Abstract
Background
Osimertinib shows higher effectiveness than first-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) in the initial treatment of EGFR-mutated non-small cell lung cancer. However, its superiority in terms of overall survival in the Asian population, especially Japanese patients, remains uncertain.
Objective
To evaluate the survival benefit of osimertinib over other EGFR-TKIs in Japanese patients, using real-world data.
Methods
As part of the Tokushukai REAl-world Data project, a retrospective multi-institutional study across 46 hospitals in Japan was conducted to evaluate the overall survival of patients with advanced EGFR-mutated non-small cell lung cancer using propensity score matching. The study involved patients receiving osimertinib as the first-line treatment (1L-Osi), those initially treated with other EGFR-TKIs (1L-non-Osi), and those receiving osimertinib after initial EGFR-TKI treatment (2L/later-Osi) between April 2010 and December 2022 and followed up until April 2023.
Results
Among 1062 Japanese patients with EGFR-mutated non-small cell lung cancer, 416 (39.2%) received 1L-Osi, while 646 (60.8%) received 1L-non-Osi, including 139 (13.1%) who received 2L/later-Osi. Within these groups, 416 (39.2%), 293 (27.6%), and 75 (7.1%) patients received first-line EGFR-TKI treatment post-osimertinib approval as a later-line treatment in Japan (March 2016). After propensity score matching, the overall survival of the 1L-Osi group was comparable to that of the 1L-non-Osi group in the post-March 2016 subset (n = 283, 42.0 vs 42.4 months). Similar trends were observed in the Del19 and L858R subgroups. The median overall survival of the 2L/later-Osi group was notably long: 60.2 months post-March 2016 (n = 75). A subgroup analysis based on initial EGFR-TKI treatment in the 1L-non-Osi and 2L/later-Osi groups revealed no significant differences among the gefitinib, erlotinib, and afatinib groups.
Conclusions
Based on real-world data, osimertinib did not show a significant improvement in overall survival compared to other EGFR-TKIs as a first-line treatment for EGFR-mutated advanced non-small cell lung cancer in the Japanese (Asian) population.
Clinical Trial Registration
This study was registered at the University Hospital Medical Information Network Clinical Trials Registry on 9 March, 2023 (identification UMIN000050552).
Key Points
| Osimertinib was approved in Japan for epidermal growth factor receptor-mutated non-small cell lung cancer as a late-line therapy in March 2016 and as a first-line therapy in August 2018. |
| Real-world data demonstrated osimertinib did not prolong overall survival in Japanese patients with untreated advanced epidermal growth factor receptor-mutated non-small cell lung cancer who started epidermal growth factor receptor -tyrosine kinase inhibitor monotherapy after March 2016, when compared to treatment with other epidermal growth factor receptor-tyrosine kinase inhibitors. |
Introduction
Epidermal growth factor receptor (EGFR) mutations are the most common driver gene mutations in lung cancer among Asian populations and directly affect treatment choices [1]. Epidermal growth factor receptor-tyrosine kinase inhibitors (TKIs) targeting these mutations are essential drugs for treating EGFR-mutated advanced non-small cell lung cancer (NSCLC) [2, 3]. First-generation (gefitinib and erlotinib), second-generation (afatinib and dacomitinib), and third-generation (osimertinib) EGFR-TKIs are used in the first-line palliative treatment of advanced NSCLC [2, 3]. Osimertinib has become the primary choice of drug in the first-line treatment of EGFR-mutated advanced NSCLC, based on the results of a randomized phase III trial in which extended progression-free survival and overall survival (OS) were achieved with osimertinib compared with those achieved through gefitinib or erlotinib treatment [4]. Our previous real-world data (RWD) study, conducted since the practical use of EGFR-TKIs in clinical settings, underscored and validated the notable OS extension observed with the use of osimertinib, a new-generation EGFR-TKI [5]. However, patients receiving initial treatment with first-generation and second-generation EGFR-TKIs were included in the era before osimertinib development [5]. Sequential administration of osimertinib is now possible, but it was not applicable to patients during that period. Moreover, a subgroup analysis of a phase III trial revealed no significant differences in survival benefits between osimertinib and first-generation EGFR-TKIs in Asian and Japanese subsets, respectively [6–8]. In the Japanese subset, there was a trend toward a lower prognostic benefit in the osimertinib group (hazard ratio [HR]: 1.39, 95% confidence interval [CI] 0.83–2.34) [7]. Furthermore, osimertinib exhibits an insufficient inhibitory effect to achieve complete remission, resulting in resistance and relapse. Therefore, developing strategies to improve the prognosis of patients with EGFR-mutated NSCLC is essential, and the sequential use of EGFR-TKIs, specifically osimertinib, in second-line and later-line therapies remains crucial.
We initially aimed to determine if osimertinib is the definitive choice for first-line therapy in an Asian population, namely a Japanese population. Additionally, we sought to assess the OS of patients receiving sequential treatment—initially treated with other EGFR-TKIs and subsequently received osimertinib. Consequently, we retrospectively examined the OS of untreated Japanese patients with EGFR-mutated advanced or recurrent NSCLC receiving osimertinib as first-line therapy and those treated with EGFR-TKIs other than osimertinib as first-line therapy, including those receiving osimertinib as second-line or later-line treatment. Propensity score matching (PSM) was performed using RWD, focusing on the time frame when osimertinib became available for use in second-line or higher-line treatment.
Materials and Methods
Study Design and Setting
We conducted a retrospective multi-institutional observational study using electronically available data from the database created by the Tokushukai REAl-world Data (TREAD) project. The Tokushukai Group is a large leading medical group in Japan that includes 71 general hospitals nationwide. The TREAD project included 46 out of the 71 hospitals that used diagnosis procedure combination data, including information on the primary diagnosis, interventions, and comorbidities. It aimed to collect RWD on systemic therapy for Japanese patients with cancer in the Tokushukai Medical Group [9]. The antecedent study within that project, named “TREAD-01,” specifically focused on EGFR-mutated NSCLC. We used the electronically available data from the Tokushukai Medical Database, which was regularly and centrally updated by Tokushukai Information Systems, Inc. before study initiation. All prescribed chemotherapy regimens were approved by the regimen review committee of the Tokushukai Medical Group, ensuring nationwide standardization of treatment schedules and dosages. The standard EGFR-TKI regimen comprised gefitinib (250 mg/day), erlotinib (150 mg/day), afatinib (40 mg/day), dacomitinib (45 mg/day), and osimertinib (80 mg/day). This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) guidelines [10, 11].
Participants
The patients were consecutively enrolled in the TREAD-01 database. Japanese patients aged ≥ 20 years at the start of the treatment were eligible for enrollment if they had cytologically or pathologically confirmed NSCLC; had illness resulting from sensitizing EGFR mutations (Del19 or L858R) and other uncommon mutations; had NSCLC categorized as untreated Stage III for which curative therapy was not feasible, Stage IV (according to the 7th or 8th edition of the American Joint Committee on Cancer staging criteria for lung cancer), or recurrent disease after curative treatment [12, 13]; and had received first-line treatment with EGFR-TKI monotherapy (gefitinib, erlotinib, afatinib, dacomitinib, or osimertinib) between April 2010 and December 2022. The exclusion criteria included the following: (1) active multiple cancer with a disease-free interval of ≤ 5 years, except carcinoma in situ/intramucosal cancer cured through local treatment or endoscopic lesions diagnosed as carcinoma in situ; (2) lack of provision of informed consent; and (3) inadequate data extraction.
