Abstract
Background
Adavosertib is a first-in-class, selective small-molecule inhibitor of Wee1. Olaparib is an inhibitor of poly(ADP-ribose) polymerase (PARP). Preclinical data suggest that adavosertib enhances the antitumor effect of PARP inhibitors.
Objective
The safety, tolerability, and efficacy of adavosertib plus olaparib were evaluated in patients with refractory solid tumors to define the maximum tolerated dose (MTD) and recommended phase II dose (RP2D).
Patients and Methods
Eligible patients in part A (dose finding) had a refractory solid tumor for which there is no established treatment and had received ≥ 1 prior course of systemic therapy; in part B (dose expansion), patients had platinum-sensitive extensive-stage or relapsed small-cell lung cancer (SCLC). Patients received adavosertib [once (qd) or twice daily (bid)] for 3 consecutive days with 4 days off treatment (3/4), or 5 consecutive days with 2 days off (5/2), plus olaparib (bid) for 14 or 21 days of a 21-day cycle.
Results
A total of 130 patients were enrolled in the study, 120 in part A and 10 in part B. The MTD for adavosertib bid was 175 mg (days 1–3, 8–10/21-day cycle) plus continuous olaparib 200 mg bid; the once-daily MTD (and RP2D) was adavosertib 200 mg (days 1–3, 8–10/21-day cycle) plus continuous olaparib 200 mg bid. In the MTD/RP2D cohort, one patient (7%) experienced a dose-limiting toxicity (DLT) of thrombocytopenia. The most common treatment-related adverse events (TRAEs) in the cohorts in which MTD/RP2D for bid dosing and RP2D for qd dosing were determined were fatigue (64.3% and 15.4%, respectively), diarrhea (42.9% and 30.8%), decreased appetite (35.7% and 23.1%), nausea (35.7% and 15.4%), and anemia (35.7% and 38.5%). In the SCLC dose-expansion cohort, TRAEs occurred in eight patients (88.9%), including thrombocytopenia (66.7%) and anemia (55.6%). In part A, objective response rate (ORR) was 14.8% [95% confidence interval (CI) 8.7–22.9] overall; for the cohorts in which MTD/RP2D for bid dosing and RP2D for qd dosing were determined, ORR was 30.8% (9.1–61.4) and 9.1% (0.2–41.3), respectively. ORR was 11.1% [95% CI 0.3–48.2; one partial response (PR)], disease control rate was 22.2% (2.8–60.0; one PR, one stable disease), and median progression-free survival was 1.5 months (1.3–4.2) in the SCLC dose-expansion cohort.
Conclusions
Adverse events and DLTs observed in the bid MTD and once-daily MTD/RP2D dosing schedules were manageable and consistent with known adavosertib and olaparib safety profiles. Limited antitumor activity was observed with adavosertib plus olaparib combination therapy.
Trial Registration
ClinicalTrials.gov, NCT02511795 (registration: 28 July 2015).
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-024-01102-8.
Key Points
| The maximum tolerated dose and recommended phase II dose of once-daily adavosertib was determined to be 200 mg (days 1–3, 8–10/21-day cycle) plus continuous olaparib 200 mg twice daily. |
| The safety profile of combination therapy was consistent with previous monotherapy studies with either agent, and adverse events were generally manageable in this heavily pretreated population of patients with refractory solid tumors. |
| Limited antitumor activity of adavosertib in combination with olaparib was observed. |
Introduction
Genomic instability and replication stress are features of cancer cells that result in the accumulation of DNA damage [1]. Wee1 is a tyrosine kinase that regulates cyclin-dependent kinases 1 and 2 (CDK1 and CDK2), which regulate the cell cycle G2/M checkpoint and intra-S replication stress response, respectively [2–4]. During S-phase, the presence of replication stress leads to the activation of Wee1 and the inactivation of CDK2 to reduce the incidence of replication-associated DNA damage. Unrepaired DNA damage in the G2-phase will also activate Wee1 leading to CDK1 phosphorylation activation of the G2/M checkpoint, thus providing time for the repair of this DNA damage before cells enter mitosis [5]. Inhibition of Wee1, therefore, has the combined potential to induce cancer cell death, either by replication catastrophe in S-phase or mitotic catastrophe in M-phase [1, 2, 6, 7].
Adavosertib (AZD1775) is an orally active, first-in-class, selective small-molecule inhibitor of Wee1 (Wee1i), investigated as a treatment for advanced solid tumors [7]. Acceptable preliminary safety, tolerability, and efficacy has been shown with adavosertib monotherapy in several phase I and II studies in patients with advanced solid tumors [8–11]. Efficacy has also been shown with adavosertib monotherapy in two phase II trials in women with recurrent uterine serous carcinoma [12, 13].
Poly(ADP-ribose) polymerase (PARP) inhibition by olaparib leads to both an inhibition of PARP enzyme activity [14] as well as trapping PARP onto DNA at the site of DNA damage [15], leading to replication fork collapse and the induction of DNA double-strand breaks. Cancer cells deficient in homologous recombination repair, including those harboring mutations in BRCA1 and/or BRCA2, are susceptible to the single-agent activity of PARP inhibitors through this concept of synthetic lethality [16, 17], and this concept has been validated in the clinic [18]. Olaparib is an inhibitor of PARP that is currently approved in a number of countries across multiple tumor types, including advanced or recurrent ovarian, early or metastatic breast, metastatic prostate, metastatic pancreatic, and mismatch repair proficient advanced or recurrent endometrial cancer (in combination with durvalumab) [19, 20]. Preclinical data demonstrate that adavosertib has the potential to both enhance the antitumor effect of PARP inhibitors such as olaparib as well as overcome PARP inhibitor resistance in patient-derived models of ovarian, triple-negative breast (TNBC), and small-cell lung cancer (SCLC) [21–23].
This phase Ib study aimed to investigate the safety, tolerability, and preliminary efficacy of adavosertib plus olaparib in patients with refractory solid tumors to define the maximum tolerated dose (MTD) and recommended phase II dose (RP2D). Archival tissue was collected for exploratory biomarker analysis to identify any genetic alterations that may predict the clinical response to the adavosertib and olaparib combination. There was no prospective selection of patients based on any known biomarkers of Wee1i or PARP inhibition.
Methods
Study Design and Patients
This was an open-label, multicenter, phase Ib study evaluating the safety, tolerability, pharmacokinetics (PK), and antitumor activity of oral adavosertib in combination with olaparib in patients with refractory solid tumors (NCT02511795). The study took place at six investigational sites: five in the USA and one in Canada.
Part A was a dose-escalation cohort. Patients received adavosertib [200, 250, or 300 mg once daily (qd) or 125, 150, or 175 mg twice daily (bid)] for 3 consecutive days with 4 days off treatment (3/4) on days 1–3 and 8–10 or days 1–3, 8–10, and 15–17, or for 5 consecutive days with 2 days off treatment (5/2; 200 or 250 mg qd cohorts only) on days 1–5 and 8–12, plus continuous oral olaparib (100, 200, or 300 mg bid) for 14 or 21 days of a 21-day cycle (detailed dosing schedules for all part A cohorts are outlined in Table 1). The MTD was the highest dose at which fewer than one-third of evaluable patients had a dose-limiting toxicity (DLT) during cycle 1 (first 21 days of treatment). The DLT observation period could be expanded by up to 2 weeks if considered appropriate in case of treatment delay due to study-drug-related adverse events (AEs). Part B was an expansion cohort of patients with relapsed extensive-stage SCLC; patients in part B received adavosertib 200 mg qd (3/4) on days 1–3 and 8–10 of a 21-day cycle, plus continuous olaparib 200 mg bid, as this was the dose and schedule selected as the RP2D during the escalation.
Table 1.
