Abstract
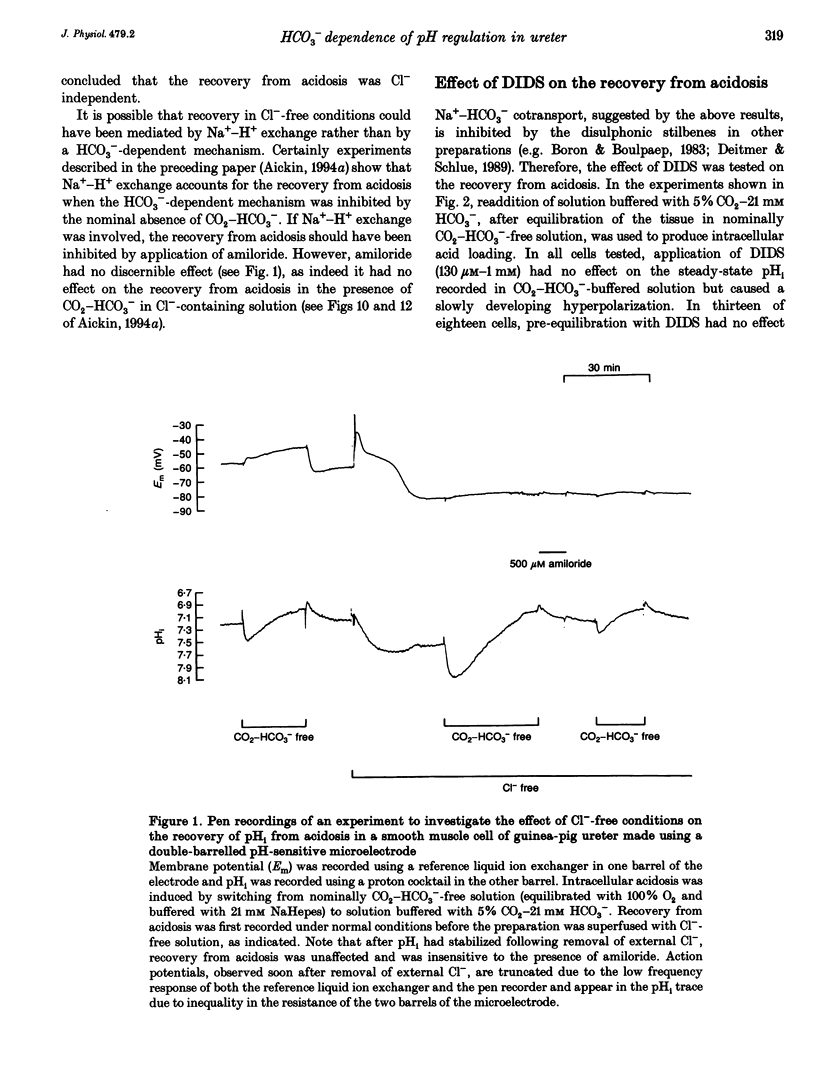
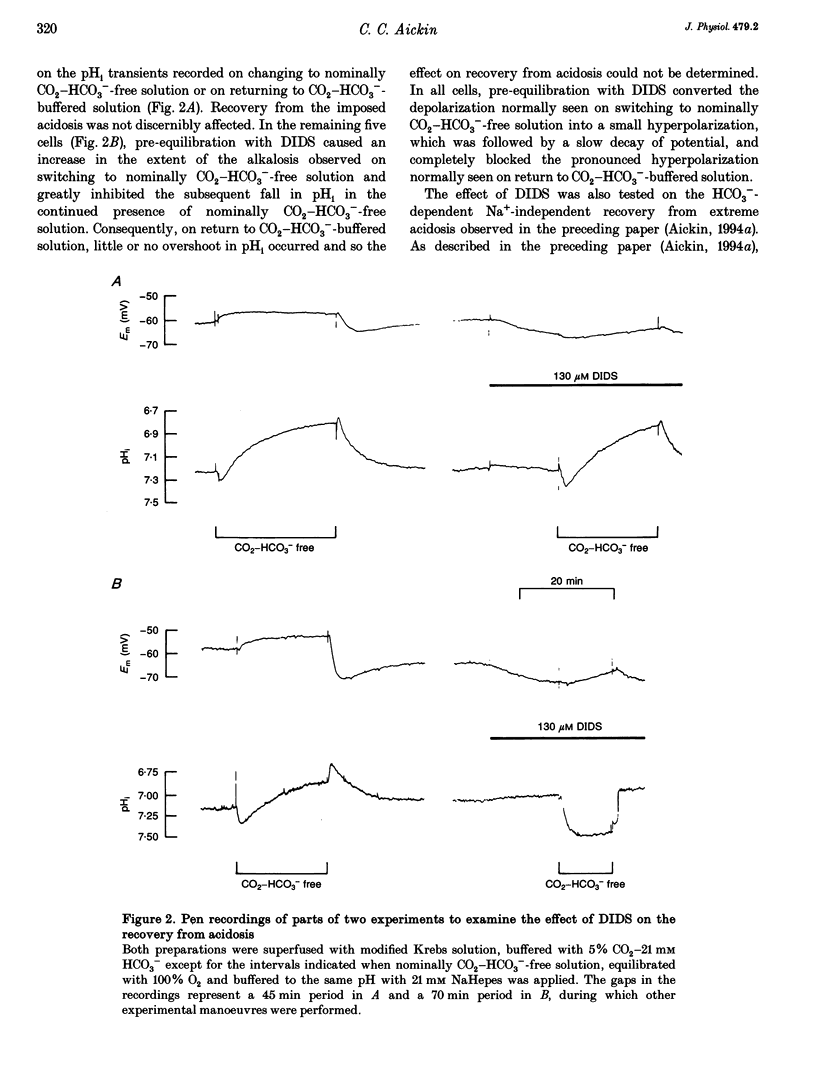
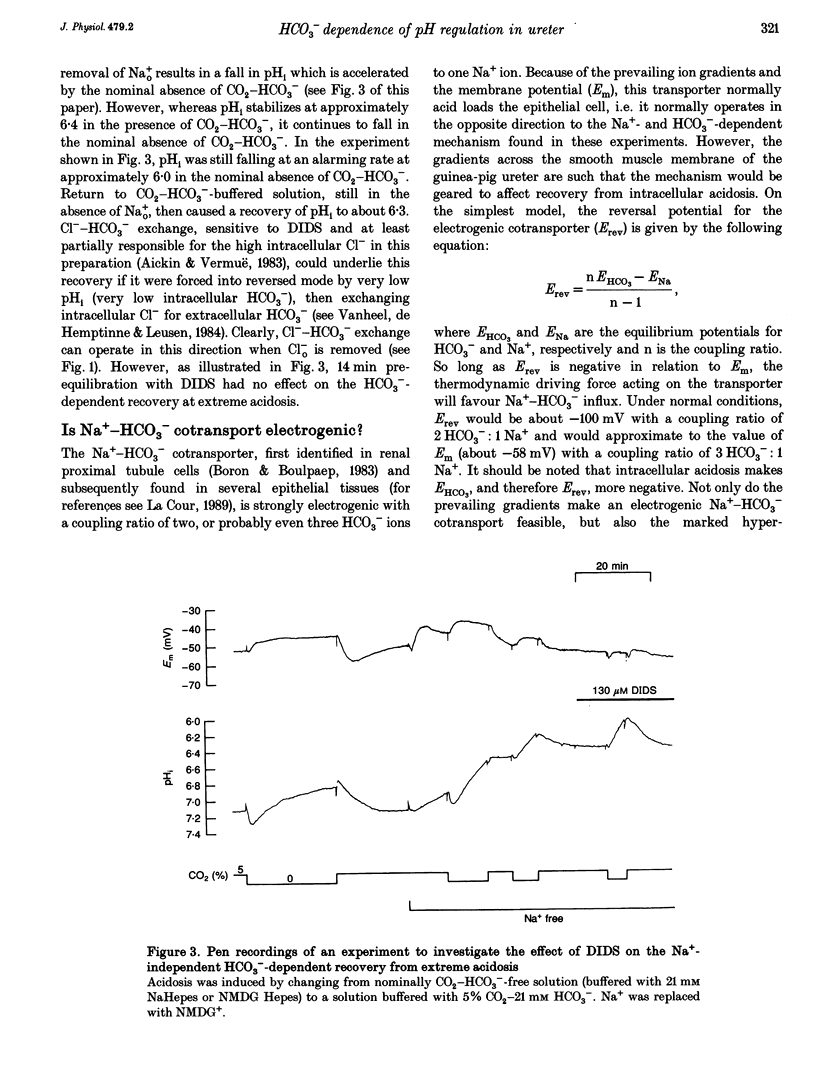
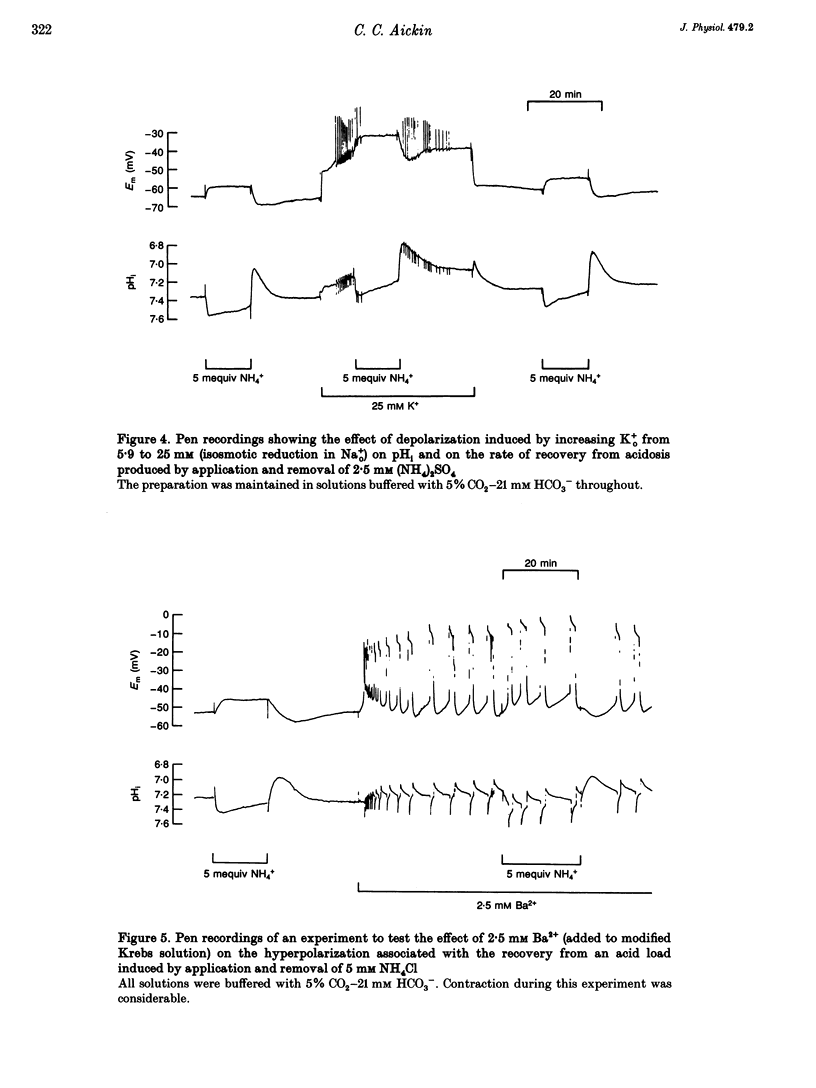
1. HCO3(-)-dependent mechanisms involved in the regulation of intracellular pH (pHi) were characterized using double-barrelled pH-sensitive microelectrodes in smooth muscle cells of the isolated guinea-pig ureter. 2. Removal of external Cl- in the presence of CO2-HCO3- caused a transient alkalosis, consistent with the presence of Cl(-)-HCO3- exchange, before pHi slowly recovered. Recovery from acidosis in the presence of CO2-HCO3- was not affected, at a time when intracellular Cl- would have been maximally depleted, indicating that a counter transport of Cl- and HCO3- was not involved. The recovery was also not affected by amiloride, indicating that Na(+)-H+ exchange was not involved. 3. A transient hyperpolarization was associated with the recovery from acidosis in the presence of CO2-HCO3-, consistent with rheogenic coupling of Na(+)-HCO3- cotransport. However, depolarization caused by elevation of the extracellular potassium (K+o) concentration, which should favour inward transport by the rheogenic mechanism, caused