Data Collection
We obtained patient background information from the electronically available Tokushukai Medical Database at the initiation of the first-line EGFR-TKI therapy. Patient background information included age, sex, smoking history, histological type, disease stage, and EGFR mutation type (in cases where sensitive mutations, Del19 and L858R, were positive as part of a compound mutation, including minor mutations turning positive, only sensitive mutations were documented). Additionally, patients with exclusive de novo T790M positivity were excluded, and Eastern Cooperative Oncology Group Performance Status (ECOG PS) scores were documented in the database or scored from unstructured narrative text up to 1 month before initiating first-line treatment. Data regarding height, weight, and body mass index were collected at the initiation of first-line EGFR-TKI therapy; if unavailable, these data were collected at the nearest alternative period to the initiation of EGFR-TKI therapy. Additionally, we collected information on prognosis (final date of survival confirmation and date of death) and treatment exposure, including the names of EGFR-TKIs and the treatment line. However, data on the reduction of initial doses, mid-treatment adjustments (involving dose reductions or extensions of dosing intervals), and treatment interruptions were not collected. The treatment lines encompassed various regimens, including EGFR-TKIs, cytotoxic anticancer agents, immune checkpoint inhibitors, and angiogenesis inhibitors, administered individually and combined. Furthermore, we collected data on the reasons for discontinuing the previous EGFR-TKI therapy and initiating osimertinib treatment in patients who received it as a second- or later-line treatment. Moreover, we gathered information on whether T790M confirmation was possible before initiating osimertinib as a second-line or later-line therapy.
To ensure that opportunities for second-line or later-line osimertinib administration were equivalent to the current situation, a cohort of patients who started EGFR-TKIs as first-line therapy after March 2016 was established. In March 2016, osimertinib was approved in Japan for use in patients with NSCLC positive for the T790M-resistance gene as second-line or later-line treatment [14, 15]. Patients for whom EGFR-TKI treatment was initiated before this approval might have missed the chance of receiving osimertinib in subsequent therapy lines. The cutoff date for prognosis-related information was 30 April, 2023.
Endpoint
The primary endpoint was OS of patients who received osimertinib as first-line therapy (1L-Osi), compared with OS of all patients who received a first-generation or second-generation EGFR-TKI other than osimertinib as first-line therapy (1L-non-Osi), with initiation dates after March 2016. The secondary endpoint was OS of patients who subsequently received osimertinib as second-line or later-line treatment after their first-line treatment with EGFR-TKIs other than osimertinib (2L/later-Osi, initiated after March 2016). Both endpoints were also assessed for the cohort throughout the study period.
A subgroup analysis of OS according to EGFR mutation type was performed in the aforementioned population. Additionally, the study investigated the reasons for regimen change to osimertinib and the T790M status in the 2L/later-Osi group. Another planned subgroup analysis of OS focused on the 1L-non-Osi and 2L/later-Osi groups, categorized by the specific EGFR-TKIs used in the first-line treatment. Overall survival was defined as the time from the date of the first EGFR-TKI prescription as first-line therapy to the date of death or last confirmed survival.
Ethical Considerations
The study was conducted following the principles of the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Tokushukai Group on 2 March, 2023 (approval number: TGE 01427-008). The requirement for obtaining informed consent from patients was waived because this was a retrospective analysis of anonymized patient data. Patients were allowed to opt out of the research use of their data, and related information is publicly available on the Tokushukai Medical Group website. This study was registered at the University Hospital Medical Information Network Clinical Trials Registry on 9 March, 2023 (identification UMIN000050552).
Statistical Analysis
To estimate median OS, we generated survival curves using the Kaplan–Meier method and compared the groups using the log-rank test. Given the presence of immortal time bias, we refrained from conducting direct statistical comparisons of OS between certain groups, such as between the 1L-Osi and 2L/later-Osi groups. Continuous variables are presented as median with range or interquartile range (IQR), and univariate between-group comparisons were conducted using the Mann–Whitney U test. Categorical variables are expressed as numbers (percentage), and they were compared using the chi-square and Fisher’s exact tests. We applied PSM to enhance comparability between the treatment groups. Covariate selection was guided by their relevance and the potential to introduce bias. We used a logistic regression model to estimate propensity scores, accounting for the selected covariates. The selected prognostic factors were age, sex, smoking history, histological type, disease stage, EGFR mutation type, and ECOG PS. In the matching process, we used nearest-neighbor matching with a specified caliper width, causing a more balanced distribution of covariates within each group. Cox proportional hazards regression analysis was performed to determine the adjusted HR and 95% CIs for OS. The possible determinants were the previously mentioned prognostic factors. Additionally, for the population that started treatment after March 2016, we included 1L-Osi or 1L-non-Osi status. For the subgroup analysis based on the specific type of EGFR-TKI used in the first-line treatment, we included the EGFR-TKI type data. Statistical significance was set at p < 0.05. p values were reported to two decimal places, except where greater precision was required. All data analyses and visualizations were performed using R (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria). Data extraction, cleaning, and analysis were conducted from May 2023 to March 2024.
Results
Patient Characteristics
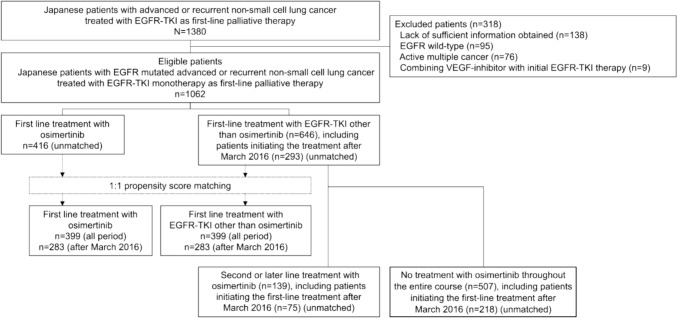
A flowchart of the study participant selection process is shown in Fig. 1. Of the 1380 patients considered for enrollment, 1062 patients with EGFR-mutated advanced or recurrent NSCLC, treated with any EGFR-TKI as first-line palliative treatment, were eligible. We identified 555 patients who received at least one dose of osimertinib during the treatment period: 416 and 139 patients in the 1L-Osi and 2L/later-Osi groups, respectively, with 103 and 36 receiving it as second-line and later-line treatments, respectively. A total of 646 patients were included in the 1L-non-Osi group: 404 received gefitinib, 119 received erlotinib, and 123 received afatinib as first-line treatment. Among the 293 patients for whom first-line treatment was initiated after March 2016 in the 1L-non-Osi group, 75 (25.6%) received osimertinib as second-line or later-line treatment. In contrast, among patients whose first-line treatment was started before this period, 64 of 353 patients (18.1%) received osimertinib as second-line or later-line treatment. The difference between these proportions was significant (p = 0.03).
Fig. 1.
Patient flowchart. EGFR epidermal growth factor receptor, TKI tyrosine kinase inhibitor, VEGF vascular endothelial growth factor
The baseline demographics and clinical characteristics of the included patients are summarized in Table 1. The predominant histological subtype was adenocarcinoma, with most patients in Stage IV and a high prevalence of patients with a good performance status. A significant proportion of patients were never smokers. Mutations in addition to Del19 and L858R included G719X (n = 44), L861Q (n = 25), S768I (n = 8), exon 20 insertions (n = 5), exon 19 insertions (n = 1), G719X with S768I (n = 6), and G719X with E709A (n = 2).
Table 1.