Summary of treatment schedules and tolerability profiles (part A)
| Cohort | Adavosertib dose | Olaparib dose | Adavosertib schedule, administration days in 21-day cycle | Olaparib schedule, days | Patients, n (evaluable,a n) | Patients with a DLT, n (%) | DLTsb (grade) |
|---|---|---|---|---|---|---|---|
| 1 | 125 mg bid (3/4) | 100 mg bid | 1–3/8–10 | 1–14 | 3 (3) | 0 | |
| 2 | 150 mg bid (3/4) | 100 mg bid | 1–3/8–10 | 1–14 | 3 (3) | 0 | |
| 3.1 | 175 mg bid (3/4) | 100 mg bid | 1–3/8–10 | 1–14 | 4 (4) | 0 | |
| 3.2 | 150 mg bid (3/4) | 200 mg bid | 1–3/8–10 | 1–14 | 7 (7) | 0 | |
| 4.1 | 175 mg bid (3/4) | 200 mg bid | 1–3/8–10 | 1–14 | 7 (7) | 0 | |
| 4.2 | 175 mg bid (3/4) | 200 mg bid | 1–3/8–10 | 1–21 | 14 (14) | 1 (7.1) | Neutrophil count decreased (4), platelet count decreased (4) |
| 4.3 | 175 mg bid (3/4) | 200 mg bid | 1–3/8–10/15–17 | 1–21 | 14 (14) | 2 (14.3) | Fatigue (3), platelet count decreased (3, 4) |
| 5 | 175 mg bid (3/4) | 300 mg bid | 1–3/8–10 | 1–14 | 5 (5) | 1 (20.0) | Alanine aminotransferase increased (2), fatigue (3) |
| 6.1 | 250 mg qd (5/2) | 200 mg bid | 1–5/8–12 | 1–21 | 8 (8) | 2 (25.0) | Neutropenia (4), thrombocytopenia (4), drug-induced liver injury (3) |
| 6.2 | 200 mg qd (5/2) | 200 mg bid | 1–5/8–12 | 1–21 | 7 (7) | 2 (28.6) | Anemia (2), neutropenia (4), thrombocytopenia (4), diarrhea (1), nausea (2), vomiting (1) |
| 7.1 | 250 mg qd (3/4) | 200 mg bid | 1–3/8–10 | 1–21 | 17 (16) | 1 (6.3) | Asthenia (2), dyspnea (2), fatigue (2), nausea (2) |
| 7.2 | 250 mg qd (3/4) | 200 mg bid | 1–3/8–10/15–17 | 1–21 | 4 (4) | 1 (25.0) | Neutrophil count decreased (4) |
| 7.3 | 300 mg qd (3/4) | 200 mg bid | 1–3/8–10 | 1–21 | 3 (3) | 1 (33.3) | Neutropenia (4) |
| 7.4 | 200 mg qd (3/4) | 200 mg bid | 1–3/8–10 | 1–21 | 13 (13) | 1 (7.7) | Thrombocytopenia (2, 3) |
| 8.1 | 200 mg qd (3/4) | 300 mg bid | 1–3/8–10 | 1–21 | 11 (11) | 2 (18.2) | Dizziness (2), platelet count decreased (3) |
The bold rows indicate the MTD for bid and qd dosing; the RP2D (cohort 7.4; adavosertib 200 mg qd + olaparib 200 mg bid) was carried forward into the dose-expansion part of this study. bid twice daily, DLT dose-limiting toxicity, MTD maximum tolerated dose, qd once daily
aEvaluable patients received > 75% of the planned dose of adavosertib and olaparib
bPreferred terms; some patients experienced > 1 DLT
Female or male patients at least 18 years of age and with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1 were eligible for inclusion in this study. Key inclusion criteria were as follows: any prior palliative radiation therapy must have been completed ≥ 7 days before study treatment, and patients must have had a histologically confirmed refractory solid tumor for which there is no established treatment with curative intent and ≥ 1 prior course of systemic therapy (part A), or extensive-stage SCLC (histologically confirmed diagnosis) and confirmed response [either partial response (PR) or complete response (CR)] to first-line platinum therapy followed by relapse after completion of treatment (part B). Key exclusion criteria included the following: prior treatment with a PARP inhibitor; use of an investigational drug during the past 30 days or five half-lives (whichever was longer) prior to the first dose of study treatment; use of anticancer drugs 21 days or five half-lives (whichever was shorter) prior to the first dose of study treatment (for drugs for which five half-lives was equal to or less than 21 days, a minimum of 10 days between termination of the prior treatment and administration of study treatment was required); use of radiotherapy (except for palliative reasons) within 21 days prior to study treatment; major surgical procedures up to 28 days, or minor surgical procedures up to 7 days, prior to beginning study treatment; and toxicity worse than grade 1 from prior therapy (except alopecia or anorexia). No other anticancer therapy [chemotherapy, immunotherapy, hormonal anticancer therapy, or radiotherapy (except palliative local radiotherapy)], biological therapy, or other novel agent was permitted while patients were receiving the study medication. Prophylactic antiemetic medication was mandatory. The institutional review boards of all participating sites approved the study, all patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Assessments
Safety and Tolerability Assessments
AEs were graded by Common Terminology Criteria for Adverse Events (CTCAE; version 4.03). AEs were assessed by physical examination, vital signs (blood pressure, pulse rate, and body temperature), and laboratory parameters starting from enrollment of the patient and throughout the post-treatment follow-up period. DLTs were recorded, and the MTD was determined during cycle 1 (first 21 days of treatment). DLTs were defined as toxicities related to adavosertib or olaparib treatment that met at least one of the following criteria: hematologic toxicities, such as infection with febrile neutropenia or thrombocytopenia (grade ≥ 4 for more than 7 days); thrombocytopenia (grade ≥ 3) with bleeding (grade ≥ 2); non-hematologic toxicity (grade ≥ 3); abnormal liver function tests (LFTs), including grade ≥ 3 elevated total bilirubin, hepatic transaminase, or alkaline phosphatase lasting more than 48 h or any change in LFT components consistent with Hy’s Law; and any other clinically significant or unacceptable toxicity that did not respond to supportive care and resulted in a disruption of the dosing schedule of more than 7 days or was judged to be a DLT by the investigator in collaboration with the medical monitor.
Efficacy Endpoints
Efficacy outcomes were based on Response Evaluation Criteria in Solid Tumors version 1.1: objective response rate (ORR), defined as rate of confirmed CR + confirmed PR divided by the number of patients in the efficacy analysis set with measurable disease at baseline; disease control rate (DCR), defined as CR + PR + stable disease; duration of response (DOR), defined as the time from the date of first documented response until date of documented progression or death in the absence of disease progression; and progression-free survival (PFS). In parts A and B, ORR and DCR were presented by dose-level cohort (part A) and RP2D expansion cohorts (part B). PFS was presented by RP2D expansion cohort(s) for part B.
Pharmacokinetic Assessments
Samples were taken on cycle 1 and beyond on day 3 or day 10 for the determination of adavosertib and olaparib plasma concentrations, and a pre-dose sample was taken every other cycle (e.g., cycle 1, 3, 5, etc.). The PK parameters of olaparib monotherapy were determined by a sub-study in which single-agent olaparib was given bid, orally, for 3 consecutive days, as determined by the adavosertib cohort assignment. Key PK parameters analyzed included area under the plasma concentration–time curve from time zero to 10 h (AUC0–10) and maximum plasma drug concentration (Cmax). The adavosertib PK sample analysis was performed by Labcorp Early Development Laboratories Inc (Madison, WI, USA), and the olaparib PK samples were analyzed by Labcorp Early Development Laboratories Limited (Harrogate, UK) on behalf of AstraZeneca. Real-time PK analysis was conducted prior to each cohort; actual/nominal sampling times were used in the parameter calculations, and PK parameters were derived using standard non-compartmental methods. The PK analysis set included all patients who received the adavosertib/olaparib dose and had at least one reportable PK sample without major protocol deviation.
Biomarker Assessments
Analysis of tumor-related genetic alterations was conducted using archived tumor tissue samples collected at baseline. Archival tumor tissue was provided in formalin-fixed paraffin-embedded (FFPE) blocks. If FFPE blocks were unavailable, archival tumor tissue sections were provided on tissue slides; all tissue was shipped at ambient temperature to a central laboratory for processing. Next-generation sequencing analyses were performed using a clinical trial assay based on FoundationOne®CDx (Foundation Medicine, Inc., Cambridge, MA, USA) [24], while immunohistochemistry analyses to assess expression of c-MYC, SLFN11, and cyclin E1 were performed by AstraZeneca.