a fall in pHi and decreased the rate of recovery from acidosis. Furthermore, ouabain abolished the transient hyperpolarization without affecting the recovery of pHi. It is concluded that Na(+)-HCO3- cotransport in the ureter is electroneutral. 4. Recovery from acidosis in the presence of CO2-HCO3- was insensitive to DIDS even after prolonged pre-equilibriation and extreme acidosis. The results suggest that Na(+)-HCO3- cotransport in the ureter is insensitive to DIDS and that Cl(-)-HCO3- exchange does not reverse to contribute to the extrusion of acid equivalents. A HCO3- conductance may account for the Na(+)-independent, HCO3(-)-dependent recovery from extreme acidosis. 5. Recovery from experimentally induced alkalosis was inhibited by Cl(-)-free conditions and by DIDS, indicating that Cl(-)-HCO3- exchange was involved. 6. It is concluded that pHi in the smooth muscle of guinea-pig ureter is controlled by three transport mechanisms. By far the most important is an electroneutral Na(+)-HCO3- cotransporter. Na(+)-H+ exchange appears to play little role in the presence of the physiological buffer. Both of these mechanisms extrude acid equivalents and so protect the cell against its fairly substantial intrinsic intracellular acid loading. Cl(-)-HCO3- exchange, on the other hand, is stimulated by intracellular alkalosis to transport acid equivalents into the cell and so restore a more normal pHi.
Full text
PDF

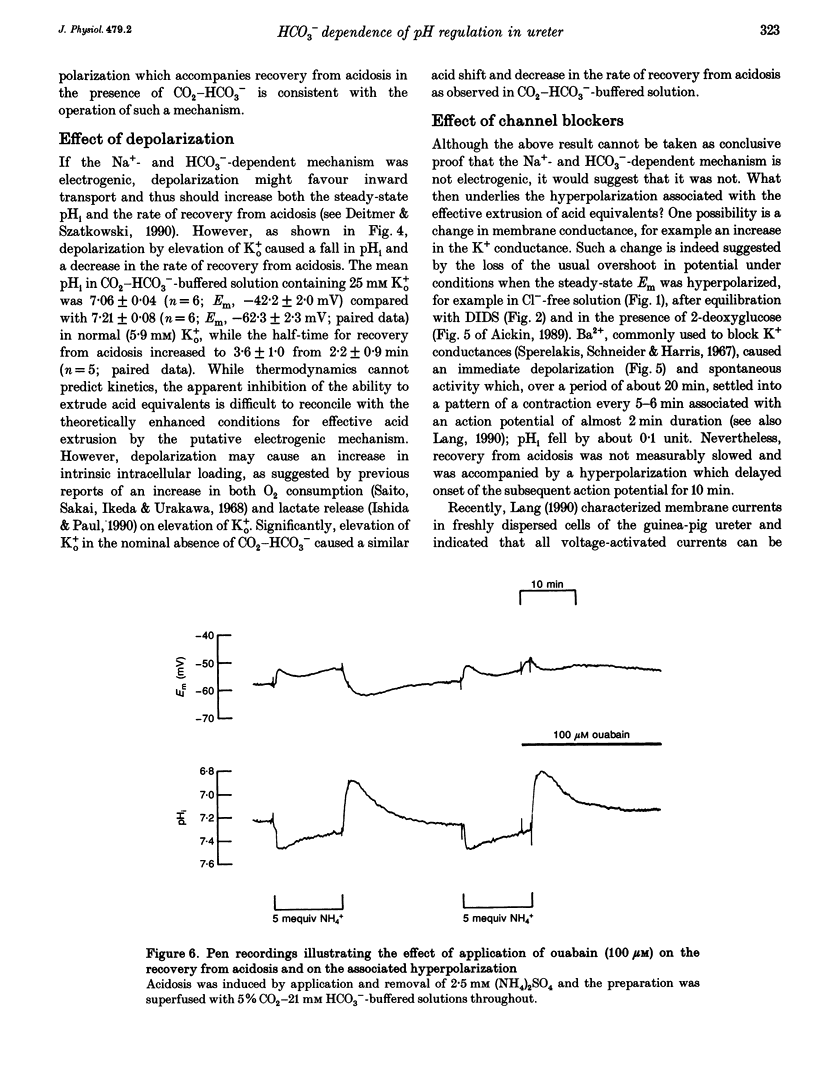
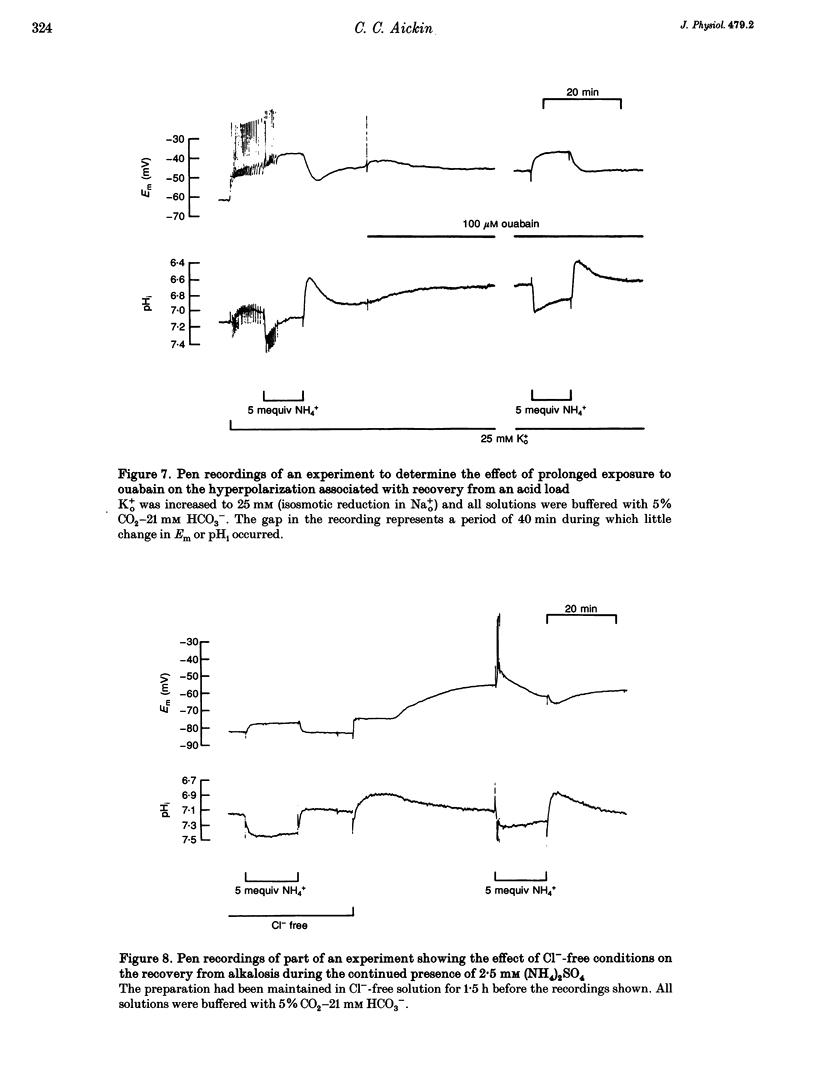
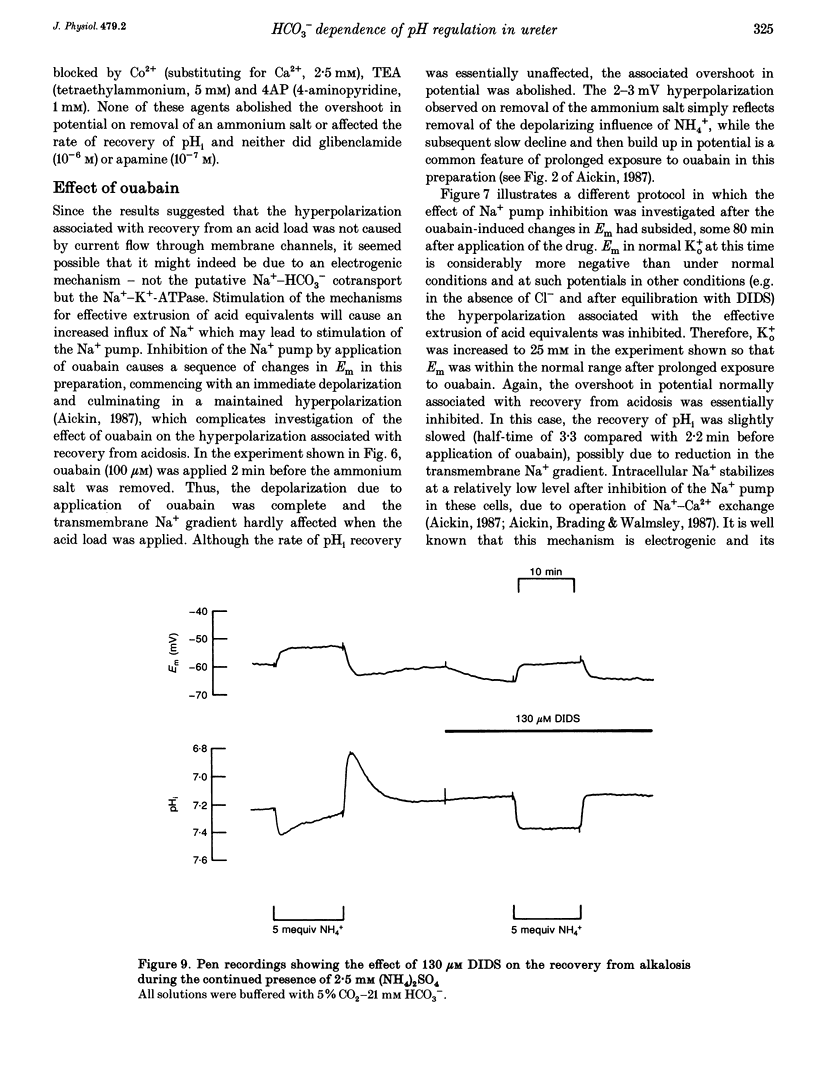




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Cragoe E. J., Jr Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol. 1988 Aug;402:391–410. doi: 10.1113/jphysiol.1988.sp017211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982 May;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. The role of chloride-bicarbonate exchange in the regulation of intracellular chloride in guinea-pig vas deferens. J Physiol. 1984 Apr;349:587–606. doi: 10.1113/jphysiol.1984.sp015175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F., Walmsley D. An investigation of sodium-calcium exchange in the smooth muscle of guinea-pig ureter. J Physiol. 1987 Oct;391:325–346. doi: 10.1113/jphysiol.1987.sp016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Investigation of factors affecting the intracellular sodium activity in the smooth muscle of guinea-pig ureter. J Physiol. 