Clinical characteristics of patients treated with osimertinib or non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitors as first-line treatment before propensity score matching adjustment
| Unmatched patients | ||||||
|---|---|---|---|---|---|---|
| Entire study period | Since March 2016 | |||||
| 1L-Osi (n = 416) | 1L-non-Osi (n = 646) | p value | 1L-Osi (n = 416) | 1L-non-Osi (n = 293) | p value | |
| Sex, n (%) | ||||||
| Male | 173 (41.6%) | 268 (41.5%) | 1.00 | 173 (41.6%) | 117 (39.9%) | 0.70 |
| Female | 243 (58.4%) | 378 (58.5%) | 243 (58.4%) | 176 (60.1%) | ||
| Age (years), median [range] | 74.0 [37.0–95.0] | 73.0 [39.0–97.0] | 0.12 | 74.0 [37.0–95.0] | 72.0 [39.0–93.0] | 0.08 |
| Smoking history (former and current), n (%) | ||||||
| Former or current | 161 (38.7%) | 195 (30.2%) | 0.005 | 161 (38.7%) | 91 (31.1%) | 0.04 |
| Never | 255 (61.3%) | 451 (69.8%) | 255 (61.3%) | 202 (68.9%) | ||
| Histological subtype of lung cancer, n (%) | ||||||
| Adenocarcinoma | 401 (96.4%) | 646 (100.0%) | <0.001 | 401 (96.4%) | 293 (100.0%) | 0.005 |
| Squamous cell carcinoma | 3 (0.7%) | 0 (0.0%) | 3 (0.7%) | 0 (0.0%) | ||
| NSCC, NOS | 7 (1.7%) | 0 (0.0%) | 7 (1.7%) | 0 (0.0%) | ||
| Others | 5 (1.2%) | 0 (0.0%) | 5 (1.2%) | 0 (0.0%) | ||
| Disease stage, n (%) | ||||||
| III | 22 (5.3%) | 52 (8.0%) | 0.21 | 22 (5.3%) | 24 (8.2%) | 0.19 |
| IV | 295 (70.9%) | 439 (68.0%) | 295 (70.9%) | 192 (65.5%) | ||
| Recurrent | 99 (23.8%) | 155 (24.0%) | 99 (23.8%) | 77 (26.3%) | ||
| EGFR mutationa, n (%) | ||||||
| Exon 19 deletion | 213 (51.2%) | 291 (45.0%) | 0.008 | 213 (51.2%) | 143 (48.8%) | 0.006 |
| Exon 21 L858R | 180 (43.3%) | 287 (44.4%) | 180 (43.3%) | 114 (38.9%) | ||
| Others | 23 (5.5%) | 68 (10.5%) | 23 (5.5%) | 36 (12.3%) | ||
| de novo T790M | 4 (1.0%) | 17 (2.6%) | 4 (1.0%) | 4 (1.4%) | ||
| ECOG performance status, n (%) | ||||||
| 0 | 136 (32.7%) | 168 (26.0%) | 0.21 | 136 (32.7%) | 91 (31.1%) | 0.99 |
| 1 | 193 (46.4%) | 326 (50.5%) | 193 (46.4%) | 137 (46.8%) | ||
| 2 | 45 (10.8%) | 72 (11.1%) | 45 (10.8%) | 34 (11.6%) | ||
| 3 | 29 (7.0%) | 55 (8.5%) | 29 (7.0%) | 21 (7.2%) | ||
| 4 | 13 (3.1%) | 25 (3.9%) | 13 (3.1%) | 10 (3.4%) | ||
| BMI, median (IQR) | 21.4 (19.4–23.7) | 21.4 (19.2–23.9) | 0.68 | 21.4 (19.4–23.7) | 21.5 (19.5–24.1) | 0.58 |
| Unknown/not documented | 8 | 16 | 8 | 10 | ||
1L-Osi first-line osimertinib, 1L-non-Osi first-line non-osimertinib, 2L/later-Osi second-line or later-line osimertinib, BMI body mass index, ECOG Eastern Cooperative Oncology Group, EGFR epidermal growth factor receptor, IQR interquartile range, NOS not otherwise specified, NSCC non-small cell carcinoma
aThe sample size pertains to the number of patients with EGFR mutations across three categories (Del19, L858R, and others). Patients with exclusive de novo T790M positivity were excluded from this study, and only those with concurrent T790M positivity and any other mutations were included
Applying PSM at a 1:1 ratio, the study cohort comprised an equal number of 399 patients each in the 1L-Osi and 1L-non-Osi groups, including 283 with initiation dates for first-line treatment after March 2016. After PSM, there were no significant differences in clinical characteristics between the groups (Table 2). The baseline demographics and clinical characteristics of patients in the 2L/later-Osi group are summarized in Table 3.
Table 2.
Clinical characteristics of patients treated with osimertinib or non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitors as first-line treatment, aligned through propensity score matching
| Propensity score-matched patients | ||||||
|---|---|---|---|---|---|---|
| Entire study period | Since March 2016 | |||||
| 1L-Osi (n = 399) | 1L-non-Osi (n = 399) | p value | 1L-Osi (n = 283) | 1L-non-Osi (n = 283) | p value | |
| Sex, n (%) | ||||||
| Male | 164 (41.1%) | 174 (43.6%) | 0.52 | 115 (40.6%) | 113 (39.9%) | 0.93 |
| Female | 235 (58.9%) | 225 (56.4%) | 168 (59.4%) | 170 (60.1%) | ||
| Age (years), median (range) | 72.0 [37.0–95.0] | 73.0 [39.0–97.0] | 0.34 | 73.0 [37.0–95.0] | 73.0 [39.0–93.0] | 0.94 |
| Smoking history (former and current), n (%) | ||||||
| Former or current | 155 (38.8%) | 161 (40.4%) | 0.71 | 95 (33.6%) | 91 (32.2%) | 0.79 |
| Never | 244 (61.2%) | 238 (59.6%) | 188 (66.4%) | 192 (67.8%) | ||
| Histological subtype of lung cancer, n (%) | ||||||
| Adenocarcinoma | 399 (100.0%) | 399 (100.0%) | NA | 283 (100.0%) | 283 (100.0%) | NA |
| Squamous cell carcinoma | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| NSCC, NOS | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Others | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Disease stage, n (%) | ||||||
| III | 21 (5.3%) | 25 (5.0%) | 0.85 | 12 (4.2%) | 23 (8.1%) | 0.13 |
| IV | 279 (69.9%) | 276 (61.9%) | 199 (70.3%) | 185 (65.4%) | ||
| Recurrent | 99 (24.8%) | 98 (33.1%) | 72 (25.4%) | 75 (26.5%) | ||
| EGFR mutation, n (%) | ||||||
| Exon 19 deletion | 204 (51.1%) | 218 (54.6%) | 0.61 | 129 (45.6%) | 143 (50.5%) | 0.06 |
| Exon 21 L858R | 172 (43.1%) | 160 (40.1%) | 135 (47.7%) | 110 (38.9%) | ||
| Others | 23 (5.8%) | 21 (5.3%) | 19 (6.7%) | 30 (10.6%) | ||
| ECOG performance status, n (%) | ||||||
| 0 | 131 (32.8%) | 118 (29.6%) | 0.70 | 89 (31.4%) | 89 (31.4%) | 0.93 |
| 1 | 185 (46.4%) | 197 (49.4%) | 138 (48.8%) | 133 (47.0%) | ||
| 2 | 41 (10.3%) | 36 (9.0%) | 28 (9.9%) | 31 (11.0%) | ||
| 3 | 29 (7.3%) | 36 (9.0%) | 17 (6.0%) | 21 (7.4%) | ||
| 4 | 13 (3.3%) | 12 (3.0%) | 11 (3.9%) | 9 (3.2%) | ||
1L-non-Osi first-line non-osimertinib, 1L-Osi first-line osimertinib, 2L/later-Osi second-line or later-line osimertinib, ECOG Eastern Cooperative Oncology Group, EGFR epidermal growth factor receptor, NA not available, NOS not otherwise specified, NSCC non-small cell carcinoma
Table 3.