Statistical Analysis
Statistical analyses were performed using SAS® (SAS Institute, Cary, NC, USA) by Sarah Cannon Development Innovations under the direction of the Biometrics Group, AstraZeneca. ORR and DCR were calculated and presented along with an exact 95% CI using the Clopper–Pearson method [25]. Kaplan–Meier median PFS and DOR was calculated, along with the 95% CI, using the Brookmeyer–Crowley method [26]. Descriptive statistics were used to summarize the safety and PK data. The PK analysis set included all patients who received the adavosertib/olaparib dose and had at least one reportable PK sample without major protocol deviation. Biomarker analysis was performed for all patients who consented and for whom a valid tumor tissue sample was obtained.
Results
Patient Disposition and Characteristics
A total of 154 patients provided informed consent, of whom 130 were enrolled in the study (part A: n = 120; part B: n = 10). Two patients (n = 1 in cohort 7.1 and the SCLC cohort) were enrolled but did not receive either study treatment. Overall, 124 patients (part A: n = 115; part B: n = 9) received both adavosertib and olaparib. Four patients (3.1%) started olaparib treatment but discontinued prior to the first dose of adavosertib (Supplementary Fig. 1). At the time of data cut-off, two patients (1.6%; n = 1 in cohorts 7.1 and 8.1) remained on treatment and continued to receive both drugs. The most common reason for treatment discontinuation was progressive disease (96 patients overall; 77.4% of the 124 patients who received adavosertib and olaparib). Of the 119 patients treated in part A, more than two-thirds were female (70.6%); median age at study entry was 59 years; the most common primary tumor sites were ovary (21.8%), breast (16.0%), and lung (12.6%); 83 (69.7%) had an ECOG PS of 1; and a median of 4.0 (range 0–16) prior systemic therapies had been received at baseline. Of the nine patients treated in part B: approximately half were female (55.6%); median age at study entry was 59 years; eight (88.9%) had an ECOG PS of 1; and a median of 2.0 (range 1–6) prior systemic therapies had been received at baseline (Table 2 and Supplementary Table 1).
Table 2.
Patient demographics and baseline characteristics
| Part A | Part B | |||
|---|---|---|---|---|
| Total population (n = 119) | Cohort 4.2 (n = 14) MTD |
Cohort 7.4 (n = 13) MTD/RP2D |
SCLC cohort (n = 9) | |
| Age | ||||
| Mean, years (SD) | 58.9 (10.7) | 56.1 (12.4) | 60.7 (11.6) | 58.6 (11.2) |
| Median, years (range) | 59 (26–80) | 56.5 (26–77) | 62 (29–76) | 59 (34–74) |
| ≥ 65 years, n (%) | 38 (31.9) | 4 (28.6) | 5 (38.5) | 2 (22.2) |
| Female, n (%) | 84 (70.6) | 10 (71.4) | 7 (53.8) | 5 (55.6) |
| Race, n (%) | ||||
| White | 105 (88.2) | 10 (71.4) | 10 (76.9) | 8 (88.9) |
| Black/African American | 6 (5.0) | 2 (14.3) | 1 (7.7) | 0 (0) |
| Asian | 5 (4.2) | 1 (7.1) | 1 (7.7) | 1 (11.1) |
| Other | 3 (2.5) | 1 (7.1) | 1 (7.7) | 0 (0) |
| Mean BMI, kg/m2 (SD) | 27.3 (6.6) | 27.0 (7.8) | 26.3 (4.9) | 28.3 (4.4) |
| ECOG PS, n (%) | ||||
| 0 (normal activity) | 36 (30.3) | 4 (28.6) | 2 (15.4) | 1 (11.1) |
| 1 (symptoms but ambulatory) | 83 (69.7) | 10 (71.4) | 11 (84.6) | 8 (88.9) |
| Primary diagnosis, n (%) | ||||
| Ovary | 26 (21.8) | 3 (21.4) | 3 (23.1) | 0 |
| Breast | 19 (16.0) | 3 (21.4) | 0 | 0 |
| Lung | 15 (12.6)a | 2 (14.3)b | 5 (38.5)c | 9 (100) |
| Prostate | 9 (7.6) | 2 (14.3) | 0 | 0 |
| Uterus | 9 (7.6) | 0 | 1 (7.7) | 0 |
| Colon | 6 (5.0) | 0 | 2 (15.4) | 0 |
| Pancreas | 6 (5.0) | 0 | 0 | 0 |
| Rectal | 4 (3.4) | 0 | 0 | 0 |
| Soft tissue | 3 (2.5) | 1 (7.1) | 0 | 0 |
| Otherd | 18 (15.1) | 2 (14.3) | 2 (15.4) | 0 |
| Median number of previous therapy regimens (range) | ||||
| Systemic | 4.0 (0–16) | 3.0 (1–11) | 4.0 (1–8) | 2.0 (1–6) |
| Radiotherapy | 1.0 (0–8) | 1.0 (0–3) | 1.0 (0–3) | 2.0 (0–3) |
Patients in cohorts 4.2 (adavosertib 175 mg bid + olaparib 200 mg bid) and 7.4 (adavosertib 200 mg qd + olaparib 200 mg bid) received the MTD for bid and qd dosing, respectively; the RP2D (cohort 7.4) was carried forward into the dose-expansion part of this study. bid twice daily, BMI body mass index, ECOG PS Eastern Cooperative Oncology Group performance status, MTD maximum tolerated dose, qd once daily, RP2D recommended phase II dose, SCLC small-cell lung cancer, SD standard deviation
a8/15 patients had a confirmed primary diagnosis of SCLC
b1/2 patients had a confirmed primary diagnosis of SCLC
c3/5 patients had a confirmed primary diagnosis of SCLC
dFurther details of the specific diagnoses are provided in Supplementary Table 1
Safety and Tolerability
Part A
A summary of tolerability by treatment schedule is provided in Table 1. A total of 14 patients [14/119 patients evaluable for DLTs (11.8%)] experienced 26 DLTs during the study. The most common (≥ 20%) DLTs were neutropenia (6/14 patients), thrombocytopenia (5/14 patients), and fatigue (3/14 patients). No bleeding events were associated with the events of thrombocytopenia. The MTD with adavosertib bid (the highest dose at which fewer than one-third of evaluable patients had a DLT), which was found in cohort 4.2, was adavosertib 175 mg bid (days 1–3 and 8–10 of a 21-day cycle) plus continuous olaparib 200 mg bid [1/14 patients (7.1%) had a DLT]. The qd MTD (which was also the RP2D) was found in cohort 7.4 to be adavosertib 200 mg qd (days 1–3 and 8–10 of a 21-day cycle) plus continuous olaparib 200 mg bid [1/13 patients (7.7%) had a DLT].
AEs considered to be causally related to either adavosertib or olaparib occurred in 12 patients (85.7%) in cohort 4.2 and 13 patients (100%) in cohort 7.4. The most common AEs causally related to either adavosertib or olaparib were fatigue (64.3% and 15.4%, respectively), diarrhea (50.0% and 38.5%), decreased appetite (50.0% and 30.8%), nausea (50.0% and 30.8%), and anemia (35.7% and 46.2%). Adavosertib- or olaparib-related AEs in cohorts 4.2 and 7.4 are shown in Table 3. Serious AEs considered to be causally related to adavosertib or olaparib occurred in three patients (21.4%) in cohort 4.2 (one patient with febrile neutropenia, one patient with decreased platelet count and decreased neutrophil count, and one patient with anemia, diarrhea, neutropenia, and thrombocytopenia) and two patients (15.4%) in cohort 7.4 (one patient with dizziness and one patient with constipation).
Table 3.
