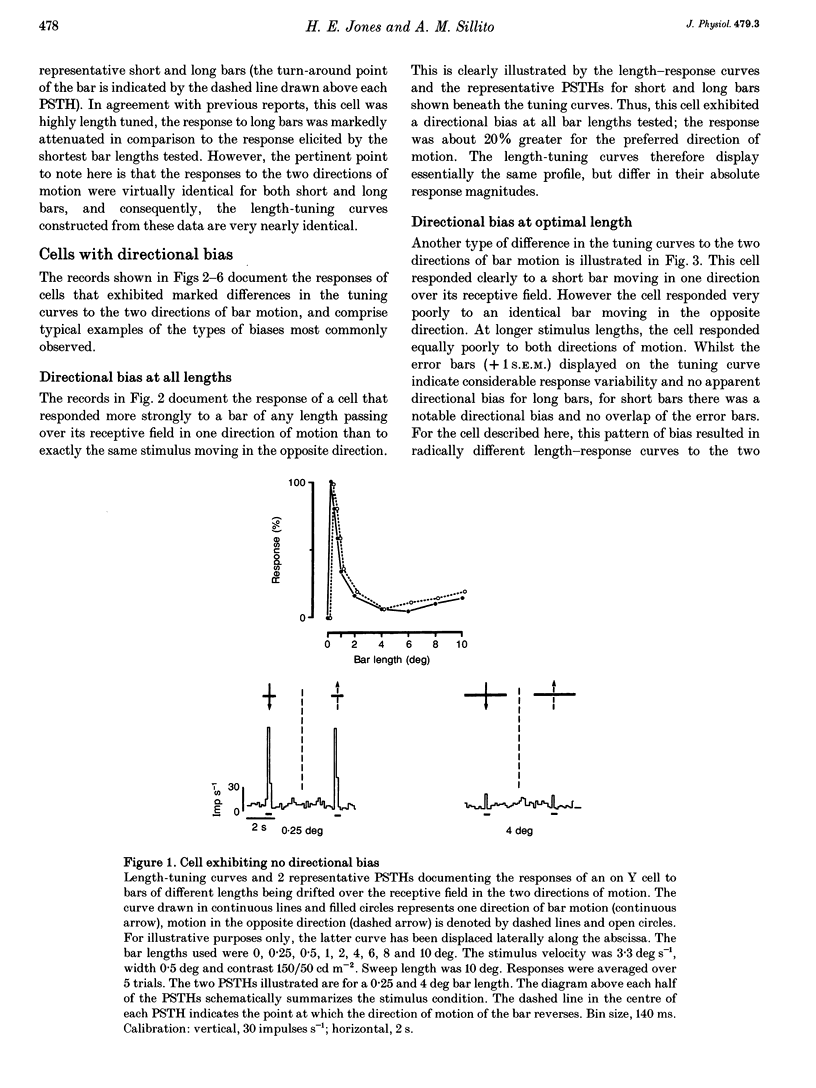
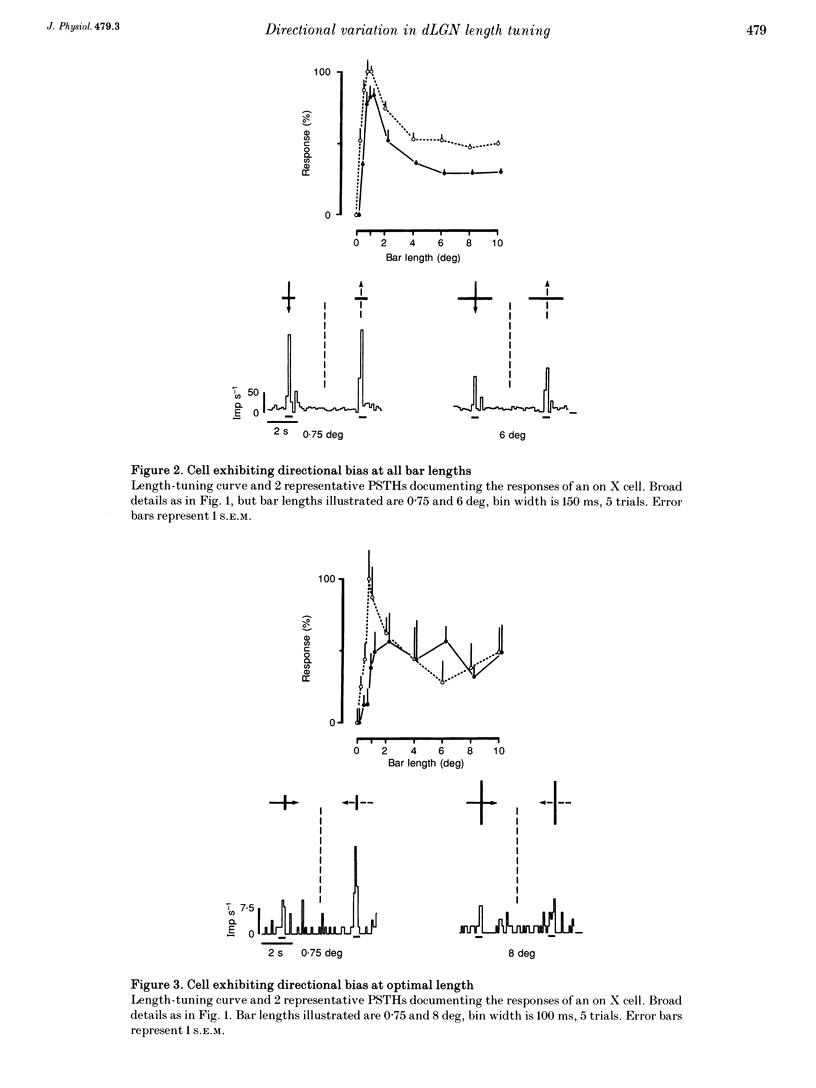
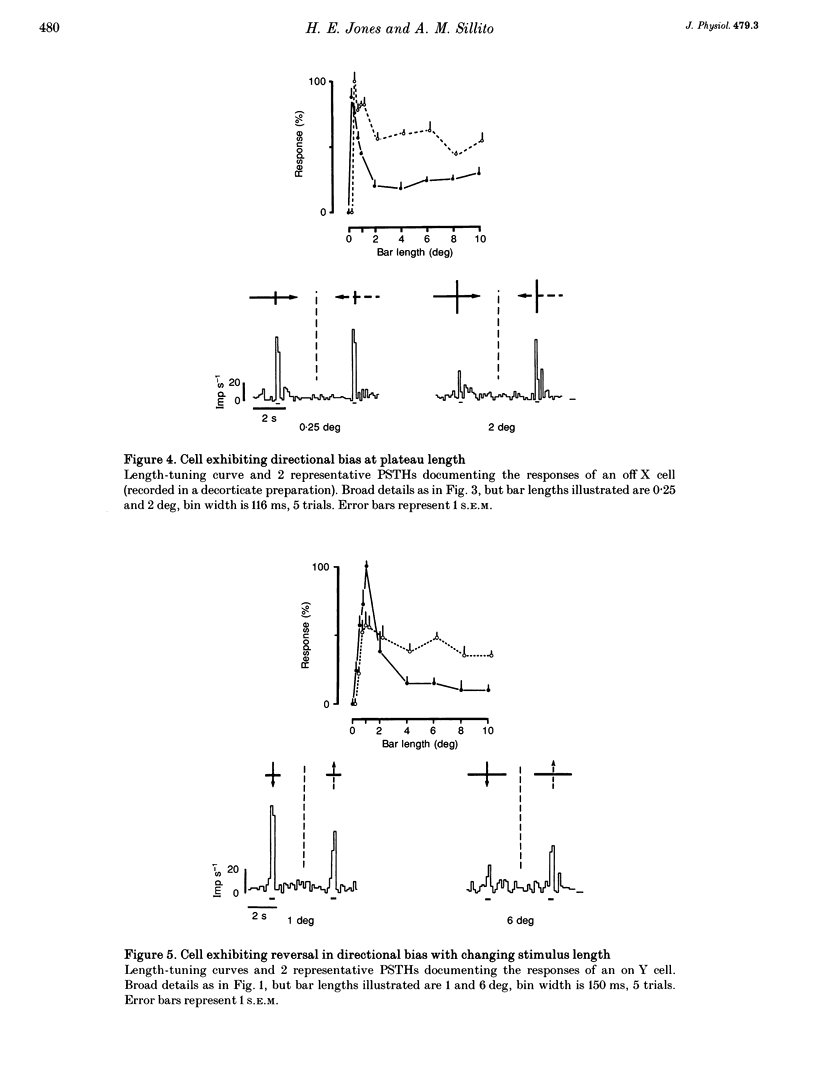
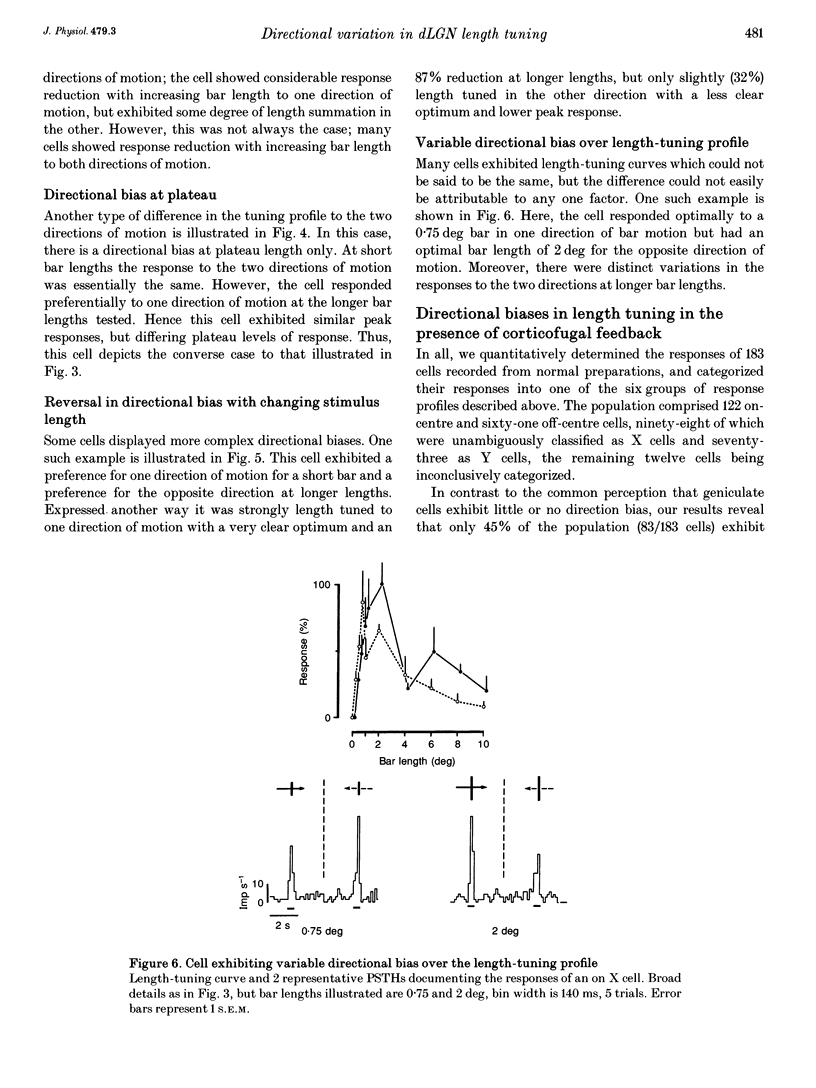
Abstract
1. The visual cortex provides a major synaptic input to the dorsal lateral geniculate nucleus (dLGN). Cortical layer VI cells giving rise to this projection are strongly influenced by stimulus orientation, length and direction of motion. In the dLGN, a significant component of the strong length tuning exhibited by most cells follows from the corticofugal influence. We have now checked whether there are directional biases in geniculate cell responses, and whether such biases are influenced by stimulus length. 2. The responses of A-laminae dLGN cells were assessed by single-unit extracellular recording. Length preference was examined by plotting multihistogram length-tuning curves to moving bars of light of various length. 3. Over half of the cells tested (100/183) exhibited directional bias and in many cases, this bias was highly dependent on bar length, resulting in radically different length response profiles for the two directions of motion. These asymmetries are similar to those documented for cortical hypercomplex cells, but do not equate to any known facet of the centre-surround organization of dLGN cell receptive fields. 