Clinical characteristics of patients treated with osimertinib as a second-line or later-line treatment following first-line treatment with non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitors
| 2L/later-Osi | ||
|---|---|---|
| Entire study period (n = 139) | Since March 2016 (n = 75) | |
| Sex, n (%) | ||
| Male | 49 (35.3%) | 29 (38.7%) |
| Female | 90 (64.7%) | 46 (61.3%) |
| Age (years), median (range) | 70.0 (39.0–90.0) | 72.0 (41.0–86.0) |
| Smoking history (former and current), n (%) | ||
| Former or current | 48 (34.5%) | 28 (37.3%) |
| Never | 91 (65.5%) | 47 (62.7%) |
| Histological subtype of lung cancer, n (%) | ||
| Adenocarcinoma | 139 (100.0%) | 75 (100.0%) |
| Squamous cell carcinoma | 0 (0.0%) | 0 (0.0%) |
| NSCC, NOS | 0 (0.0%) | 0 (0.0%) |
| Others | 0 (0.0%) | 0 (0.0%) |
| Disease stage, n (%) | ||
| III | 7 (5.0%) | 3 (4.0%) |
| IV | 86 (61.9%) | 49 (65.3%) |
| Recurrent | 46 (33.1%) | 23 (30.7%) |
| EGFR mutationa, n (%) | ||
| Exon 19 deletion | 84 (60.4%) | 46 (61.3%) |
| Exon 21 L858R | 50 (36.0%) | 25 (33.3%) |
| Others | 5 (3.6%) | 4 (5.3%) |
| de novo T790M | 3 (2.2%) | 2 (2.7%) |
| ECOG performance status, n (%) | ||
| 0 | 47 (33.8%) | 29 (38.7%) |
| 1 | 72 (51.8%) | 35 (46.7%) |
| 2 | 11 (7.9%) | 6 (8.0%) |
| 3 | 7 (5.0%) | 4 (5.3%) |
| 4 | 2 (1.4%) | 1 (1.3%) |
| BMI, median (IQR) | 20.9 (19.2–23.5) | 20.9 (18.8–23.9) |
| Unknown/not documented | 3 | 3 |
1L-non-Osi first-line non-osimertinib, 1L-Osi first-line Osimertinib, 2L/later-Osi second-line or later-line osimertinib, BMI body mass index, ECOG Eastern Cooperative Oncology Group, EGFR epidermal growth factor receptor, IQR interquartile range, NOS not otherwise specified, NSCC non-small cell carcinoma
aThe sample size pertains to the number of patients with EGFR mutations across three categories (Del19, L858R, and others). Patients with exclusive de novo T790M positivity were excluded from this study, and only those with concurrent T790M positivity and any other mutations were included
Survival Analysis
The median follow-up period for OS was 16.2 (IQR, 6.2–27.0), 20.3 (IQR 7.9–40.2), and 44.6 months (IQR 25.5–60.3) for the 416 patients in the 1L-Osi, 646 in the 1L-non-Osi, and 139 in the 2L/later-Osi groups, respectively. At the data cutoff point, the number of deaths in each group was as follows: 114 (27.4%) in the 1L-Osi group, 410 (63.5%) in the 1L-non-Osi group, and 84 (60.4%) in the 2L/later-Osi group.
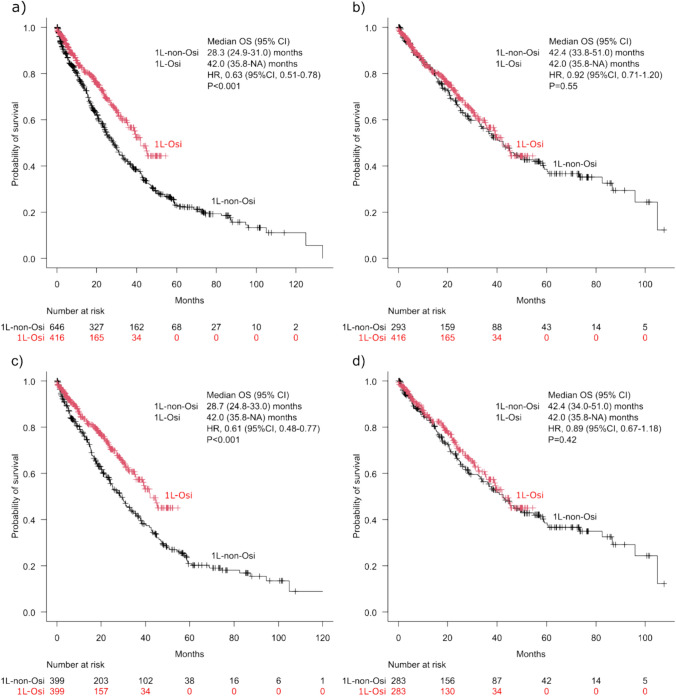
The 1L-Osi group demonstrated an extended median OS of 42.0 months (95% CI 35.8 to not available [NA]), which significantly exceeded the median OS of 28.3 months (95% CI 24.9–31.0) in the 1L-non-Osi group (HR 0.63; 95% CI 0.51–0.78, p < 0.001) (Fig. 2a). However, in the cohort of patients for whom first-line EGFR-TKI treatment was initiated after March 2016, there were no significant differences in OS between the 1L-Osi (42.0 months: 95% CI 35.8 to NA) and 1L-non-Osi (42.4 months: 95% CI 33.8–51.0) groups when comparing all patients (HR 0.92; 95% CI 0.71–1.20, p = 0.55) (Fig. 2b). These trends for the 1L-Osi and 1L-non-Osi groups remained in the PSM cohort, where the median OS reached 42.0 months (95% CI 35.8 to NA) and 28.7 months (95% CI 24.8–33.0), respectively (HR 0.61; 95% CI 0.48–0.77, p < 0.001) (Fig. 2c), and the median OS reached 42.0 months (95% CI 35.8 to NA) and 42.4 months (95% CI 34.0–51.0) in the post-March 2016 cohort, respectively, with an HR of 0.89 (95% CI 0.67–1.18, p = 0.42) (Fig. 2d).
Fig. 2.
Kaplan–Meier curves for overall survival (OS) in the first-line treatment with osimertinib (1L-Osi) and first-line treatment with non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitors (1L-non-Osi) groups: a throughout the study period, b after March 2016, c throughout the study period using propensity score matching, and d after March 2016 using propensity score matching. CI confidence interval, HR hazard ratio, NA not available
The median OS of the 2L/later-Osi group over the entire study period was 54.1 months (95% CI 47.0–60.2), and that for the cohort of patients who initiated first-line EGFR-TKI treatment after March 2016 was 60.2 months (95% CI 49.9–95.9) (Fig. 3) Conversely, for the 1L-non-Osi group without sequential osimertinib over the entire study period (n = 507), the median OS was 22.3 months (95% CI 19.7–25.0). For this group after March 2016 (n = 218), the median OS was 32.2 months (95% CI 24.0–40.0).