Adavosertib- or olaparib-related adverse events in cohort 4.2, cohort 7.4, and the SCLC cohort
| Parameter, n (%) | Part A | Part B | |
|---|---|---|---|
| Cohort 4.2 (n = 14) | Cohort 7.4 (n = 13) | SCLC cohort (n = 9) | |
| Any AE causally related to adavosertib or olaparib (any grade) | 12 (85.7) | 13 (100) | 8 (88.9) |
| Any grade ≥ 3 AE causally related to adavosertib or olaparib | 6 (42.8) | 4 (30.8) | 6 (66.7) |
| Deaths causally related to adavosertib or olaparib | 0 | 0 | 0 |
| Any AE resulting in death (any grade) | 1 (7.1) | 0 | 1 (11.1) |
| AEs causally related to adavosertib or olaparib occurring in > 10% of patients (any grade) | |||
| Infections and infestations | |||
| Escherichia bacteremia | 0 | 0 | 1 (11.1) |
| Blood and lymphatic system disorders | |||
| Anemia | 5 (35.7) | 6 (46.2) | 5 (55.6) |
| Febrile neutropenia | 1 (7.1) | 0 | 1 (11.1) |
| Leukopenia | 1 (7.1) | 0 | 2 (22.2) |
| Neutropenia | 2 (14.3) | 0 | 2 (22.2) |
| Thrombocytopenia | 3 (21.4) | 2 (15.4) | 6 (66.7) |
| Metabolism and nutrition disorders | |||
| Decreased appetite | 7 (50.0) | 4 (30.8) | 2 (22.2) |
| Dehydration | 1 (7.1) | 2 (15.4) | 0 |
| Nervous system disorders | |||
| Dizziness | 1 (7.1) | 2 (15.4) | 0 |
| Headache | 3 (21.4) | 0 | 0 |
| Taste disorder | 3 (21.4) | 0 | 0 |
| Cardiac disorders | |||
| Tachycardia | 2 (14.3) | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | |||
| Dyspnea | 0 | 2 (15.4) | 0 |
| Gastrointestinal disorders | |||
| Abdominal distension | 0 | 2 (15.4) | 0 |
| Abdominal pain | 1 (7.1) | 2 (15.4) | 0 |
| Constipation | 3 (21.4) | 1 (7.7) | 0 |
| Diarrhea | 7 (50.0) | 5 (38.5) | 2 (22.2) |
| Mouth ulceration | 0 | 0 | 1 (11.1) |
| Nausea | 7 (50.0) | 4 (30.8) | 1 (11.1) |
| Vomiting | 3 (21.4) | 2 (15.4) | 2 (22.2) |
| Skin and subcutaneous tissue disorders | |||
| Pruritus | 3 (21.4) | 0 | 1 (11.1) |
| Musculoskeletal and connective tissue disorders | |||
| Joint swelling | 0 | 2 (15.4) | 0 |
| Muscle spasms | 2 (14.3) | 0 | 0 |
| Myalgia | 3 (21.4) | 0 | 0 |
| General disorders and administration-site conditions | |||
| Fatigue | 9 (64.3) | 2 (15.4) | 1 (11.1) |
| Peripheral edema | 0 | 2 (15.4) | 0 |
Patients in cohorts 4.2 (adavosertib 175 mg bid + olaparib 200 mg bid) and 7.4 (adavosertib 200 mg qd + olaparib 200 mg bid) received the MTD for bid and qd dosing, respectively; the RP2D (cohort 7.4) was carried forward into the dose-expansion part of this study. AE adverse event, bid twice daily, MTD maximum tolerated dose, qd once daily, RP2D recommended phase II dose, SCLC small-cell lung cancer
AEs considered to be causally related to adavosertib occurred in 12 patients (85.7%) in cohort 4.2 and 12 patients (92.3%) in cohort 7.4. The most common AEs causally related to adavosertib in cohorts 4.2 and 7.4 were fatigue (64.3% and 15.4%, respectively), diarrhea (50.0% and 38.5%), decreased appetite (50.0% and 30.8%), nausea (50.0% and 23.1%), and anemia (35.7% and 38.5%). Serious AEs considered to be causally related to adavosertib occurred in three patients (21.4%) in cohort 4.2 (one patient with febrile neutropenia, one patient with decreased platelet count and decreased neutrophil count, and one patient with anemia, diarrhea, neutropenia, and thrombocytopenia) and one patient (7.7%) in cohort 7.4 (dizziness).
Overall, CTCAE grade ≥ 3 events causally related to adavosertib were observed in 52 patients (43.7%). There were four AEs with an outcome of death [sepsis (n = 2) and respiratory failure and cardiovascular disease (both n = 1)]; one (sepsis) was considered causally related to adavosertib but was not a result of a DLT. One AE (cardiovascular disorder) led to discontinuation of adavosertib and olaparib in cohort 4.2 (not considered related to adavosertib or olaparib); there were no discontinuations due to AEs in cohort 7.4. Five (35.7%) patients in cohort 4.2 and three (23.1%) patients in cohort 7.4 experienced an AE leading to dose reduction of adavosertib, while three (21.4%) and three (23.1%) patients, respectively, experienced an AE leading to dose reduction of olaparib. Seven (50.0%) patients in cohort 4.2 and five (38.5%) patients in cohort 7.4 experienced an AE leading to dose interruption of adavosertib, while nine (64.3%) and eight (61.5%) patients, respectively, experienced an AE leading to dose interruption of olaparib.
Part B
AEs considered to be causally related to adavosertib or olaparib occurred in eight patients (88.9%; Table 3). The most common AEs causally related to adavosertib or olaparib were thrombocytopenia (66.7%) and anemia (55.6%). Six patients (66.7%) experienced grade 3 AEs related to adavosertib or olaparib (grade 4 or 5 AEs related to adavosertib or olaparib, n = 0). Three patients (33.3%) experienced serious AEs [febrile neutropenia, pneumonia, and urinary tract infection, n = 1 (11.1%) each]; the serious AE of febrile neutropenia was considered related to olaparib but not to adavosertib. There was one AE that resulted in death (pneumonia), which was not considered by investigators to be treatment related. AEs led to dose reductions of adavosertib in one patient and interruptions of adavosertib in three patients; AEs led to interruptions of olaparib in five patients. There were no AEs that resulted in discontinuation of adavosertib or olaparib.
Clinical Response
Part A
In part A, ORR was 14.8% (17/117; 95% CI 8.7–22.9). For the cohorts in which MTD for bid dosing (cohort 4.2) and RP2D for qd dosing (cohort 7.4) were determined, ORR was 30.8% (4/13; 9.1–61.4) and 9.1% (1/11; 0.2–41.3), respectively. This included four patients with PR in cohort 4.2 [ovarian cancer (n = 2), SCLC (n = 1), and TNBC (n = 1)] and one patient with PR in cohort 7.4 (ovarian cancer).
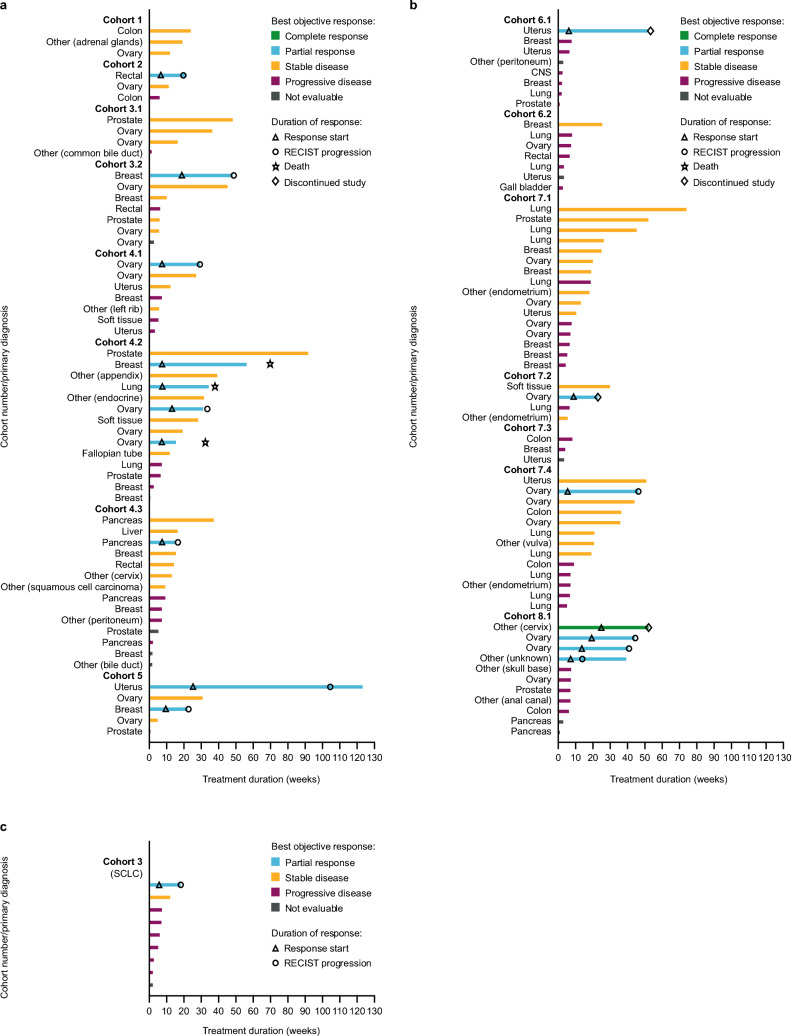
In part A, median PFS was 3.5 months (n = 115; 95% CI 2.4–4.7) and median DOR was 6.4 months (n = 17; interquartile range 1.4–3.1). Of the 124 patients treated with adavosertib and olaparib, one patient (cohort 8.1) had a confirmed CR and 17 patients (13.7%) had a confirmed PR. The patient with a confirmed CR had measurable disease at baseline but did not have target lesions at baseline. Four patients in part A had an unconfirmed CR or PR (one patient each in cohorts 3.2, 4.2, 4.3, and 7.1). For cohorts 4.2 and 7.4, median PFS was 7.4 months (95% CI 1.2–15.8) and 4.0 months (95% CI 1.4–10.6), respectively. At the time of data cut-off (25 April 2019), 36 patients (29.0%) were censored for PFS. For cohorts 4.2 and 7.4, DCR was 76.9% (46.2–95.0) and 61.5% (31.6–86.1), respectively. Supplementary Table 2 summarizes clinical response by cohort (parts A and B); individual patient clinical responses are presented for part A in Fig. 1.