1987 Apr;385:483–505. doi: 10.1113/jphysiol.1987.sp016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Movement of acid equivalents across the mammalian smooth muscle cell membrane. Ciba Found Symp. 1988;139:3–22. doi: 10.1002/9780470513699.ch2. [DOI] [PubMed] [Google Scholar]
- Aickin C. C. Regulation of intracellular pH in smooth muscle cells of the guinea-pig femoral artery. J Physiol. 1994 Sep 1;479(Pt 2):331–340. doi: 10.1113/jphysiol.1994.sp020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Regulation of intracellular pH in the smooth muscle of guinea-pig ureter: Na+ dependence. J Physiol. 1994 Sep 1;479(Pt 2):301–316. doi: 10.1113/jphysiol.1994.sp020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Vermuë N. A. Microelectrode measurement of intracellular chloride activity in smooth muscle cells of guinea-pig ureter. Pflugers Arch. 1983 Apr;397(1):25–28. doi: 10.1007/BF00585163. [DOI] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983 Mar;81(3):373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart C., Vaughan-Jones R. D. Na(+)-HCO3- symport in the sheep cardiac Purkinje fibre. J Physiol. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989 Apr;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Szatkowski M. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J Physiol. 1990 Feb;421:617–631. doi: 10.1113/jphysiol.1990.sp017965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Effects of hypoxia on high-energy phosphagen content, energy metabolism and isometric force in guinea-pig taenia caeci. J Physiol. 1990 May;424:41–56. doi: 10.1113/jphysiol.1990.sp018054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H., Schlue W. R. Intracellular pH regulation in cultured mouse oligodendrocytes. J Physiol. 1988 Dec;406:147–162. doi: 10.1113/jphysiol.1988.sp017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbmacher C., Helbig H., Stahl F., Wiederholt M. Evidence for Na/H exchange and Cl/HCO3 exchange in A10 vascular smooth muscle cells. Pflugers Arch. 1988 Jul;412(1-2):29–36. doi: 10.1007/BF00583728. [DOI] [PubMed] [Google Scholar]
- La Cour M. Rheogenic sodium-bicarbonate co-transport across the retinal membrane of the frog retinal pigment epithelium. J Physiol. 1989 Dec;419:539–553. doi: 10.1113/jphysiol.1989.sp017885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Buckler K. J., Vaughan-Jones R. D. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992 Dec;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. J. The whole-cell Ca2+ channel current in single smooth muscle cells of the guinea-pig ureter. J Physiol. 1990 Apr;423:453–473. doi: 10.1113/jphysiol.1990.sp018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon C. B., Little P. J., Cragoe E. J., Jr, Bobik A. Intracellular pH in human arterial smooth muscle. Regulation by Na+/H+ exchange and a novel 5-(N-ethyl-N-isopropyl)amiloride-sensitive Na(+)- and HCO3(-)-dependent mechanism. Circ Res. 1990 Oct;67(4):814–825. doi: 10.1161/01.res.67.4.814. [DOI] [PubMed] [Google Scholar]
- Putnam R. W. pH regulatory transport systems in a smooth muscle-like cell line. Am J Physiol. 1990 Mar;258(3 Pt 1):C470–C479. doi: 10.1152/ajpcell.1990.258.3.C470. [DOI] [PubMed] [Google Scholar]
- Saito Y., Sakai Y., Ikeda M., Urakawa N. Oxygen consumption during potassium-induced contracture in guinea pig taenia coli. Jpn J Pharmacol. 1968 Sep;18(3):321–331. doi: 10.1254/jjp.18.321. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F., Harris E. J. Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J Gen Physiol. 1967 Jul;50(6):1565–1583. doi: 10.1085/jgp.50.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C., Cohen C. J. A liquid ion-exchanger alternative to KCl for filling intracellular reference microelectrodes. Pflugers Arch. 1981 Apr;390(1):96–98. doi: 10.1007/BF00582719. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Analysis of Cl- -HCO3(-) exchange during recovery from intracellular acidosis in cardiac Purkinje strands. Am J Physiol. 1984 May;246(5 Pt 1):C391–C400. doi: 10.1152/ajpcell.1984.246.5.C391. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. An investigation of chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. J Physiol. 1986 Oct;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Chloride activity and its control in skeletal and cardiac muscle. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):537–548. doi: 10.1098/rstb.1982.0150. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P., Breittmayer J. P., Frelin C., Lazdunski M. Dual control of the intracellular pH in aortic smooth muscle cells by a cAMP-sensitive HCO3-/Cl- antiporter and a protein kinase C-sensitive Na+/H+ antiporter. J Biol Chem. 1988 Dec 5;263(34):18023–18029. [PubMed] [Google Scholar]