Fig. 3.
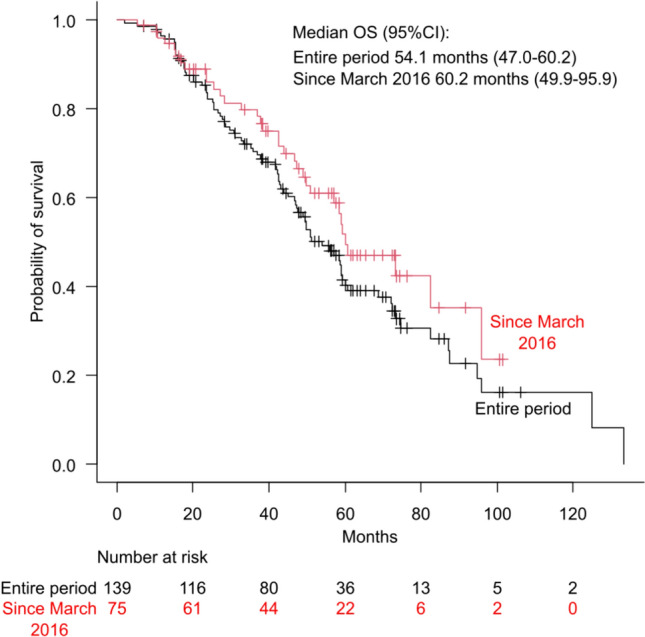
Kaplan–Meier curves for overall survival (OS) in the second-line/later-osimertinib (following first-line treatment with non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitors) group. The black line represents data from the entire study period, whereas the red line represents data from patients who received first-line treatment after March 2016. CI confidence interval
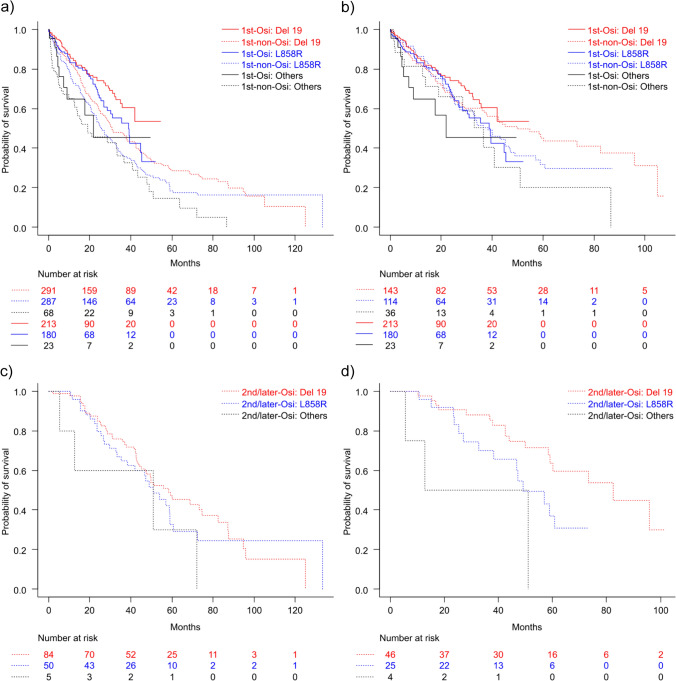
Subgroup analysis of OS was conducted based on three EGFR mutations: Del19, L858R, and other EGFR mutations. Throughout the study period, for patients with Del19, the 1L-Osi group showed better OS outcomes (median OS, NA; 95% CI 35.8 to NA) than the 1L-non-Osi group (median OS, 30.9 months; 95% CI 27.5–38.4; HR 0.61; 95% CI 0.45–0.84; p = 0.002). Similarly, for patients with L858R, the 1L-Osi group had longer OS (median OS, 38.8 months; 95% CI 27.0–45.3) than the 1L-non-Osi group (median OS, 25.8 months; 95% CI 22.3–30.8; HR 0.67; 95% CI 0.49–0.91; p = 0.01) (Fig. 4a). No significant differences were observed for other mutations (1L-Osi: median OS, 22.0 months; 95% CI 7.3 to NA vs 1L-non-Osi: median OS, 19.0 months; 95% CI 12.3–36.7; HR 0.73; 95% CI 0.35–1.51, p = 0.39) (Fig. 4a).
Fig. 4.
Kaplan–Meier curves for overall survival by epidermal growth factor receptor mutation type. a First-line treatment with osimertinib (1L-Osi) and first-line treatment with non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitor (1L-non-Osi) groups during the entire study period, b 1L-Osi and 1L-non-Osi groups after March 2016, c 2L/later-Osi (osimertinib in second-line or later-line treatment following first-line treatment with non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitors) group during the entire study period, and d 2L/later-Osi group after March 2016
In the cohort where initial treatment was initiated after March 2016, for Del19, there were no significant differences in OS between the 1L-Osi (median OS, NA; 95% CI 35.8 months to NA) and 1L-non-Osi (median OS, 49.9 months; 95% CI 36.1–82.5 months) [HR 0.83; 95% CI 0.56–1.21; p = 0.33] groups of patients. Similarly, for L858R, there were no significant differences in OS between the 1L-Osi (median OS, 38.8 months; 95% CI 27.0–45.3) and 1L-non-Osi (median OS, 38.4 months; 95% CI 27.1–47.3) (HR 1.04; 95% CI 0.70–1.54; p = 0.85) groups. For other mutations, there were no significant differences in OS between the 1L-Osi (median OS, 22.0 months; 95% CI 7.33 to NA) and 1L-non-Osi (median OS, 36.7 months; 95% CI 13.7–51.0) (HR 1.25; 95% CI 0.53–2.94, p = 0.61) groups (Fig. 4b). In the entire 2L/later-Osi group, the median OS was 58.4 months (95% CI 45.1–74.5), 50.9 months (95% CI 35.3–59.0), and 51.0 months (95% CI 5.5 to NA) for Del19, L858R, and other mutations, respectively, with no significant differences among the groups (Fig. 4c). A comparison of the L858R mutation with the Del19 mutation yielded an HR of 1.22 (95% CI 0.77–1.94, p = 0.39), and a comparison of other mutations with the Del19 mutation yielded an HR of 1.55 (95% CI 0.92–2.59, p = 0.10). Additionally, a comparison of other mutations with the L858R mutation yielded an HR of 1.79 (95% CI 0.63–5.12, p = 0.28). However, in the subset of patients where initial treatment was initiated after March 2016 in the 2L/later-Osi group, the median OS was 82.5 months (95% CI 58.4 to NA), for 49.1 months (95% CI 32.8 to NA), and 31.9 months (95% CI 5.5 to NA) for Del19, L858R, and other mutations, respectively (Fig. 4d). In the subset, there were significant differences between the L858R and Del19 groups, with an HR of 2.13 (95% CI 1.01–4.48, p = 0.046) and between the other mutation and Del19 groups, with an HR of 2.47 (95% CI 1.29–4.70, p = 0.006). However, the difference between the other mutation and L858R groups was not significant (HR 3.28; 95% CI 0.90–11.94; p = 0.07). In the population that began treatment after March 2016, a multivariate analysis identified several significant factors associated with OS. These factors include EGFR mutation type (HR 1.36, 95% CI 1.11–1.66, p = 0.003), histological type (HR 1.46, 95% CI 1.06–2.00, p = 0.02), ECOG PS (HR 1.39, 95% CI 1.22–1.57, p < 0.001), and disease stage (HR 1.76, 95% CI 1.37–2.26, p < 0.001). Conversely, the use of osimertinib as a first-line treatment was not a significant predictor of OS (HR 0.86, 95% CI 0.66–1.12, p = 0.27).
