular smooth muscle cells. J Cell Biol. 1986 Nov;103(5):2053–2060. doi: 10.1083/jcb.103.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam R. W., Grubbs R. D. Steady-state pHi, buffering power, and effect of CO2 in a smooth muscle-like cell line. Am J Physiol. 1990 Mar;258(3 Pt 1):C461–C469. doi: 10.1152/ajpcell.1990.258.3.C461. [DOI] [PubMed] [Google Scholar]
- Putnam R. W. pH regulatory transport systems in a smooth muscle-like cell line. Am J Physiol. 1990 Mar;258(3 Pt 1):C470–C479. doi: 10.1152/ajpcell.1990.258.3.C470. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Brock T. A. Analysis of angiotensin-stimulated sodium transport in cultured smooth muscle cells from rat aorta. J Cell Physiol. 1983 Mar;114(3):284–290. doi: 10.1002/jcp.1041140306. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Cohen C. J. A liquid ion-exchanger alternative to KCl for filling intracellular reference microelectrodes. Pflugers Arch. 1981 Apr;390(1):96–98. doi: 10.1007/BF00582719. [DOI] [PubMed] [Google Scholar]
- Vigne P., Breittmayer J. P., Frelin C., Lazdunski M. Dual control of the intracellular pH in aortic smooth muscle cells by a cAMP-sensitive HCO3-/Cl- antiporter and a protein kinase C-sensitive Na+/H+ antiporter. J Biol Chem. 1988 Dec 5;263(34):18023–18029. [PubMed] [Google Scholar]
- Weissberg P. L., Little P. J., Cragoe E. J., Jr, Bobik A. Na-H antiport in cultured rat aortic smooth muscle: its role in cytoplasmic pH regulation. Am J Physiol. 1987 Aug;253(2 Pt 1):C193–C198. doi: 10.1152/ajpcell.1987.253.2.C193. [DOI] [PubMed] [Google Scholar]
- de Hemptinne A., Marrannes R., Vanheel B. Surface pH and the control of intracellular pH in cardiac and skeletal muscle. Can J Physiol Pharmacol. 1987 May;65(5):970–977. doi: 10.1139/y87-154. [DOI] [PubMed] [Google Scholar]


