Abstract
1. An in vitro brainstem slice preparation of the superior olivary complex has been developed permitting patch recording from a presynaptic terminal (calyx of Held) and from its postsynaptic target--the principal neurone of the medial nucleus of the trapezoid body (MNTB). 2. The fluorescent stain DiI (1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate) was used in fixed tissue and Lucifer Yellow in living slices, to identify calices enclosing single MNTB neuronal somata. 3. Whole-cell recording from the MNTB neurone shows evoked EPSCs preceded by a prespike, corresponding to the presynaptic action potential (AP). In some cases one patch pipette recorded from both pre- and postsynaptic elements, but confirmation of exclusively presynaptic recording was obtained using pipettes containing Lucifer Yellow in a further eleven cases. 4. Under current clamp, the pre- and postsynaptic sites could be distinguished by their response to step depolarizations; presynaptic terminals generated a train of APs at frequencies up to 200 Hz, while MNTB neurones gave a single AP. Each presynaptic AP had an after-hyperpolarization lasting less than 2 ms. 5. Under voltage clamp, step depolarizations of presynaptic terminals generated a tetrodotoxin-sensitive inward current followed by rapidly activating outward potassium currents at potentials more positive than -60 mV. The outward current exhibited little inactivation over the 150 ms steps and 4-aminopyridine (200 microM) blocked 63.0 +/- 14.5% (mean +/- S.D., n = 3) of the sustained current at 0 mV. Like the squid giant synapse, mammalian terminals express rapidly activating 'delayed rectifier'-type potassium currents.
Full text
PDF
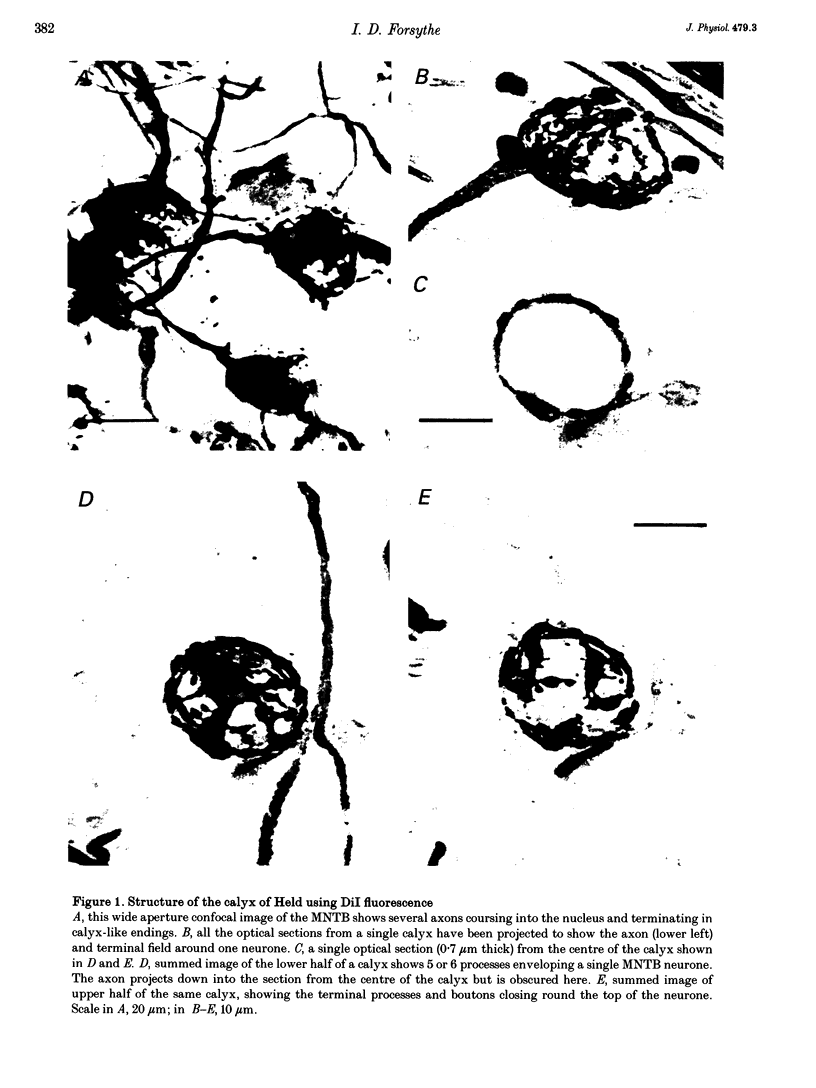

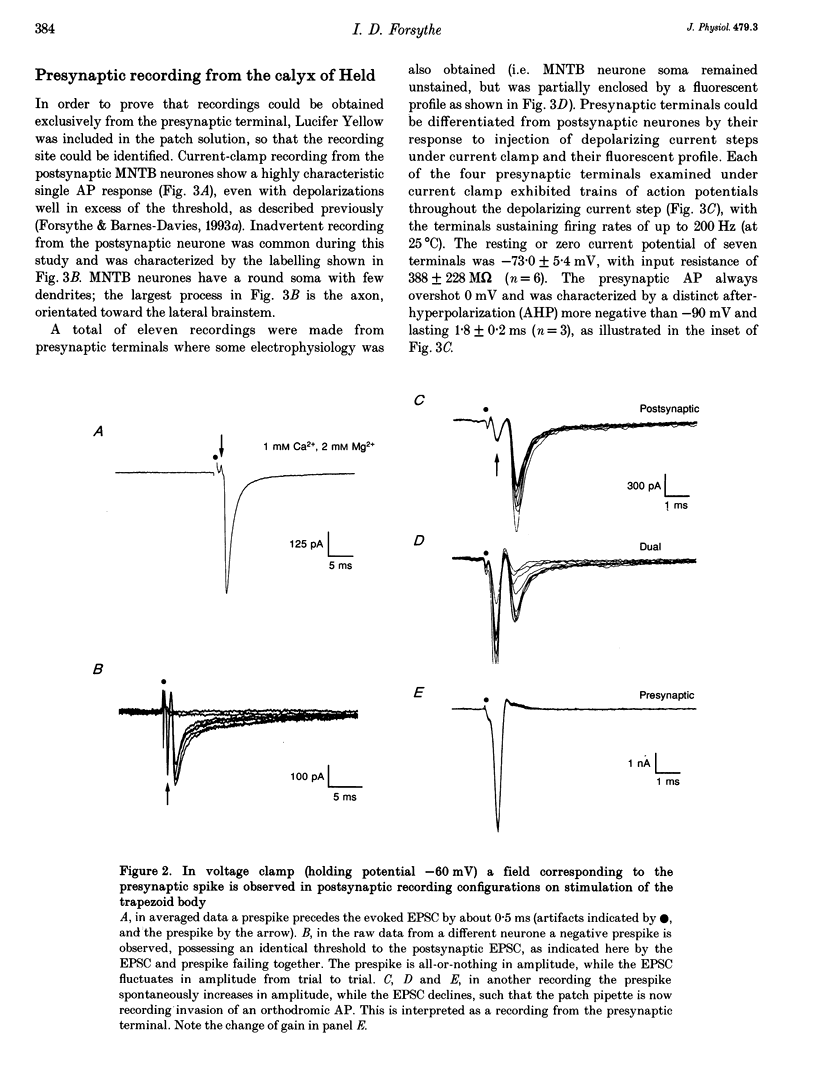
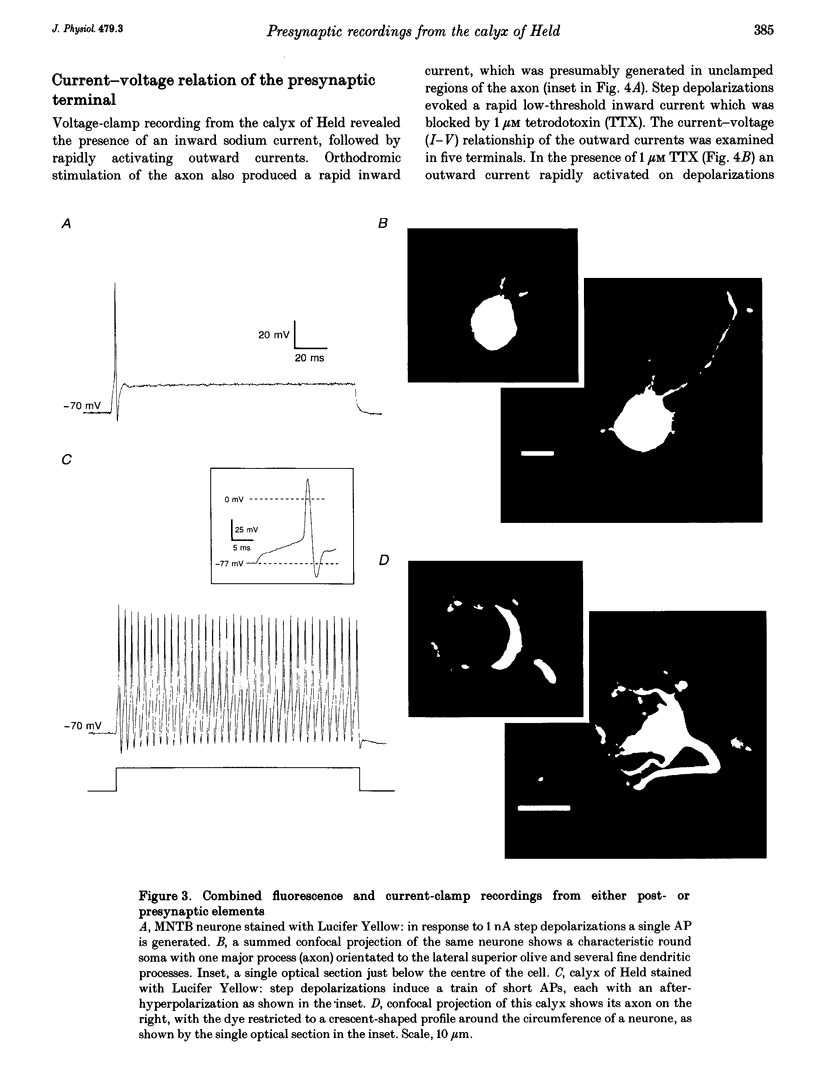
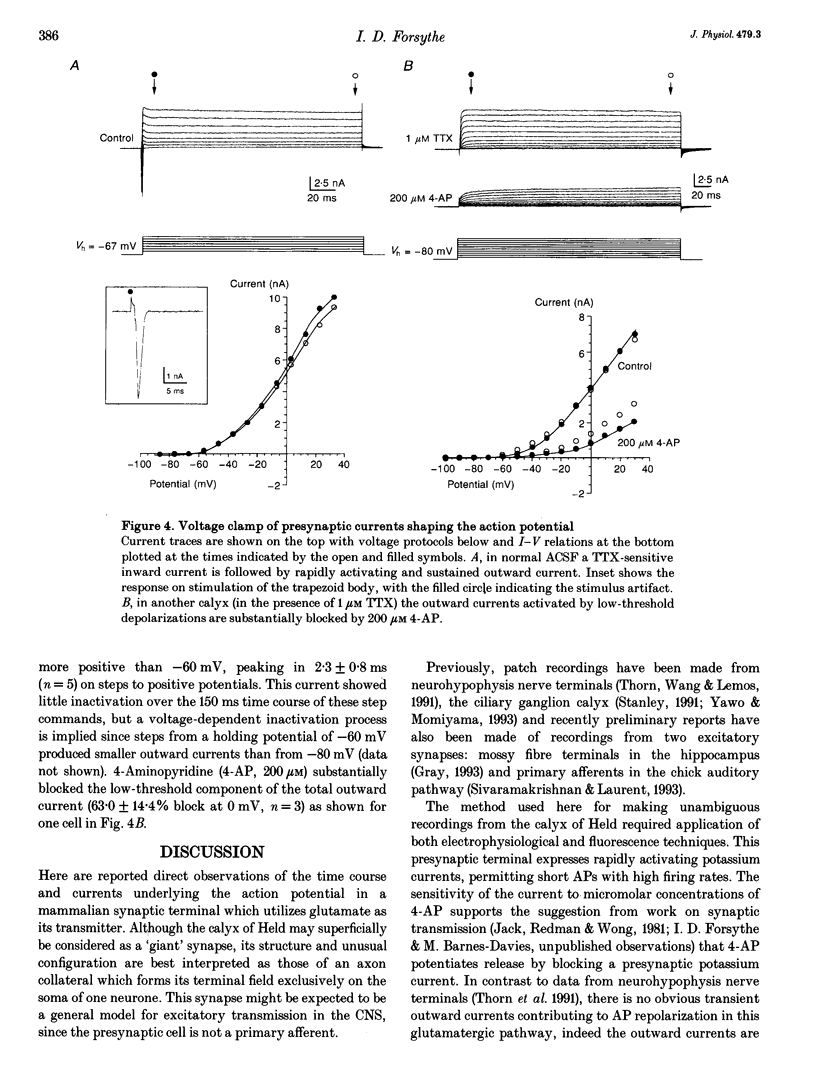

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J Physiol. 1990 Dec;431:343–364. doi: 10.1113/jphysiol.1990.sp018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Barrett J. N., Barrett E. F. Activation of internodal potassium conductance in rat myelinated axons. J Physiol. 1993 Dec;472:177–202. doi: 10.1113/jphysiol.1993.sp019942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Barnes-Davies M. The binaural auditory pathway: excitatory amino acid receptors mediate dual timecourse excitatory postsynaptic currents in the rat medial nucleus of the trapezoid body. Proc Biol Sci. 1993 Feb 22;251(1331):151–157. doi: 10.1098/rspb.1993.0022. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Barnes-Davies M. The binaural auditory pathway: membrane currents limiting multiple action potential generation in the rat medial nucleus of the trapezoid body. Proc Biol Sci. 1993 Feb 22;251(1331):143–150. doi: 10.1098/rspb.1993.0021. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. Modifications to synaptic transmission at group Ia synapses on cat spinal motoneurones by 4-aminopyridine. J Physiol. 1981 Dec;321:111–126. doi: 10.1113/jphysiol.1981.sp013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K., Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J Comp Neurol. 1993 Feb 8;328(2):161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A. Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. J Gen Physiol. 1993 Nov;102(5):797–816. doi: 10.1085/jgp.102.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis P. B., Marx S. O. Outward currents in isolated ventral cochlear nucleus neurons. J Neurosci. 1991 Sep;11(9):2865–2880. doi: 10.1523/JNEUROSCI.11-09-02865.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara N. M., Muniz Z. M., Wilkin G. P., Dolly J. O. Prominent location of a K+ channel containing the alpha subunit Kv 1.2 in the basket cell nerve terminals of rat cerebellum. Neuroscience. 1993 Dec;57(4):1039–1045. doi: 10.1016/0306-4522(93)90047-j. [DOI] [PubMed] [Google Scholar]
- Perney T. M., Marshall J., Martin K. A., Hockfield S., Kaczmarek L. K. Expression of the mRNAs for the Kv3.1 potassium channel gene in the adult and developing rat brain. J Neurophysiol. 1992 Sep;68(3):756–766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- Raman I. M., Trussell L. O. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992 Jul;9(1):173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- Sheng M., Tsaur M. L., Jan Y. N., Jan L. Y. Contrasting subcellular localization of the Kv1.2 K+ channel subunit in different neurons of rat brain. J Neurosci. 1994 Apr;14(4):2408–2417. doi: 10.1523/JNEUROSCI.14-04-02408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels on a cholinergic presynaptic nerve terminal. Neuron. 1991 Oct;7(4):585–591. doi: 10.1016/0896-6273(91)90371-6. [DOI] [PubMed] [Google Scholar]
- Thorn P. J., Wang X. M., Lemos J. R. A fast, transient K+ current in neurohypophysial nerve terminals of the rat. J Physiol. 1991 Jan;432:313–326. doi: 10.1113/jphysiol.1991.sp018386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Momiyama A. Re-evaluation of calcium currents in pre- and postsynaptic neurones of the chick ciliary ganglion. J Physiol. 1993 Jan;460:153–172. doi: 10.1113/jphysiol.1993.sp019464. [DOI] [PMC free article] [PubMed] [Google Scholar]