In the 1L-non-Osi group, in which the initial treatment was initiated after March 2016, a subgroup analysis of OS was conducted based on the EGFR-TKI used as first-line treatment. The median OS of patients initially treated with gefitinib, erlotinib, and afatinib was 40.2 months (95% CI 28.5–57.1), 33.1 (95% CI 23.6–45.2), and 46.8 months (95% CI 32.2 months to NA), respectively (Fig. 5). The differences among the three groups were not significant (p = 0.33). In the subgroup of patients who began their treatment with agents other than osimertinib after March 2016, a multivariate analysis identified several significant factors associated with OS. EGFR mutation type (HR 1.35, 95% CI 1.04–1.75, p = 0.03), ECOG PS (HR 1.28, 95% CI 1.07–1.53, p = 0.007), and disease stage (HR 1.71, 95% CI 1.26–2.32, p < 0.001) were significant predictors. Conversely, the type of EGFR-TKI used as first-line treatment was not a significant predictor (HR 0.93, 95% CI 0.76–1.14, p = 0.49).
Fig. 5.
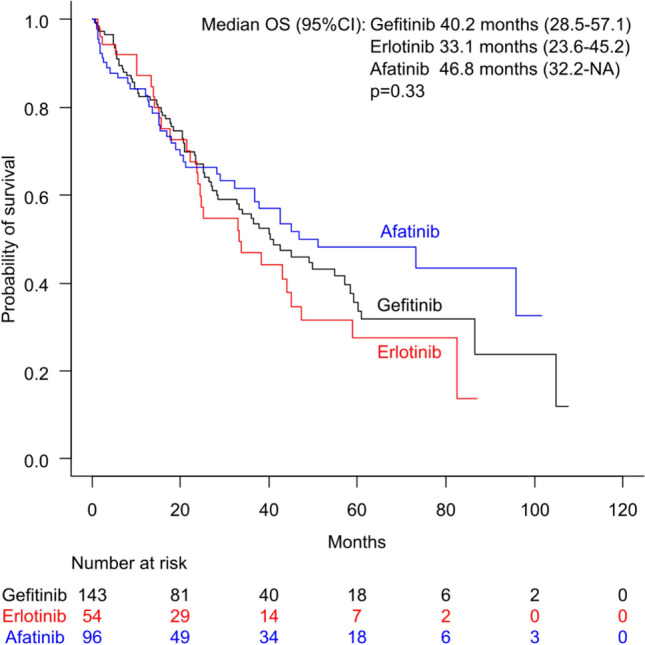
Kaplan–Meier curves for overall survival (OS) based on the epidermal growth factor receptor-tyrosine kinase inhibitors used in first-line therapy among patients treated with non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitors as their initial treatment (treatment initiated after March 2016). CI confidence interval, NA not available
In the 2L/later-Osi group, a subgroup analysis of OS was conducted based on the EGFR-TKI used as a first-line treatment. The median OS for patients initially treated with gefitinib, erlotinib, and afatinib was 54.1 months (95% CI 43.2–59.2), 41.8 months (95% CI 25.2–82.5), and 73.3 months (95% CI 46.8 to NA), respectively (Fig. 6). The differences among the three groups were not significant (p = 0.21). In this cohort, multivariate analysis identified the disease stage as a significant factor associated with OS (HR 1.68, 95% CI 1.06–2.65, p = 0.03). Other factors, including the type of first-line treatment used (HR 0.79, 95% CI 0.61–1.03, p = 0.08), were not significant predictors.
Fig. 6.
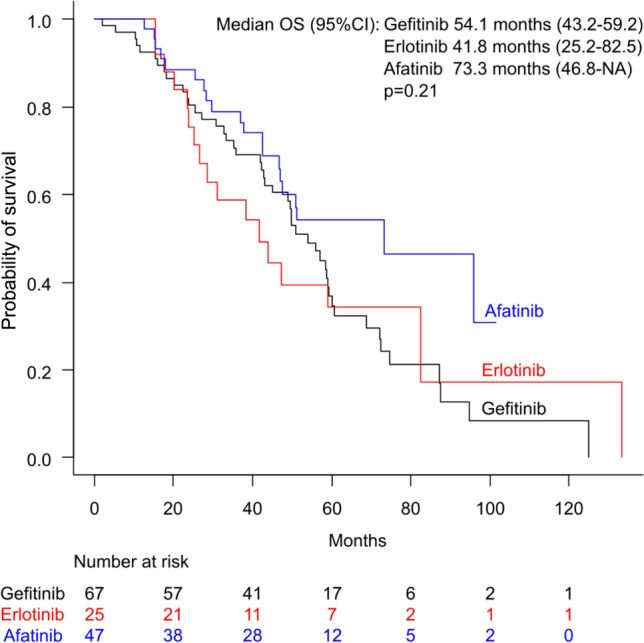
Kaplan–Meier curves for overall survival (OS) based on the epidermal growth factor receptor-tyrosine kinase inhibitors used in first-line therapy among patients treated with osimertinib as second-line or later-line treatment following first-line treatment with non-osimertinib epidermal growth factor receptor-tyrosine kinase inhibitors. CI confidence interval, NA not available
Rationale Behind Transitioning Treatment to Osimertinib
The reasons for changing the treatment regimen to osimertinib in the 2L/later-Osi group (osimertinib as second-line or later-line treatment following first-line treatment with non-osimertinib EGFR-TKIs) were the following: disease progression in 111 cases (79.9%), of which 91 (65.5%) had T790M confirmed through rebiopsy before initiating second-line or later-line osimertinib treatment, 17 (12.2%) had unconfirmed T790M, and 3 (2.2%) were de novo T790M-positive cases; adverse events during first-line treatment in 25 cases (18.0%); and availability of osimertinib, recently launched in Japan, during the effectiveness of first-line treatment in three cases (2.2%).
Discussion
In this study, in the cohort where first-line treatment was initiated after March 2016, when osimertinib became available as a second-line treatment, there were no significant differences in OS between the groups using osimertinib and other EGFR-TKIs as first-line treatments. Furthermore, the group receiving osimertinib as a second-line or later-line treatment showed extended OS, emphasizing the extended survival observed in this cohort. The subgroup analysis based on EGFR gene mutations, specifically Del19 and L858R, indicated similar trends irrespective of the genetic mutation, in line with the aforementioned results.
The primary reason for the improved OS in the group using other EGFR-TKIs as first-line treatment after March 2016 compared with that during the entire study period is the enhanced adoption rate of osimertinib as the second-line or later-line treatment. In the group using an EGFR-TKI other than osimertinib as first-line treatment, many individuals could not receive osimertinib as second-line or later-line treatment, regardless of T790M expression, owing to timing constraints. Therefore, the cohort during the study period differs from that in the current environment where osimertinib is available as a second-line or later-line treatment. However, the period since March 2016 has been similar, as osimertinib has become available as a second-line or later-line treatment. The most significant finding is that after osimertinib became available as a second-line or later-line treatment, there were no differences in OS between the group receiving osimertinib and that receiving non-osimertinib drugs as a first-line treatment.