4. We suspected the directional biases followed from the influence of the corticofugal projection. To test this, we recorded from preparations where areas 17 and 18 of the visual cortex had been removed. Surprisingly, a similar proportion of cells exhibited directional biases after removal of the corticofugal input, suggesting that the biases are generated subcortically. 5. The widespread presence of systematic biases in the response profiles of dLGN cells further underlines the possibility that geniculate mechanisms may make a far greater contribution to visual processing than hitherto suspected.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. The detection of movement direction and effects of contrast reversal in the cat's striate cortex. Vision Res. 1980;20(4):289–293. doi: 10.1016/0042-6989(80)90015-2. [DOI] [PubMed] [Google Scholar]
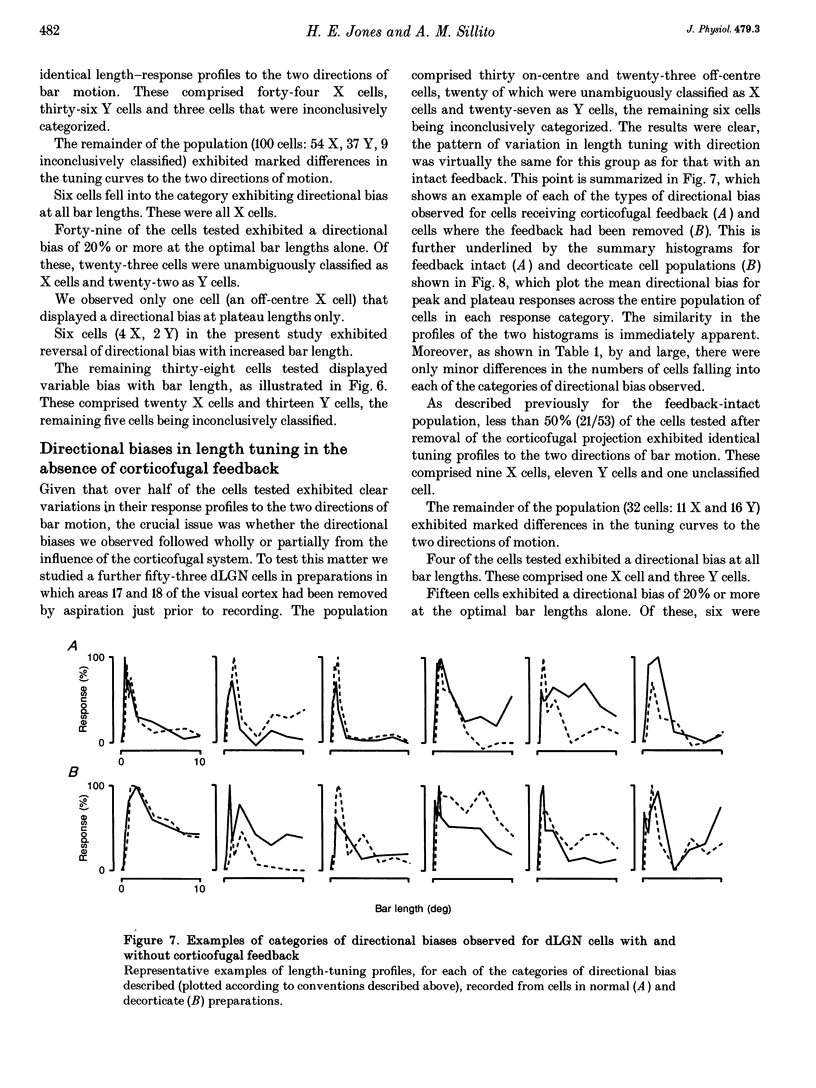
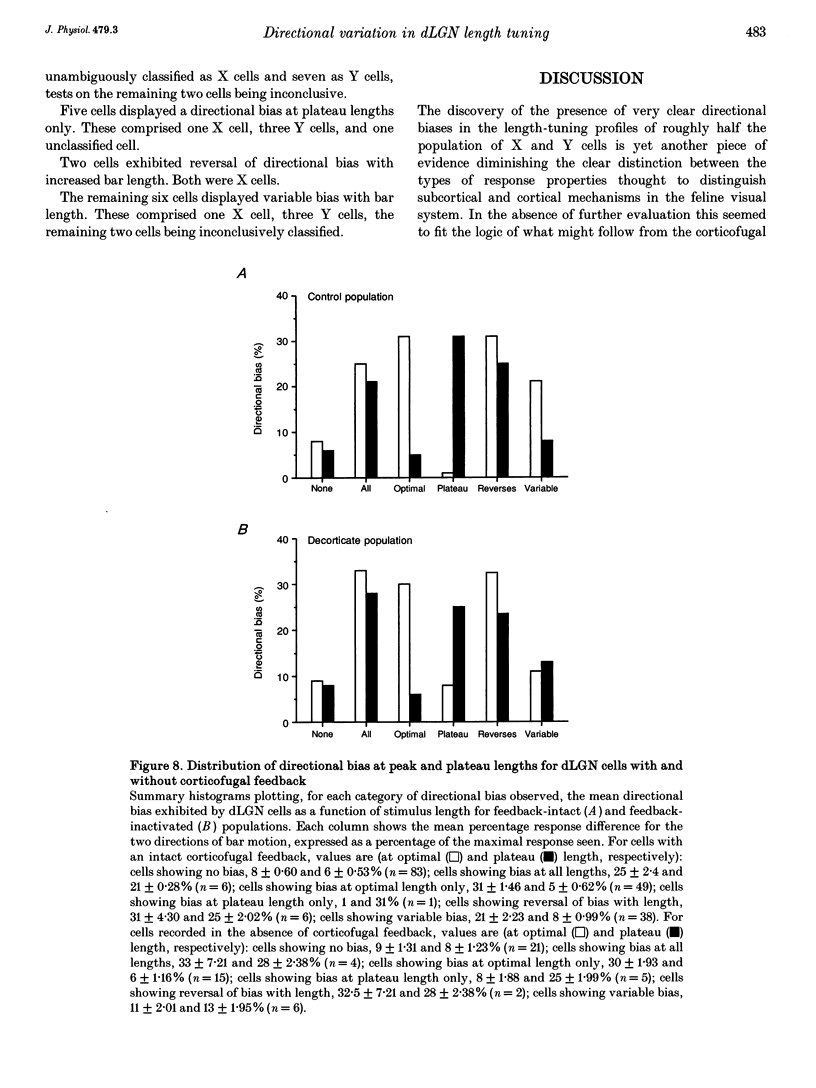
- Albus K., Wolf W., Beckman R. Orientation bias in the response of kitten LGNd neurons to moving light bars. Brain Res. 1983 Feb;282(3):308–313. doi: 10.1016/0165-3806(83)90071-8. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Gilbert C. D. Generation of end-inhibition in the visual cortex via interlaminar connections. 1986 Mar 27-Apr 2Nature. 320(6060):362–365. doi: 10.1038/320362a0. [DOI] [PubMed] [Google Scholar]
- Bowling D. B., Wieniawa-Narkiewicz E. The distribution of on- and off-centre X- and Y-like cells in the A layers of the cat's lateral geniculate nucleus. J Physiol. 