Fig. 1.
Swimmer plots of duration of treatment and best objective response for: a part A, dose frequency qd; b part A, dose frequency bid; and c part B. bid twice daily, qd once daily, RECIST response evaluation criteria in solid tumors
Part B
In the SCLC cohort, ORR was 11.1% (95% CI 0.3–48.2); there was one PR, and DCR was 22.2% (95% CI 2.8–60.0; one PR, one stable disease). Median PFS was 1.5 months (95% CI 1.3–4.2); DOR in the responding patient was 2.9 months. Supplementary Table 2 summarizes clinical response by cohort (parts A and B); individual patient clinical responses are presented for part B in Fig. 1.
Pharmacokinetics—Part A
Changes in olaparib PK following combination with adavosertib (part A) are shown in Supplementary Table 3, and a comparison of PK across adavosertib doses in combination with olaparib 200 mg bid (part A) is shown in Supplementary Fig. 2. Following dosing on cycle 1, day 3, adavosertib 175 mg bid plus olaparib 200 mg bid produced the longest duration of plasma adavosertib concentration above the phosphorylated CDK1 half-maximal inhibitory concentration (IC50) and the greatest coverage within the preclinically determined target adavosertib plasma concentration range for cell kill activity (0.5–1 µM; Supplementary Fig. 2). Assessment of the PK results for olaparib indicated that there was generally no statistically significant difference between exposure to olaparib in the presence of adavosertib and to olaparib alone. Adavosertib 175 mg bid increased olaparib AUC0–10 by 33% and Cmax by 18%. The ratio of olaparib plus adavosertib to olaparib alone for AUC0–10 and/or Cmax exceeded 2 in some patients; however, there was no evidence that these data were unreliable, so data from these high outliers were included in the statistical analyses. Olaparib clinical minimum steady-state plasma drug concentration (Cmin,ss; measured in part A) was comparable with the 95% inhibitory concentration (IC95) in preclinical studies, as shown in Supplementary Fig. 3.
Biomarker Analysis
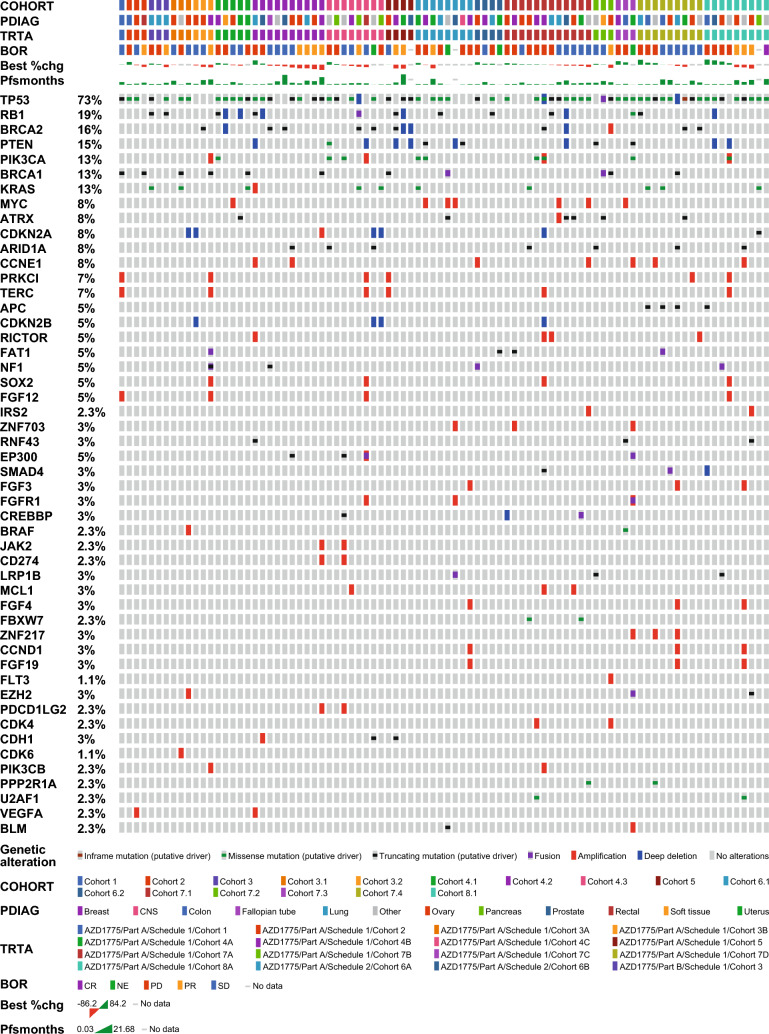
Preliminary analyses of samples showed that the TP53 mutation was the most common genetic aberration, found in 73% of all patients with response data and next-generation sequencing data regardless of tumor type (Fig. 2). The RB1 mutation was found in 19% of patients; BRCA2 and BRCA1 mutations were found in 16% and 13% of patients, respectively. No clear correlation was observed between genomic markers and best objective response in the overall cohort with mixed tumor types. The small number of patients in the expansion cohort (part B) with available next-generation sequencing data (n = 3) precluded a firm conclusion. A total of 63 samples were evaluable for c-MYC expression, 58 for cyclin E1, and 61 for SLFN11. No statistically significant differences were observed between the expression of these proteins and the best objective response in the overall cohort (Supplementary Fig. 4), although only a small number of patients achieved a PR.
Fig. 2.
Biomarker analysis: all patients with next-generation sequencing and response data available (n = 88). BOR best objective response, CR complete response, NE not evaluable, PD progressive disease, PFS progression-free survival, PR partial response, SD stable disease
Discussion
This study investigated 15 possible treatment schedules to identify a tolerable dosing schedule for adavosertib in combination with olaparib. The qd MTD [cohort 7.4; adavosertib 200 mg qd (days 1–3 and 8–10 of a 21-day cycle) plus continuous olaparib 200 mg bid] was established as the RP2D and was used for patients treated in part B (relapsed extensive-stage SCLC).
The PK results indicated that there was generally no statistically significant difference between exposure to olaparib in the presence of adavosertib and to olaparib alone. Some patients had a greater-than-twofold increase in olaparib exposure in the presence of adavosertib. As the number of patients in each cohort was small, the interpatient variability in olaparib exposure when co-administered with adavosertib was moderate to high and the study was not sized to show lack of effect; therefore, a drug–drug interaction cannot be ruled out. Greater olaparib exposure in the presence of adavosertib may underlie the fact the MTDs were determined using olaparib at a dose of 200 mg as opposed to its standard dose of 300 mg.