In contrast, a large phase III trial strongly endorsed first-line treatment with osimertinib, as recommended in the Japanese lung cancer treatment and international guidelines [2–4]. However, despite consistent results across various subgroups in the trial, the efficacy of first-line treatment with osimertinib in terms of survival was not superior in Asian populations, particularly the Japanese population [6–8]. Moreover, a single-institution retrospective study of 117 cases within an American–Asian population with Stage IV NSCLC treated with osimertinib demonstrated no significant differences in 2-year OS between the groups treated with first-line osimertinib and earlier-generation EGFR-TKI before osimertinib [16]. This multi-center RWD-based study involving PSM reaffirmed that first-line osimertinib treatment does not necessarily demonstrate absolute superiority in Japanese (Asian) individuals.
The advantage of using non-osimertinib EGFR-TKIs as first-line treatment lies in the sequential treatment with EGFR-TKIs, particularly the introduction of osimertinib in later-line settings [17]. There is a concern that the optimal treatment sequence would involve the initial use of first-generation or second-generation EGFR-TKIs, followed by osimertinib as second-line or later-line treatment, particularly in cases where T790M is detected in rebiopsy [17]. In fact, a retrospective single-center study with a limited number of Japanese patients with NSCLC reported that upfront use of first/second-generation EGFR-TKIs followed by osimertinib (n = 17) resulted in better OS than upfront osimertinib therapy alone (n = 57) [18], consistent with the extended OS observed in the group receiving osimertinib as a second-line or later-line treatment in the present study, which involved a larger participant pool.
If the optimal sequence is followed, osimertinib will not be indicated as a second-line treatment if the patient has progressive disease and T790M is negative after first-line treatment with a first-generation or second-generation EGFR-TKI [17]. Additionally, the indication for secondary therapy may be lost if performance status drops rapidly during primary therapy [17]. This phenomenon results in a group of patients who do not benefit from osimertinib. Indeed, a previous phase III study has demonstrated that the introduction rate for osimertinib as a second-line or later-line treatment is 24% in the Japanese population and 30.7% in all participants [6, 7]. In this RWD study, the introduction rate of osimertinib was 25.6% in the group that initiated treatment after March 2016, which was comparable to previously reported rates [6, 7]. However, under the conditions reported in this study, our findings indicate that using non-osimertinib EGFR-TKIs as initial treatment did not result in a lower OS than that of the group using osimertinib as a first-line treatment, suggesting a deviation from the one-sided perspective that osimertinib is the mainstay of first-line treatment. Furthermore, in the subgroup analysis of the cohort in which treatment was initiated with EGFR-TKIs other than osimertinib and the cohort where osimertinib was introduced as a second-line or later-line treatment, the results suggest that gefitinib, erlotinib, or afatinib used as a first-line treatment are viable options. In addition to monotherapy with first-generation or second-generation EGFR-TKIs, combination therapies with angiogenesis inhibitors or cytotoxic anticancer agents are feasible, and their use remains an option as a first-line treatment.
In the genetic subgroup analysis of the present study, osimertinib did not demonstrate superiority for L858R or Del19 mutations. In contrast, in the subgroup analysis of a previous trial, the superiority of osimertinib was not observed among Asian populations or for the L858R mutation, but was evident for the Del19 mutation [6]. In Asian patients with EGFR-mutated NSCLC residing in the USA, the 2-year OS was reportedly reduced in the group receiving first-line treatment with osimertinib for patients with Del19 compared with that in the group receiving first-line treatment with first-generation or second-generation EGFR-TKIs [14]. These results imply that investigations on the effect of first-line treatment with osimertinib on OS should consider differences in race besides known genetic mutations. The reason for the lack of observed superiority in OS with osimertinib as a first-line treatment in the Asian population remains unclear; however, it is speculated to be attributable to differences in the effectiveness of the comparator EGFR-TKI. In the Japanese subset of a previous trial, there were no significant differences in OS between the osimertinib and comparator EGFR-TKI groups [7, 8]. However, the 3-year OS of the osimertinib group was nearly identical for the Japanese (59%) and overall (54%) populations [7, 8]. Conversely, in the comparator EGFR-TKI group, the 3-year OS rate in the Japanese population was 63%, compared with 44% in the overall population [7, 8]. Moreover, in this RWD study, the introduction rate of osimertinib as a second-line treatment or beyond was comparable to previously reported rates [6, 7]. These findings suggest that Japanese (Asian) individuals might derive greater benefits from the comparator EGFR-TKI than the overall population. Other factors to consider include differences in adverse-effect profiles and drug approval statuses. In Japanese patients, comparator EGFR-TKIs have been linked to lower interstitial pneumonia rates and fewer treatment discontinuations than those linked to osimertinib [19]. Furthermore, EGFR-TKI retreatment is reimbursed in Japan, potentially prolonging the exposure to various EGFR-TKIs in patients initially treated with comparator EGFR-TKIs. These factors may contribute to the OS advantage associated with comparator EGFR-TKIs [20].
The efficacy of EGFR-TKIs in patients with EGFR uncommon mutations is variable and generally lower than that in those with common mutations, such as Del19 and L858R—a trend also observed in our study [21, 22]. According to the latest National Comprehensive Cancer Network guidelines [2], the recommended first-line therapy for patients with EGFR Exon 20 insertion mutations includes a combination of amivantamab-vmjw, carboplatin, and pemetrexed [23]. For other uncommon EGFR mutations, afatinib or osimertinib is recommended. Given the notable limitations of current treatments, there is a pressing need for the development of new targeted therapies and innovative strategies to improve outcomes for this critical patient population.
Our study has some limitations. First, the broad patient enrollment period may have caused variations in the accessibility to immune checkpoint inhibitor (ICI) therapy, potentially influencing OS. The cohort receiving first-line osimertinib treatment had more favorable access to ICI therapy, whereas cases of first-line therapies other than osimertinib usually involved instances before ICI approval, potentially hampering access to ICI treatments. Additionally, the subgroup that received osimertinib in the second-line or later-line treatment usually underwent a rebiopsy for T790M confirmation. This suggests a population with a high likelihood of good performance status after pre-EGFR-TKI treatment and the potential for inherently longer survival. Furthermore, although we conducted PSM at the initiation of the first-line treatment, the inherent characteristics of this subgroup could influence the results, potentially leading to bias. Detailed information regarding programmed death-ligand 1 expression within tumor tissues and the specifics of distant metastasis before treatment initiation is not structurally represented in the electronic data. Hence, these factors could not be included in the PSM analysis. Similarly, despite efforts to align various backgrounds through PSM, complete homogeneity was not achieved. In our subgroup analyses, the relatively small sample size limited our ability to adjust for background factors. Consequently, potential confounding factors may not have been fully accounted for, possibly influencing the interpretation of the results. Finally, the primary focus was the hard endpoint, OS. However, factors such as economic toxicity, detailed adverse events, and changes in quality of life have not been considered owing to concerns related to data extraction.
Conclusions
This large-scale RWD study indicated that osimertinib, in the initial treatment of EGFR-mutated advanced NSCLC in the Japanese (Asian) population, did not show better efficacy in terms of OS than other EGFR-TKIs. Furthermore, the use of osimertinib in second-line or later-line treatments after the initial EGFR-TKI therapy resulted in notably longer OS. With the current possibility to conduct liquid biopsies for confirming T790M mutations, reserving osimertinib as a sequential treatment option for the Asian population, specifically the Japanese population, is a valuable strategy. Further investigation is essential to optimize the first-line treatment in patients with EGFR-mutated NSCLC.