1986 Jun;375:561–572. doi: 10.1113/jphysiol.1986.sp016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyapati J., Henry G. H. The duplex character of the corticofugal pathway from the striate cortex to the lateral geniculate complex of the cat. Vision Res. 1987;27(5):723–726. doi: 10.1016/0042-6989(87)90069-1. [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Laminar distribution of first-order neurons and afferent terminals in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1271–1281. doi: 10.1152/jn.1979.42.5.1271. [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Neural path taken by afferent streams in striate cortex of the cat. J Neurophysiol. 1979 Sep;42(5):1264–1270. doi: 10.1152/jn.1979.42.5.1264. [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Ordinal position of neurons in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1251–1263. doi: 10.1152/jn.1979.42.5.1251. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Harding T. H., Tulunay-Keesey U. Response to the length of moving visual stimuli of the brisk classes of ganglion cells in the cat retina. J Physiol. 1983 Dec;345:27–45. doi: 10.1113/jphysiol.1983.sp014963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Lee B. B., Vidyasagar T. R. Response of neurons in the cat's lateral geniculate nucleus to moving bars of different length. J Neurosci. 1983 Jan;3(1):108–116. doi: 10.1523/JNEUROSCI.03-01-00108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J. D., Norman J. L., Pettigrew J. D. Biases for oriented moving bars in lateral geniculate nucleus neurons of normal and stripe-reared cats. Exp Brain Res. 1977 Aug 31;29(2):155–172. doi: 10.1007/BF00237039. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. Spatial and temporal properties of X and Y cells in the cat lateral geniculate nucleus. J Physiol. 1979 Aug;293:347–364. doi: 10.1113/jphysiol.1979.sp012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A., Whitteridge D. Selective responses of visual cortical cells do not depend on shunting inhibition. Nature. 1988 Apr 14;332(6165):642–644. doi: 10.1038/332642a0. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysel U. T., Wörgötter F., Pape H. C. Local cortical lesions abolish lateral inhibition at direction selective cells in cat visual cortex. Exp Brain Res. 1987;68(3):606–612. doi: 10.1007/BF00249803. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Berson D. M. Autoradiographic evidence for a projection from the pretectal nucleus of the optic tract to the dorsal lateral geniculate complex in the cat. Brain Res. 1980 Aug 11;195(1):1–12. doi: 10.1016/0006-8993(80)90861-6. [DOI] [PubMed] [Google Scholar]
- Grieve K. L., Sillito A. M. The length summation properties of layer VI cells in the visual cortex and hypercomplex cell end zone inhibition. Exp Brain Res. 1991;84(2):319–325. doi: 10.1007/BF00231452. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Harvey A. R. A physiological analysis of subcortical and commissural projections of areas 17 and 18 of the cat. J Physiol. 1980 May;302:507–534. doi: 10.1113/jphysiol.1980.sp013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J. Retinal input to the nucleus of the optic tract of the cat assessed by antidromic activation of ganglion cells. Exp Brain Res. 1985;59(2):395–403. doi: 10.1007/BF00230920. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Sur M., Uhlrich D. J., Sherman S. M. Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol. 1985 Mar 8;233(2):159–189. doi: 10.1002/cne.902330203. [DOI] [PubMed] [Google Scholar]
- Jones H. E., Sillito A. M. The length-response properties of cells in the feline dorsal lateral geniculate nucleus. J Physiol. 1991 Dec;444:329–348. doi: 10.1113/jphysiol.1991.sp018881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T., Morimoto M., Kanaseki T., Inomata H. Projection from the pretectal nuclei to the dorsal lateral geniculate nucleus in the cat: a wheat germ agglutinin-horseradish peroxidase study. Brain Res. 1987 Sep 22;421(1-2):30–40. doi: 10.1016/0006-8993(87)91271-6. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Willshaw D. J. Responses of the various types of cat retinal ganglion cells to moving contours. Vision Res. 1978;18(7):757–765. doi: 10.1016/0042-6989(78)90114-1. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Oyster C. W., Takahashi E. Rabbit lateral geniculate nucleus: sharpener of directional information. Science. 1969 Aug 15;165(3894):712–714. doi: 10.1126/science.165.3894.712. [DOI] [PubMed] [Google Scholar]