The safety profile of adavosertib plus olaparib was acceptable for this heavily pre-treated patient population (for which standard-of-care therapy does not exist or has proven ineffective or intolerable), and the nature of the AEs observed was consistent with the known safety profiles of adavosertib and olaparib [8, 21, 27–37].
Preliminary antitumor activity was demonstrated with adavosertib in combination with olaparib in patients with BRCA-mutant and BRCA-wild-type solid tumor malignancies, contributing to the growing body of evidence of antitumor activity following treatment with adavosertib. In the EFFORT trial, adavosertib administered alone (300 mg on days 1–5 and 8–12 of a 21-day cycle) or in combination with olaparib (150 mg bid on days 1–3 and 8–10 and olaparib at 200 mg bid on days 1–21 of a 21-day cycle) demonstrated efficacy in patients with PARP inhibitor-resistant ovarian cancer [37]. Results from a recent phase Ib trial in patients with DNA damage response aberrant advanced tumors indicated that alternating adavosertib and olaparib treatment may be better tolerated than concurrent drug administration [38]. Phase I and II studies have also been performed with adavosertib in combination with gemcitabine, cisplatin, docetaxel, paclitaxel, pegylated liposomal doxorubicin, carboplatin, or durvalumab for the treatment of advanced pancreatic cancer, advanced solid tumors, refractory TP53-mutated ovarian cancer, platinum-resistant ovarian cancer, and head and neck squamous-cell carcinoma [29–31, 33, 39–41]. A dosing schedule for adavosertib in combination with irinotecan has also been determined for the treatment of refractory solid tumors in children [28]. Recent studies have provided evidence of clinical efficacy for adavosertib as monotherapy [12, 13, 42] and in combination with gemcitabine [43] in high-grade serous ovarian and uterine cancer, for which there is currently no effective therapy. Results from two phase II studies assessing the efficacy and safety of adavosertib as treatment for recurrent uterine serous carcinoma have also been reported [12, 13]. Data from the most recent of these, the ADAGIO trial, indicated a narrow therapeutic window for adavosertib. While the clinical development of adavosertib is not currently proceeding, the scientific rationale for exploring Wee1 inhibition as a potential treatment target remains valid, and other Wee1is remain in clinical development.
Although a number of patients in this trial responded to combination therapy and achieved a robust DOR, there was no clear correlation with their genomic profiles. BRCA1/2 status was not found to be involved in treatment response across all cohorts, and further genomic biomarkers as well as expression levels of potentially predictive protein biomarkers also failed to find any significant association. However, the lack of clear correlation detected is not unexpected when taking into consideration the range of dosing schedules, heterogeneous cohorts, and the small number of clinical responses. Nevertheless, evidence from the IGNITE trial, where Cyclin E1 over-expression in high-grade serous ovarian cancer was used to pre-select patients for adavosertib monotherapy, and where there was a 53% ORR observed, suggests that targeting Wee1 is still a valid approach in selected patients. In addition, the preclinical data suggesting that replication stress is enriched in BRCA-mutant PARP inhibitor-resistant cancers [22] are consistent with the adavosertib monotherapy and olaparib combination efficacy seen in the EFFORT trial [37]. All patients participating in the EFFORT trial had progressed previously on a PARP inhibitor, and just under half of the ovarian cancer patients had germline or somatic BRCA mutations [37]. Both adavosertib monotherapy and olaparib combination arms demonstrated clinical benefit with a clinical benefit rate of 63% and ORR of 23% for monotherapy, and clinical benefit rate of 89% and ORR of 29% for the olaparib and adavosertib combination [37]. These data suggest that a post-PARP inhibitor or PARP inhibitor-resistant setting is another patient population where adavosertib could be beneficial.
Conclusions
Preliminary data from this trial indicate only limited antitumor activity of adavosertib in combination with olaparib in patients with solid tumors where there was no preselection for replication-stress-associated biomarkers or a population enriched for replication stress, such as those who had progressed on PARP inhibitors. An overall DCR of 56.5% and a median DOR of 5.8 months (DOR up to 18.2 months) was seen across a variety of tumors in both BRCA-mutated and BRCA wild-type patients who were heavily pre-treated. These data indicate the need for appropriate selection of patients for Wee1i in combination with a PARP inhibitor. The safety profile observed in the study was consistent with the known safety profiles of adavosertib and olaparib. The toxicity was manageable with effective toxicity management, including dose delays, dose reductions, dose interruptions, and/or the use of supportive care approach in the patients with poor tolerance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank all participating patients and their families. This study was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA, who are codeveloping olaparib. Medical writing support was provided by C.L. Attwell, Ph.D., of AMICULUM Ltd and was funded by AstraZeneca.
Declarations
Funding
This study was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA, who are codeveloping olaparib.
Disclosure of Potential Conflicts of Interests
Erika P. Hamilton: Institutional funding (AstraZeneca, Pfizer, Genentech/Roche, Lilly, Puma Biotechnology, Daiichi Sankyo, Mersana, Boehringer Ingelheim, Cascadian Therapeutics, Hutchinson MediPharma, OncoMed, MedImmune, Stem CentRx, Curis, Verastem, Zymeworks, Syndax, Lycera, Rgenix, Millennium, TapImmune, BerGenBio, Medivation, Tesaro, Eisai, H3 Biomedicine, Radius Health, Acerta Pharma, Takeda, Macrogenics, Abbvie, Immunomedics, Fujifilm, eFFECTOR Therapeutics, Merus, Nucana, Regeneron, Leap Therapeutics, Taiho Pharmaceutical, EMD Serono, Clovis, CytomX, InventisBio, Novartis, Silverback, Black Diamond, ArQule, Sermonix Pharmaceuticals, Sutro Biopharma, Zenith Epigenetics, Arvinas, Torque Therapeutics, Harpoon Therapeutics, Fochon Pharmaceuticals, Orinove, Molecular Templates, Unum Therapeutics, Aravive, Dana Farber Cancer Institute, G1 Therapeutics, Karyopharm Therapeutics, and Compugen) and non-financial support (Amgen, Bayer, Bristol-Myers Squibb, Genzyme, Helsinn Therapeutics, HERON, Lexicon, Medivation, Merck, Novartis, Roche, Sysmex, Guardant Health, Foundation Medicine, Deciphera, and NanoString). Gerald S. Falchook: Royalties, self (Wolters Kluwer); advisory role, institution (AbbVie, Fujifilm, Silicon, Navire, Turning Point, Predicine, Inspirna, Regeneron, Jubilant, BostonGene, Teon, Merck, Sanofi, and BridgeBio); advisory role, self (EMD Serono); speaker’s honorarium for CME (Total Health Conferencing and Rocky Mountain Oncology Society); travel, self, for work and/or research related to institution [Amgen, Bristol-Myers Squibb, EMD Serono, Fujifilm, Millennium, Sarah Cannon Research Institute (employer), and Synthorx/Sanofi]; research funding, to institution (3-V Biosciences, Abbisko, Abbvie, ABL Bio, ADC Therapeutics, Accutar, Agenus, Aileron, American Society of Clinical Oncology, Amgen, ARMO/Eli Lilly, Artios, AstraZeneca, Bayer, BeiGene, Bioatla, Bioinvent, Biothera, Bicycle, Black Diamond, Boehringer Ingelheim, Celgene, Celldex, Ciclomed, Curegenix, Curis, Cyteir, Daiichi, DelMar, eFFECTOR, Eli Lilly, EMD Serono, Epizyme, Erasca, Exelixis, Freenome, Fujifilm, Genmab, GlaxoSmithKline, Hutchison MediPharma, IGM Biosciences, Ignyta, Immunitas, ImmunoGen/MacroGenics, Incyte, Jacobio, Jazz, Jounce, Jubilant, Kolltan, Loxo/Bayer, MedImmune, Merck, Metabomed, Millennium, Mirati, miRNA Therapeutics, Molecular Templates, National Institutes of Health, Navire/BridgeBio, NGM Bio, NiKang, Novartis, OncoMed, Oncorus, Oncothyreon, Poseida, Precision Oncology, Prelude, PureTech, Pyramid, RasCal, Regeneron, Relay, Rgenix, Ribon, Samumed, Sapience, Seagen, Silicon, Simcha, Sirnaomics, Strategia, Syndax, Synthorx/Sanofi, Taiho, Takeda, Tallac, Tarveda, Teneobio, Tesaro, Tocagen, Turning Point, U.T. MD Anderson Cancer Center, Vegenics, Xencor, and Zhuhai Yufan). Judy S. Wang: Consulting fees (Janssen, Stemline/Menarini, BioNTech, and Kanaph Therapeutics); honoraria for speakers’ bureau (AstraZeneca and Eisai); research funding to institution only (Syndax, AstraZeneca, Phoenix, Accutar, Celgene, BMS, Tenebio, LOXO, Nurix, Step Pharma, BioNTech, Moderna, Merck, Compugen, Boehringer Ingelheim, Kymab, Sanofi, Bicycle, Cyteir, Daiichi Sankyo, Xencor, Ribon, Klus Pharma, Artios, Genentech, Treadwell, MabSpace, IGM, Immunogen, PureTech, Erasca, Bayer, 7.8 Pharma, NGMBio, Zymeworks, Immuno-Onc, Astellas, Immunitas, Prelude, Blueprint, Beigene, Pionyr, Agenus, Adagene, Macrogenics, Jazz, StingThera, Coherus, Takeda, Taiho, Jounce, Evelo, Vedanta, Stemline, GSK, Janssen, Black Diamond, Vigeo, Relay, and Jacobio). Siqing Fu: Clinical trial research support/grant funding, institution {National Institutes of Health [NIH]/National Cancer Institute [NCI] P30CA016672 – Core Grant [Cancer Center Support Grant (CCSG) Shared Resources]; Abbisko; BeiGene; BioAtla, LLC.; Boehringer Ingelheim; CUE Biopharma, Inc.; Eli Lilly & Co.; Exelisis; Greenfire Bio, Inc.; Hookipa Biotech; IMV, Inc.; Innovent Biologics, Co., Ltd.; K-Group Beta; Lyvgen Biopharm, Co., Ltd.; MacroGenics; MediLink Therapeutics, Co. Ltd.; Millennium Pharmaceuticals, Inc.; Nerviano Medical Sciences; NeuPharma, Inc.; NextCure, Inc.; Ningbo NewBay Technology Development Co., Ltd.; Novartis; NovoCure; Nykode Therapeutics AS.; Parexel International, LLC; Pionyr Immunotherapeutics, Inc.; PureTech Health, LLC; Sellas Life Sciences Group; Soricimed Biopharma, Inc.; SQZ Biotechnologies; Sumitomo Dainippon; Taiho Oncology and NCCN; Treadwell Therapeutics; Turnstone Biologics; Tyligand Bioscience, Ltd.; and Virogin Biotech, Ltd}. Amit M. Oza: principal investigator (PI) and steering committees (AstraZeneca, GSK, and Clovis); advisory boards (AstraZeneca, Morphosys); CEO [Ozmosis Research (uncompensated)], institutional support for clinical trials (AstraZeneca, GSK, Esperas Pharm, Clovis, Karyopharm, Ocellaris, Senhwa, Merck, ImmunoGen, Immunovaccine, Pfizer, Aravive, Mersana, Seagen, Alkermes, Verastem, Zentalis, Shuttck Labs, Sutro, NovoCure, Plexxikon, and Amgen). Esteban Rodrigo Imedio: Former employee (AstraZeneca). Sanjeev Kumar: Employee and stock ownership (AstraZeneca). Lone Ottesen: Former employee and stock ownership (AstraZeneca). Ganesh M. Mugundu: Former employee and stock ownership (AstraZeneca). Elza C. de Bruin: Employee and stock ownership (AstraZeneca). Mark J. O’Connor: Employee and stock ownership (AstraZeneca). Suzanne F. Jones: Consulting/advisory role (Amgen) – all payments to institution; stock ownership (HCA Healthcare). David R. Spigel: Institutional funding (AstraZeneca, Genentech/Roche, Novartis, Celgene, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Abbvie, Foundation Medicine, GlaxoSmithKline, Lilly, Merck, Moderna Therapeutics, Nektar, Takeda, Amgen, University of Texas Southwestern Medical Center – Simmons Cancer Center, G1 Therapeutics, Neon Therapeutics, Celldex, Clovis Oncology, Daiichi Sankyo, EMD Serono, Acerta Pharma, Oncogenex, Astellas Pharma, GRAIL, Transgene, Aeglea Biotherapeutics, Tesaro, Ipsen, ARMO BioSciences, and Millennium); consultation (AstraZeneca, TRM Oncology, Precision Oncology, Evelo Therapeutics, Illumina, PharmaMar, Genentech/Roche, Novartis, Celgene, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Abbvie, Foundation Medicine, GlaxoSmithKline, Lilly, and Merck); travel and expenses (AstraZeneca, Genzyme, Intuitive Surgical, Purdue Pharma, Spectrum Pharmaceuticals, Sysmex, EMD Serono, Genentech/Roche, Novartis, Celgene, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Abbvie, Foundation Medicine, GlaxoSmithKline, Lilly, and Merck). Bob T. Li: Uncompensated advisor and consultant (Amgen, AstraZeneca, Boehringer Ingelheim, Bolt Biotherapeutics, Daiichi Sankyo, Genentech, and Lilly); research grants, institution (Amgen, AstraZeneca, Bolt Biotherapeutics, Daiichi Sankyo, Genentech, Hengrui USA, and Lilly); academic travel support (Jiangsu Hengrui Medicine and MORE Health); inventor on two institutional patents at MSK (US62/685,057 and US62/514,661); intellectual property rights as a book author at Karger Publishers and Shanghai Jiao Tong University Press.
Ethics Approval and Consent to Participate
The institutional review boards of all participating sites approved the study, all patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Consent to Publish
Not applicable.
Availability of Data and Material
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Code Availability
Not applicable.
Author Contributions
Writing – original draft; writing – review and editing: E.P. Hamilton, G.S. Falchook, J.S. Wang, S. Fu, A.M. Oza, E.R. Imedio, S. Kumar, L. Ottesen, G.M. Mugundu, E. de Bruin, M. J. O’Connor, S.F. Jones, D.R. Spigel, and B.T. Li. Investigation: E.P. Hamilton, G.S. Falchook, J.S. Wang, S. Fu, A.M. Oza, and B.T. Li. Methodology: E.R. Imedio, S. Kumar, G.M. Mugundu, S.F. Jones, and D.R. Spigel. Conceptualization: E.R. Imedio, S. Kumar, G.M. Mugundu, M. J. O’Connor, S.F. Jones, and D.R. Spigel. Supervision: E.R. Imedio, S. Kumar, and G.M. Mugundu. Project administration: E.R. Imedio, S. Kumar, and G.M. Mugundu.