Acknowledgements
We extend our sincere gratitude to the patients, their families, and caregivers for their participation in this study. We also thank all physicians responsible for comprehensive patient care. We thank Yoshiaki Fujimura and Megumi Shiragami from the Tokushukai Information System for their collaborative efforts in extracting data from the electronic medical record system. Additionally, we extend our gratitude to Maki Hayashi of Mirai Iryo Research Center Inc. for her valuable support at our research office. We acknowledge Editage (www.editage.jp) for the meticulous English language editing, which enhanced the clarity and coherence of the manuscript.
Declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Makoto Hibino received research funding from and engaged in collaborative research with Shionogi & Co., Ltd. outside the submitted work. Tomoya Fukui received speakers’ bureau fees/honoraria from AstraZeneca, Boehringer Ingelheim, Chugai Pharm, Eli Lilly, Nippon Kayaku, Novartis Pharma, Ono Pharm, Pfizer, and Taiho Pharm outside the submitted work. Imamura received speakers’ bureau fees/honoraria from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Pfizer, and Ono Pharm outside the submitted work. Minami received speakers’ bureau fees/honoraria from Daiichi-Sankyo and Ono Pharm; research funding from Astelas-Amgen Biopharma, Bayer, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Incite, Novartis, Ono Pharm, Pfizer, and Rakuten Medical; and scholarship donations from Bayer, Chugai, Daiichi-Sankyo, Eisai, Kyowa-Kirin, Lilly, Ono Pharmaceutical, Pfizer, Taiho Pharma, and Takeda outside the submitted work. These organizations had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. Rai Shimoyama, Ryuta Fukai, Akihiko Iwase, Yukihiro Tamura, Yusuke Chihara, Takafumi Okabe, Uryu Kiyoaki, Tadahisa Okuda, and Masataka Taguri have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study was conducted following the principles of the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Tokushukai Group on 2 March, 2023 (approval number: TGE 01427-008). This study was registered at the University Hospital Medical Information Network Clinical Trials Registry on 9 March, 2023 (identification UMIN000050552).
Consent to participate
The requirement for obtaining informed consent from patients was waived because this was a retrospective analysis of anonymized patient data. Patients were allowed to opt out of the research use of their data, and related information is publicly available on the Tokushukai Medical Group website.
Consent for publication
Not applicable.
Availability of data and material
The data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors’ contributions
Conceptualization, data curation, formal analysis, investigation, methodology, resources, writing (original draft): MH. Resources, writing (review and editing): KU. Resources, writing (review and editing): RS. Resources, writing (review and editing): TF. Resources, writing (review and editing): RF. Resources, writing (review and editing): AI. Resources, writing (review and editing): YT. Resources, writing (review and editing): YC. Resources, writing (review and editing): MF. Formal analysis: TO. Formal analysis: MT. Writing (review and editing): YI. Writing (review and editing): HM.
References
- 1.Shi Y, Au JS-K, Thongprasert S, Srinivasan S, Tsai C-M, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154–62. 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, et al. Non-small cell lung cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024;22:249–74. 10.6004/jnccn.2204.0023. [DOI] [PubMed] [Google Scholar]
- 3.Akamatsu H, Ninomiya K, Kenmotsu H, Morise M, Daga H, Goto Y, et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731–70. 10.1007/s10147-019-01431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, FLAURA Investigators, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25. 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 5.Uryu K, Imamura Y, Shimoyama R, Mase T, Fujimura Y, Hayashi M, et al. Stepwise prolongation of overall survival from first to third generation EGFR-TKIs for EGFR mutation-positive non-small-cell lung cancer: the Tokushukai REAl-world Data project (TREAD 01). Jpn J Clin Oncol. 2024;54:319–28. 10.1093/jjco/hyad162. [DOI] [PubMed] [Google Scholar]
- 6.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, FLAURA Investigators, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 7.Nogami N, Ramalingam SS, Imamura F, Okamoto I, Kurata T, Kato T, et al. Osimertinib as first-line therapy for EGFRm advanced NSCLC (FLAURA): final OS in Japanese subset. 60th Annual Meeting of the Japan Lung Cancer Society, Presidential Symposium; 6-8 December, 2019; Osaka.
- 8.Tsukita Y, Inoue A. First-line therapy in non-small cell lung cancer patients with EGFR activating mutations: a consideration of the clinical position of osimertinib based on the subset of Japanese patients in the FLAURA study. Jpn J Clin Oncol. 2022;52:405–10. 10.1093/jjco/hyac012. [DOI] [PubMed] [Google Scholar]
- 9.Shimoyama R, Imamura Y, Uryu K, Mase T, Fujimura Y, Hayashi M, et al. Real-world outcomes of systemic therapy in Japanese patients with cancer (Tokushukai REAl-World Data project: TREAD): study protocol for a nationwide cohort study. Healthcare (Basel). 2022;10:2146. 10.3390/healthcare10112146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10): e1001885. 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, International Association for the Study of Lung Cancer International Staging Committee, Participating Institutions, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 13.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Japan Pharmaceuticals and Medical Devices Agency (PMDA). Tagrisso: package insert. https://www.info.pmda.go.jp/go/pack/4291045F1027_1_12/. Accessed 14 Mar 2024.
- 15.Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, AURA3 Investigators, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40. 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Bi J, Moreira A, Chachoua A, Velcheti V, Lau SCM, et al. Real-world clinical outcomes in a US Asian population with stage IV NSCLC treated with osimertinib (Osi) stratified by EGFR subtype. J Clin Oncol. 2023;41:e21128. 10.1200/JCO.2023.41.16_suppl.e211. [Google Scholar]
- 17.Haratake N, Misumi T, Yamanaka T, Seto T. Optimizing sequential treatment with EGFR tyrosine kinase inhibitor with a simulation of the T790M mutation rate in EGFR-mutated lung cancer. JTO Clin Res Rep. 2020;1: 100085. 10.1016/j.jtocrr.2020.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori T, Yamamoto K, Ito T, Ikushima S, Omura T, Yano I. Upfront use of first-/second-generation EGFR-TKI followed by osimertinib shows better prognosis than upfront osimertinib therapy in Japanese patients with non-small-cell lung cancer with exon 19 deletion: a single-center retrospective study. Biol Pharm Bull. 2023;46:788–95. 10.1248/bpb.b22-00794. [DOI] [PubMed] [Google Scholar]
- 19.Ohe Y, Imamura F, Nogami N, Okamoto I, Kurata T, Kato T, et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49:29–36. 10.1093/jjco/hyy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michelon I, Vilbert M, Castro C, Stecca C, Dacoregio MI, Rizzo MM, et al. Retreatment with EGFR inhibitor in non-small cell lung cancer patients previously exposed to EGFR-TKI: a systematic review and meta-analysis. J Clin Oncol. 2023;41: e21159. 10.1200/JCO.2023.41.16_suppl.e21159. [Google Scholar]
- 21.Friedlaender A, Subbiah V, Russo A, Banna GL, Malapelle U, Rolfo C, et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat Rev Clin Oncol. 2022;19:51–69. 10.1038/s41571-021-00558-1. [DOI] [PubMed] [Google Scholar]
- 22.Borgeaud M, Parikh K, Banna GL, Kim F, Olivier T, Le X, et al. Unveiling the landscape of uncommon EGFR mutations in NSCLC: a systematic review. J Thorac Oncol. 2024;19:973–83. 10.1016/j.jtho.2024.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhou C, Tang KJ, Cho BC, Liu B, Paz-Ares L, Cheng S, PAPILLON Investigators, et al. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N Engl J Med. 2023;389:2039–51. 10.1056/NEJMoa2306441. [DOI] [PubMed] [Google Scholar]