Abstract
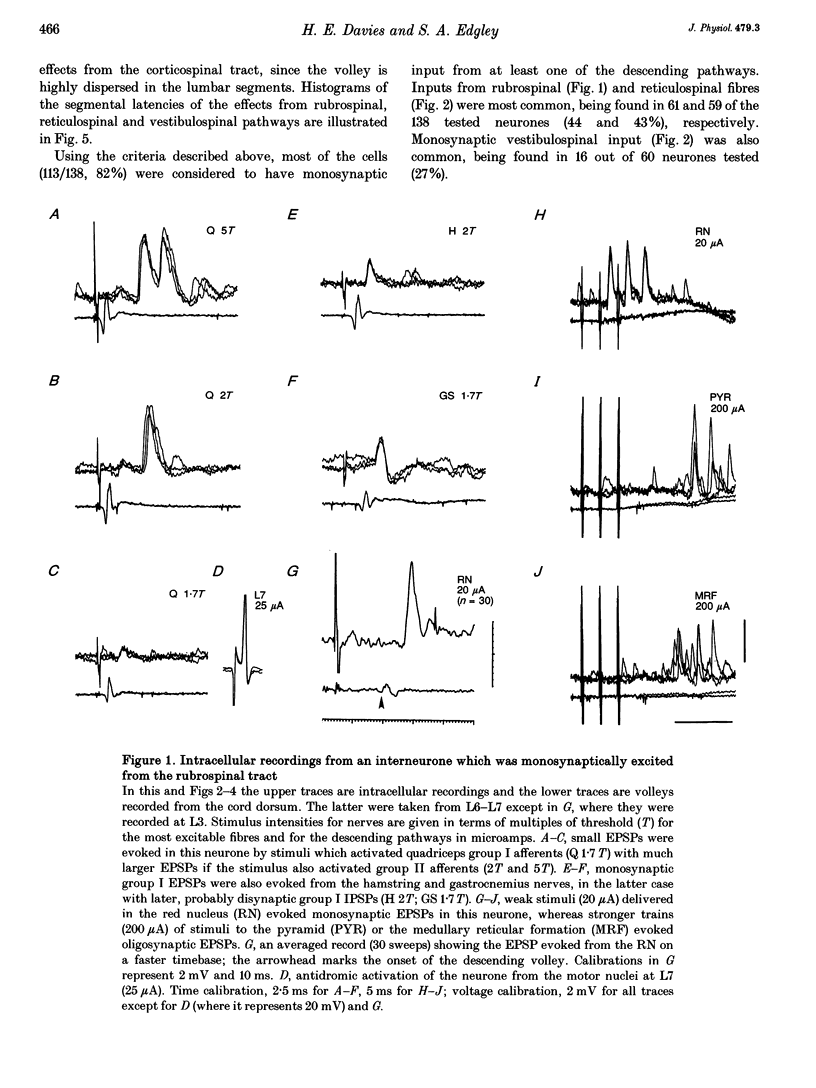
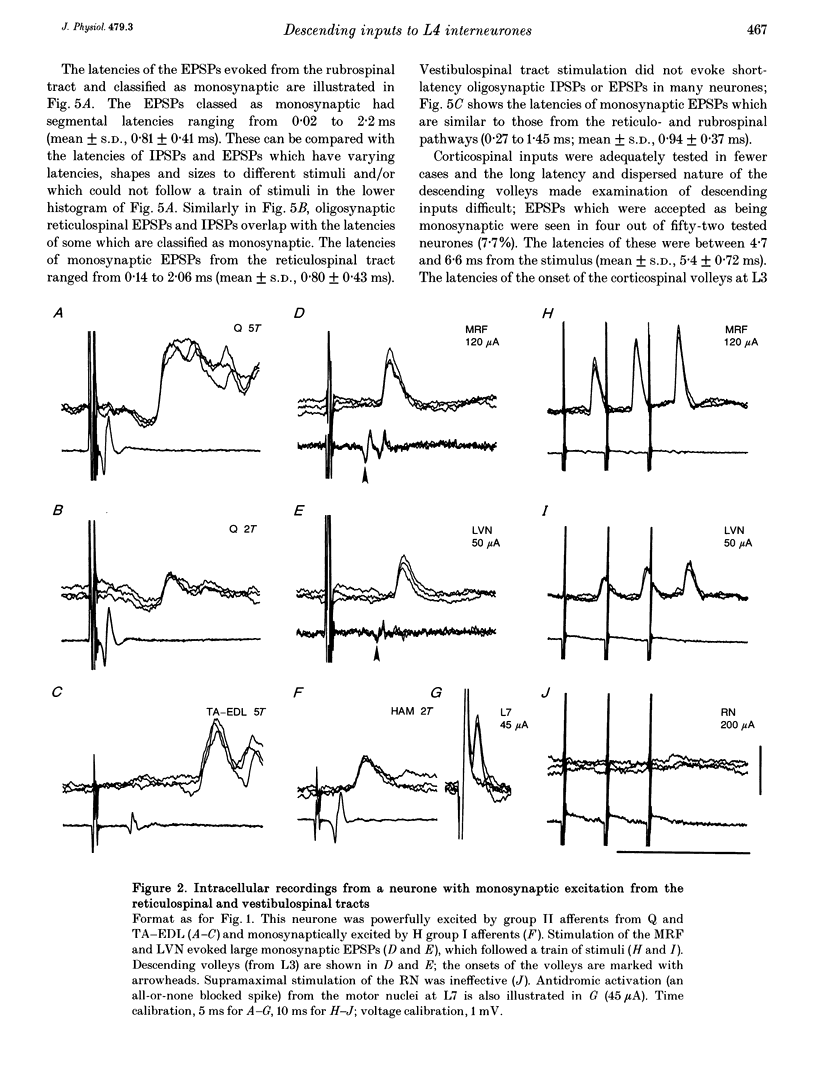
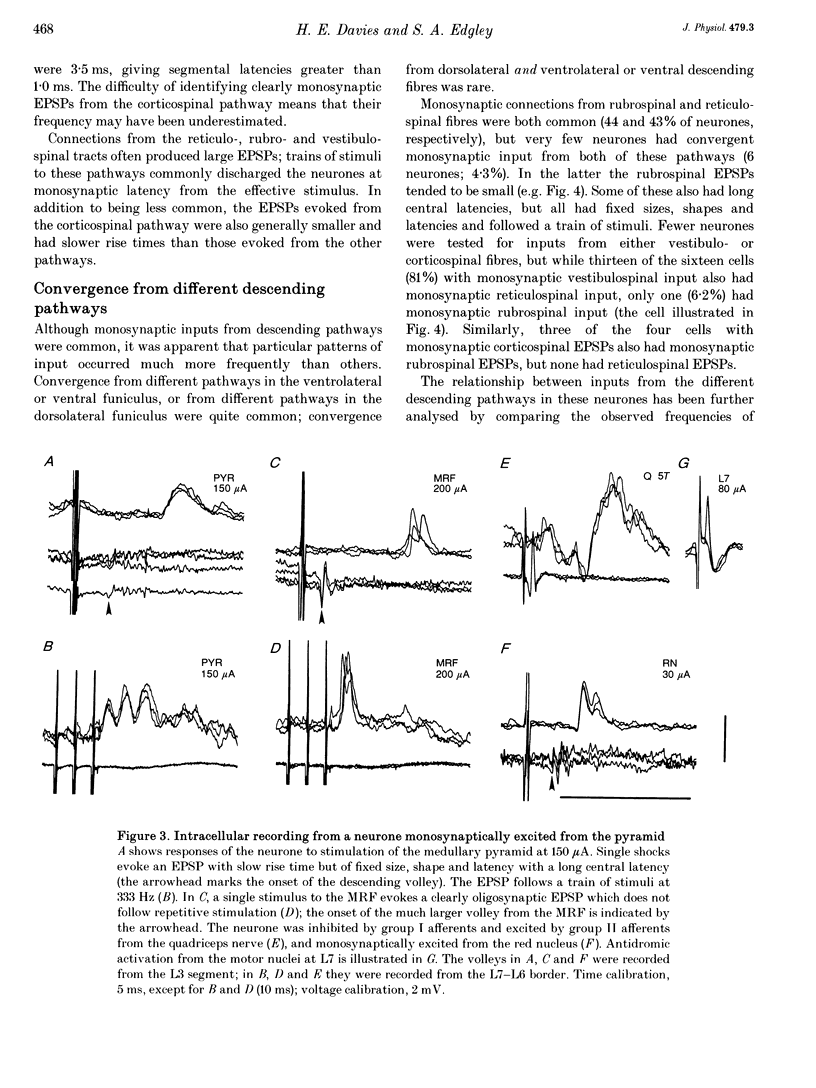
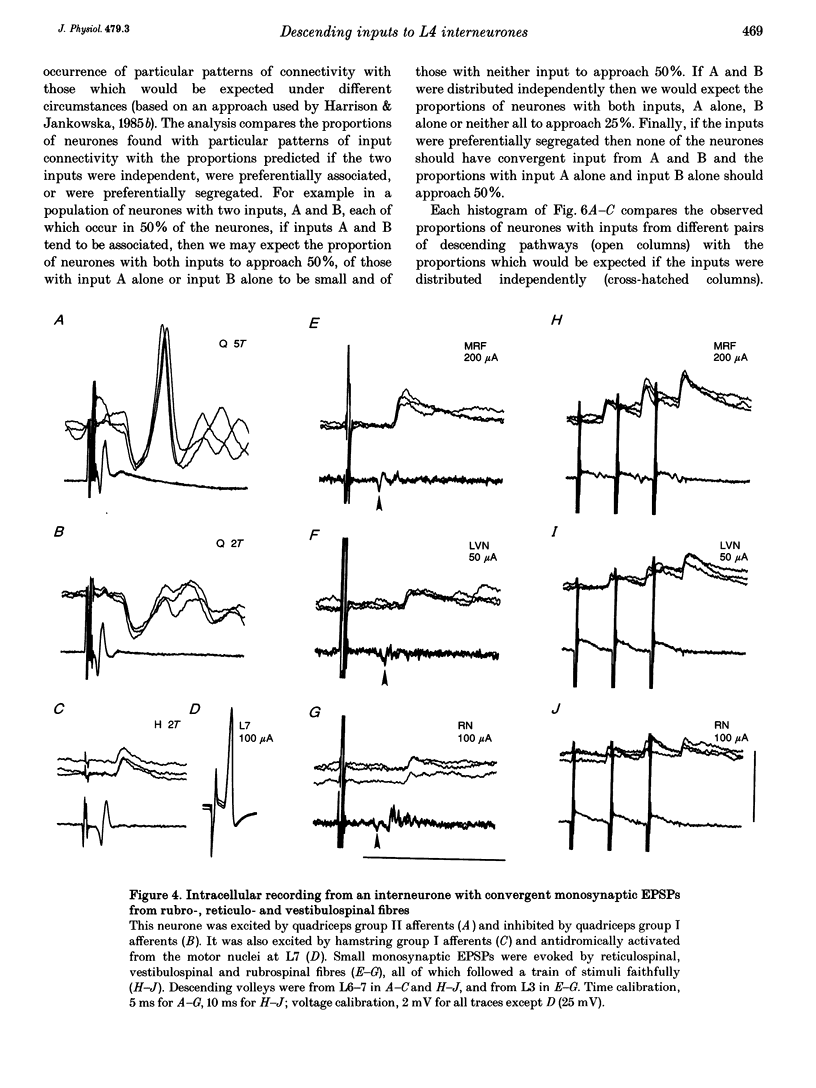
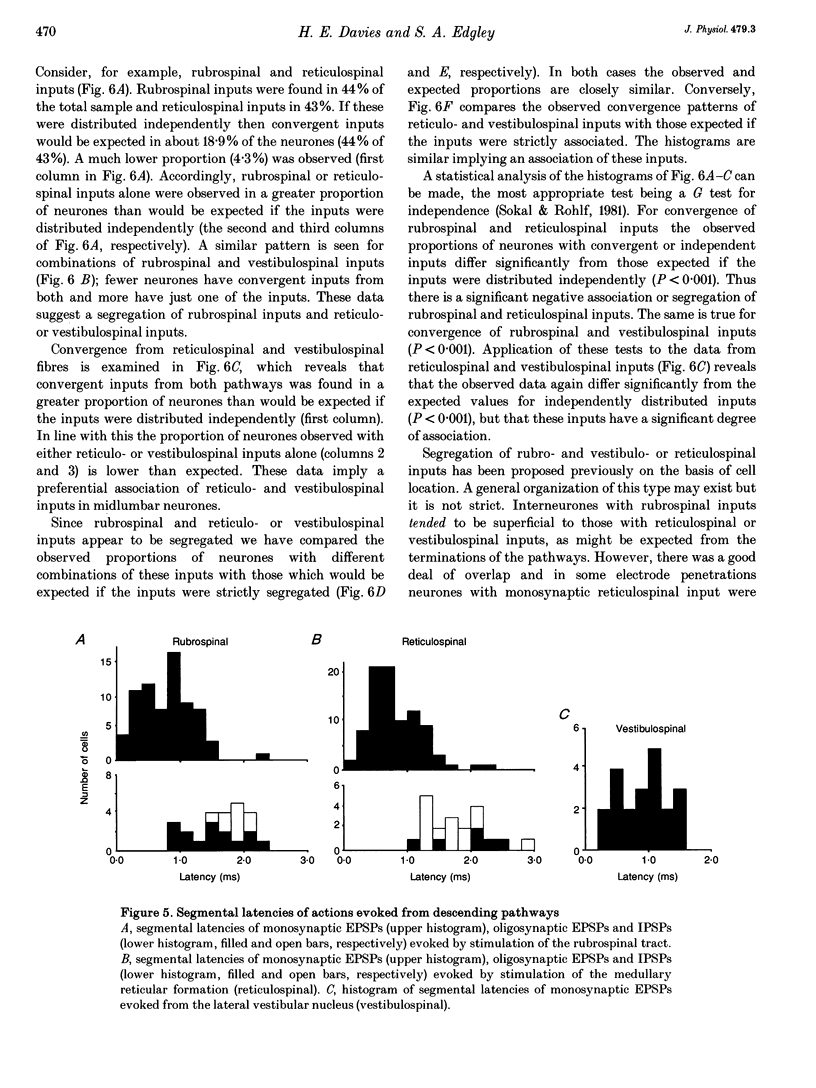
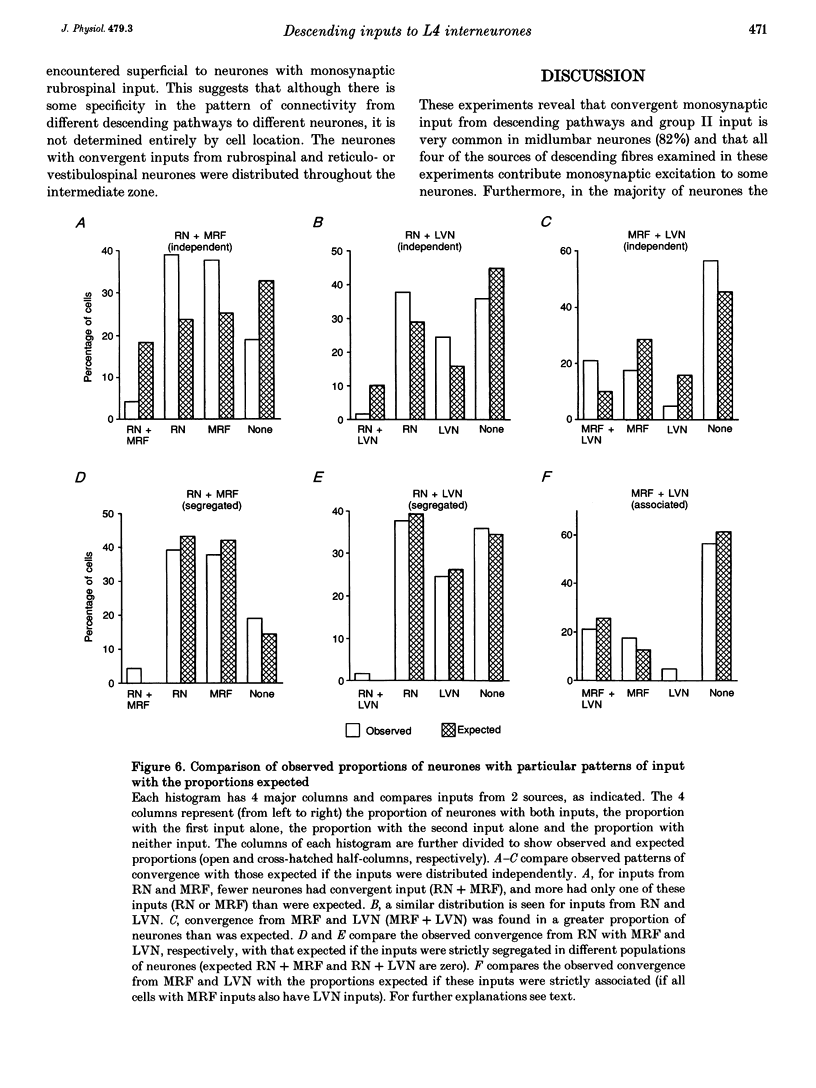
1. Connections from descending motor pathways to group II-activated interneurones in the midlumbar segments of the spinal cord have been examined by intracellular recording. Interneurones, many of which had axonal projections to the hindlimb motor nuclei, were tested for inputs from rubro-, reticulo-, vestibulo- or corticospinal fibres. 2. Of 138 cells, 113 were monosynaptically excited by electrical stimulation of at least one of the descending motor pathways. Monosynaptic excitation from reticulo-, vestibulo- and rubrospinal pathways was common. Monosynaptic corticospinal EPSPs were identified in fewer neurones. 3. Convergent monosynaptic inputs from pathways which descend in the ventrolateral and ventral funiculi were common. Although few neurones with monosynaptic input from the corticospinal tract were identified, most also had monosynaptic