References
- 1.O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–60. 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Aarts M, Sharpe R, Garcia-Murillas I, et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov. 2012;2(6):524–39. 10.1158/2159-8290.Cd-11-0320. [DOI] [PubMed] [Google Scholar]
- 3.Gérard C, Goldbeter A. The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface Focus. 2014;4(3):20130075. 10.1098/rsfs.2013.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forment JV, O’Connor MJ. Targeting the replication stress response in cancer. Pharmacol Ther. 2018;188:155–67. 10.1016/j.pharmthera.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Do K, Doroshow JH, Kummar S. WEE1 kinase as a target for cancer therapy. Cell Cycle. 2013;12(19):3159–64. 10.4161/cc.26062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck H, Nähse-Kumpf V, Larsen MS, et al. Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol Cell Biol. 2012;32(20):4226–36. 10.1128/mcb.00412-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirai H, Iwasawa Y, Okada M, et al. Small-molecule inhibition of WEE1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8(11):2992–3000. 10.1158/1535-7163.Mct-09-0463. [DOI] [PubMed] [Google Scholar]
- 8.Do K, Wilsker D, Ji J, et al. Phase I study of single-agent AZD1775 (MK-1775), a WEE1 kinase inhibitor, in patients with refractory solid tumors. J Clin Oncol. 2015;33(30):3409–15. 10.1200/jco.2014.60.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer TM, Moore KN, Rader JS, et al. A phase Ib study assessing the safety, tolerability, and efficacy of the first-in-class Wee1 inhibitor adavosertib (AZD1775) as monotherapy in patients with advanced solid tumors. Target Oncol. 2023;18:517–30. 10.1007/s11523-023-00965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falchook GS, Sachdev J, Imedio ER, et al. A phase Ib study of adavosertib, a selective Wee1 inhibitor, in patients with locally advanced or metastatic solid tumors. Invest New Drugs. 2023;41:493–502. 10.1007/s10637-023-01371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu S, Yao S, Yuan Y, et al. Multicenter phase II trial of the WEE1 inhibitor adavosertib in refractory solid tumors harboring CCNE1 amplification. J Clin Oncol. 2023;41(9):1725–34. 10.1200/jco.22.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JF, Xiong N, Campos SM, et al. Phase II study of the Wee1 inhibitor adavosertib in recurrent uterine serous carcinoma. J Clin Oncol. 2021;39(14):1531–9. 10.1200/jco.20.03167. [DOI] [PubMed] [Google Scholar]
- 13.Liu JF, Colombo N, Oza AM, et al. ADAGIO: a Phase IIb, open-label, single-arm, multicenter study assessing the efficacy and safety of adavosertib (AZD1775) as treatment for recurrent or persistent uterine serous carcinoma. J Clin Oncol. 2021;39(15 Suppl):abst TPS5612. 10.1200/JCO.2021.39.15_suppl.TPS5612. [Google Scholar]
- 14.Menear KA, Adcock C, Boulter R, et al. 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem. 2008;51(20):6581–91. 10.1021/jm8001263. [DOI] [PubMed] [Google Scholar]
- 15.Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8(362):362–317. 10.1126/scitranslmed.aaf9246. [DOI] [PubMed] [Google Scholar]
- 16.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 17.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 18.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 19.FDA. Lynparza prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf. Accessed Apr 2024.
- 20.EMA. Lynparza summary of product characteristics. 2024. https://www.ema.europa.eu/en/documents/product-information/lynparza-epar-product-information_en.pdf. Accessed Apr 2024.
- 21.Lallo A, Frese KK, Morrow CJ, et al. The combination of the PARP inhibitor olaparib and the WEE1 inhibitor AZD1775 as a new therapeutic option for small cell lung cancer. Clin Cancer Res. 2018;24(20):5153–64. 10.1158/1078-0432.Ccr-17-2805. [DOI] [PubMed] [Google Scholar]
- 22.Serra V, Wang AT, Castroviejo-Bermejo M, et al. Identification of a molecularly-defined subset of breast and ovarian cancer models that respond to WEE1 or ATR inhibition, overcoming PARP inhibitor resistance. Clin Cancer Res. 2022;28(20):4536–50. 10.1158/1078-0432.Ccr-22-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez MK, Illuzzi G, Colomer C, et al. Identifying and overcoming mechanisms of PARP inhibitor resistance in homologous recombination repair-deficient and repair-proficient high grade serous ovarian cancer cells. Cancers (Basel). 2020;12(6):1503. 10.3390/cancers12061503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–31. 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 26.Brookmeyer R, Crowley JA. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 27.Balmana J, Tung NM, Isakoff SJ, et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol. 2014;25(8):1656–63. 10.1093/annonc/mdu187. [DOI] [PubMed] [Google Scholar]
- 28.Cole KA, Pal S, Kudgus RA, et al. Phase I clinical trial of the WEE1 inhibitor adavosertib (AZD1775) with irinotecan in children with relapsed solid tumors: a COG Phase I consortium report (ADVL1312). Clin Cancer Res. 2020;26(6):1213–9. 10.1158/1078-0432.Ccr-19-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuneo KC, Morgan MA, Sahai V, et al. Dose escalation trial of the WEE1 inhibitor adavosertib (AZD1775) in combination with gemcitabine and radiation for patients with locally advanced pancreatic cancer. J Clin Oncol. 2019;37(29):2643–50. 10.1200/jco.19.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leijen S, van Geel RM, Pavlick AC, et al. Phase I study evaluating WEE1 inhibitor AZD1775 as monotherapy and in combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. J Clin Oncol. 2016;34(36):4371–80. 10.1200/jco.2016.67.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leijen S, van Geel RM, Sonke GS, et al. Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patients with TP53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J Clin Oncol. 2016;34(36):4354–61. 10.1200/jco.2016.67.5942. [DOI] [PubMed] [Google Scholar]
- 32.Lin X, Chen D, Zhang C, et al. Augmented antitumor activity by olaparib plus AZD1775 in gastric cancer through disrupting DNA damage repair pathways and DNA damage checkpoint. J Exp Clin Cancer Res. 2018;37(1):129. 10.1186/s13046-018-0790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendez E, Rodriguez CP, Kao MC, et al. A Phase I clinical trial of AZD1775 in combination with neoadjuvant weekly docetaxel and cisplatin before definitive therapy in head and neck squamous cell carcinoma. Clin Cancer Res. 2018;24(12):2740–8. 10.1158/1078-0432.Ccr-17-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajan A, Carter CA, Kelly RJ, et al. A Phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors. Clin Cancer Res. 2012;18(8):2344–51. 10.1158/1078-0432.Ccr-11-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samol J, Ranson M, Scott E, et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a Phase I study. Invest New Drugs. 2012;30(4):1493–500. 10.1007/s10637-011-9682-9. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto N, Nokihara H, Yamada Y, et al. A Phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors. Cancer Sci. 2012;103(3):504–9. 10.1111/j.1349-7006.2011.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westin SN, Coleman RL, Fellman BM, et al. EFFORT: EFFicacy Of adavosertib in parp ResisTance: a randomized two-arm non-comparative phase II study of adavosertib with or without olaparib in women with PARP-resistant ovarian cancer. J Clin Oncol. 2021;39(15_suppl):5505–5505. 10.1200/JCO.2021.39.15_suppl.5505. [Google Scholar]
- 38.Yap TA, Ngoi N, Dumbrava EE, et al. NCI10329: phase Ib sequential trial of agents against DNA repair (STAR) study to investigate the sequential combination of the poly (ADP-Ribose) polymerase inhibitor (PARPi) olaparib (ola) and WEE1 inhibitor (WEE1i) adavosertib (ada) in patients (pts) with DNA damage response (DDR)-aberrant advanced tumors, enriched for BRCA1/2 mutated and CCNE1 amplified cancers. Eur J Cancer. 2022;174:S7. 10.1016/S0959-8049(22)00822-X. [Google Scholar]
- 39.Patel MR, Falchook GS, Wang JS-Z, et al. Open-label, multicenter, phase I study to assess safety and tolerability of adavosertib plus durvalumab in patients with advanced solid tumors. J Clin Oncol. 2019;37(15 Suppl):abst2562. 10.1200/JCO.2019.37.15_suppl.2562. [Google Scholar]
- 40.Embaby A, Kutzera J, Geenen JJ, et al. WEE1 inhibitor adavosertib in combination with carboplatin in advanced TP53 mutated ovarian cancer: a biomarker-enriched phase II study. Gynecol Oncol. 2023;174:239–46. 10.1016/j.ygyno.2023.05.063. [DOI] [PubMed] [Google Scholar]
- 41.Moore KN, Chambers SK, Hamilton EP, et al. Adavosertib with chemotherapy in patients with primary platinum-resistant ovarian, fallopian tube, or peritoneal cancer: an open-label, four-arm, phase II study. Clin Cancer Res. 2022;28(1):36–44. 10.1158/1078-0432.Ccr-21-0158. [DOI] [PubMed] [Google Scholar]
- 42.Au-Yeung G, Bressel M, Prall O, et al. IGNITE: a phase II signal-seeking trial of adavosertib targeting recurrent high-grade, serous ovarian cancer with cyclin E1 overexpression with and without gene amplification. J Clin Oncol. 2022;40(16):5515–5515. 10.1200/JCO.2022.40.16_suppl.5515. [Google Scholar]
- 43.Lheureux S, Cristea MC, Bruce JP, et al. Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: a double-blind, randomised, placebo-controlled, Phase 2 trial. Lancet. 2021;397(10271):281–92. 10.1016/s0140-6736(20)32554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.