- Martin K. A. The lateral geniculate nucleus strikes back. Trends Neurosci. 1988 May;11(5):192–194. doi: 10.1016/0166-2236(88)90121-x. [DOI] [PubMed] [Google Scholar]
- Murphy P. C., Sillito A. M. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature. 1987 Oct 22;329(6141):727–729. doi: 10.1038/329727a0. [DOI] [PubMed] [Google Scholar]
- Murphy P. C., Sillito A. M. The binocular input to cells in the feline dorsal lateral geniculate nucleus (dLGN). J Physiol. 1989 Aug;415:393–408. doi: 10.1113/jphysiol.1989.sp017727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustari M. J., Bullier J., Henry G. H. Comparison of response of properties of three types of monosynaptic S-cell in cat striate cortex. J Neurophysiol. 1982 Mar;47(3):439–454. doi: 10.1152/jn.1982.47.3.439. [DOI] [PubMed] [Google Scholar]
- Orban G. A., Kato H., Bishop P. O. Dimensions and properties of end-zone inhibitory areas in receptive fields of hypercomplex cells in cat striate cortex. J Neurophysiol. 1979 May;42(3):833–849. doi: 10.1152/jn.1979.42.3.833. [DOI] [PubMed] [Google Scholar]
- Orban G. A., Kato H., Bishop P. O. End-zone region in receptive fields of hypercomplex and other striate neurons in the cat. J Neurophysiol. 1979 May;42(3):818–832. doi: 10.1152/jn.1979.42.3.818. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rose D. Mechanisms underlying the receptive field properties of neurons in cat visual cortex. Vision Res. 1979;19(5):533–544. doi: 10.1016/0042-6989(79)90138-x. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. I. Spatiotemporal organization of receptive fields. J Neurophysiol. 1976 Nov;39(6):1288–1319. doi: 10.1152/jn.1976.39.6.1288. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Cudeiro J., Murphy P. C. Orientation sensitive elements in the corticofugal influence on centre-surround interactions in the dorsal lateral geniculate nucleus. Exp Brain Res. 1993;93(1):6–16. doi: 10.1007/BF00227775. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat's visual cortex. J Physiol. 1977 Oct;271(3):699–720. doi: 10.1113/jphysiol.1977.sp012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soodak R. E., Shapley R. M., Kaplan E. Linear mechanism of orientation tuning in the retina and lateral geniculate nucleus of the cat. J Neurophysiol. 1987 Aug;58(2):267–275. doi: 10.1152/jn.1987.58.2.267. [DOI] [PubMed] [Google Scholar]
- Uhlrich D. J., Cucchiaro J. B., Humphrey A. L., Sherman S. M. Morphology and axonal projection patterns of individual neurons in the cat perigeniculate nucleus. J Neurophysiol. 1991 Jun;65(6):1528–1541. doi: 10.1152/jn.1991.65.6.1528. [DOI] [PubMed] [Google Scholar]
- Vidyasagar T. R., Urbas J. V. Orientation sensitivity of cat LGN neurones with and without inputs from visual cortical areas 17 and 18. Exp Brain Res. 1982;46(2):157–169. doi: 10.1007/BF00237172. [DOI] [PubMed] [Google Scholar]