rubrospinal input. In contrast, few neurones (4.3%) had convergent monosynaptic input both from pathways in the dorsolateral funiculus and from fibres in the ventral/ventrolateral funiculi. 4. The patterns of convergence from the different descending motor pathways differ from the patterns expected if the descending connections were distributed independently. Thus there is a significant segregation between rubrospinal and reticulo- or vestibulospinal inputs, and a significant association of reticulo- and vestibulospinal inputs. 5. Since descending motor pathways make monosynaptic connections with most group II-activated midlumbar neurones, many of which project to the hindlimb motor nuclei, some of these neurones provide a disynaptic pathway for the supraspinal control of hindlimb movements. The distribution of descending connections is consistent with the hypothesis that pathways descending in the dorsal part of the lateral funiculus and those descending in the ventrolateral or ventral funiculi contact different sets of interneurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alstermark B., Lundberg A., Norrsell U., Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Exp Brain Res. 1981;42(3-4):299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- Arya T., Bajwa S., Edgley S. A. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol. 1991 Dec;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa S., Edgley S. A., Harrison P. J. Crossed actions on group II-activated interneurones in the midlumbar segments of the cat spinal cord. J Physiol. 1992 Sep;455:205–217. doi: 10.1113/jphysiol.1992.sp019297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H., Cavallari P., Jankowska E., Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol. 1989 Dec 1;290(1):1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Cavallari P., Edgley S. A., Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987 Aug;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T., Dubuc R., Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986 Feb;55(2):375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987 Aug;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:403–418. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Kudo N., Tanaka R. The vestibulospinal tract: crossed and uncrossed effects on hindlimb motoneurones in the cat. Exp Brain Res. 1975 Nov 28;24(1):37–55. doi: 10.1007/BF00236016. [DOI] [PubMed] [Google Scholar]
- Illert M., Jankowska E., Lundberg A., Odutola A. Integration in descending motor pathways controlling the forelimb in the cat. 7. Effects from the reticular formation on C3-C4 propriospinal neurones. Exp Brain Res. 1981;42(3-4):269–281. doi: 10.1007/BF00237494. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Padel Y., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic excitatory convergence on C3--C4 propriospinal neurones. Exp Brain Res. 1978 Sep 15;33(1):101–130. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- Kozhanov V. M., Shapovalov A. I. Sinapticheskaia organizatsiia supraspinal'nogo kontrolia propriospinal'nykh neironov ventral'nogo roga spinnogo mozga koshki i obez'iany. Neirofiziologiia. 1977;9(2):177–184. [PubMed] [Google Scholar]
- Kuypers H. G. A new look at the organization of the motor system. Prog Brain Res. 1982;57:381–403. doi: 10.1016/S0079-6123(08)64138-2. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of rubrospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of vestibulospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:85–98. doi: 10.1016/0006-8993(72)90007-8. [DOI] [PubMed] [Google Scholar]
- Petras J. M. Cortical, tectal and tegmental fiber connections in the spinal cord of the cat. Brain Res. 1967 Oct;6(2):275–324. doi: 10.1016/0006-8993(67)90196-5. [DOI] [PubMed] [Google Scholar]
- Shefchyk S., McCrea D., Kriellaars D., Fortier P., Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Exp Brain Res. 1990;80(2):290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Timerick S. J., Wilson V. J. Body position with respect to the head or body position in space is coded by lumbar interneurons. J Neurophysiol. 1985 Jul;54(1):123–133. doi: 10.1152/jn.1985.54.1.123. [DOI] [PubMed] [Google Scholar]
- Yates B. J., Kasper J., Wilson V. J. Effects of muscle and cutaneous hindlimb afferents on L4 neurons whose activity is modulated by neck rotation. Exp Brain Res. 1989;77(1):48–56. doi: 10.1007/BF00250566. [DOI] [PubMed] [Google Scholar]


