Abstract
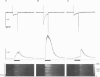
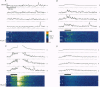
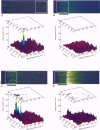
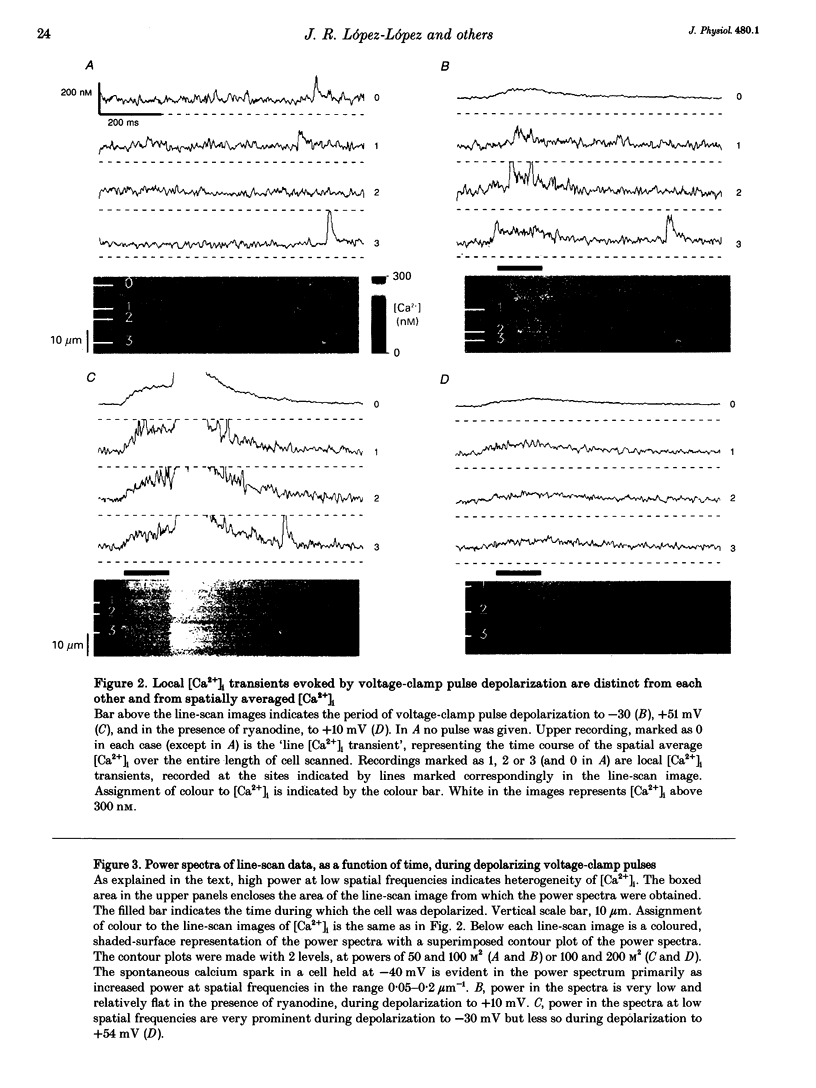
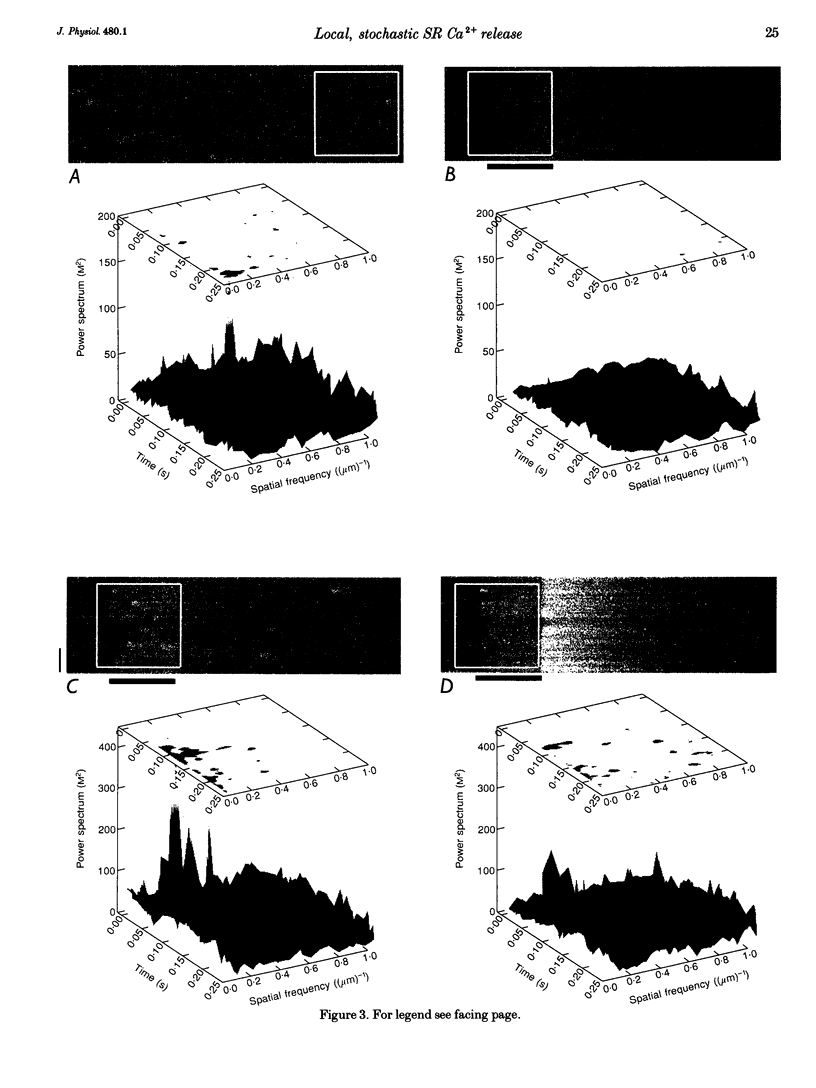
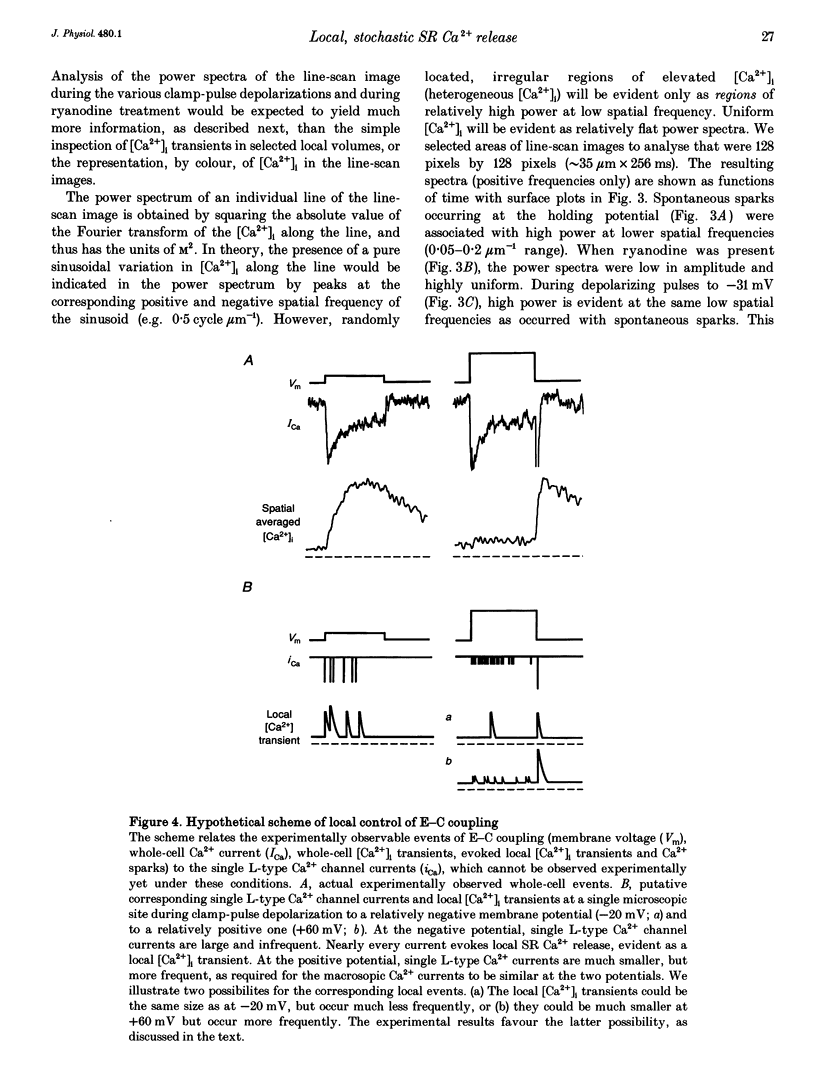
1. Confocal microscopy and the fluorescent Ca2+ indicator fluo-3 (K+ salt) were used to measure cytosolic free calcium ion concentration ([Ca2+]) during excitation-contraction (E-C) coupling in single, voltage-clamped, rat cardiac ventricular cells. 2. Local [Ca2+]i transients were measured nearly simultaneously in different, separate, subcellular volumes of approximately 2.0 microns 3. During depolarization, local [Ca2+]i transients were distinctly different from each other and from whole-cell [Ca2+]i transients. These differences were particularly apparent during small depolarizations, and were substantially reduced by ryanodine. 3. Components of the local [Ca2+]i transients, particularly those evoked by small depolarizations, were closely similar, in time course and amplitude, to spontaneous local [Ca2+]i transients, or 'sparks' (which have been shown previously to be Ca2+ released from sarcoplasmic reticulum). 4. Analysis of local [Ca2+]i transients in the spatial frequency domain (power spectrum) revealed that high power at spatial frequencies of 0.05-0.2 microns-1 was always associated with spontaneous calcium 'sparks' and with local [Ca2+]i transients evoked by small depolarizing pulses (e.g. to -31 mV). Evoked local [Ca2+]o transients in the presence of ryanodine, and those evoked by depolarization to very positive clamp-pulse potentials (+45 mV), were associated with considerably lower power at this frequency. 5. The results suggest that whole-cell [Ca2+]i transients evoked by voltage-clamp depolarization, and thus by L-type Ca2+ current, are comprised of local [Ca2+]i transients that are similar to the spontaneous calcium 'sparks'. At very positive clamp-pulse potentials, however, the electrically evoked local [Ca2+]i transients may be smaller, perhaps as a result of smaller unitary L-type Ca2+ current.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke C. W., Egan T. M., Wier W. G. Processes that remove calcium from the cytoplasm during excitation-contraction coupling in intact rat heart cells. J Physiol. 1994 Feb 1;474(3):447–462. doi: 10.1113/jphysiol.1994.sp020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke C. W., Wier W. G. Modulation of L-type calcium channels by sodium ions. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4417–4421. doi: 10.1073/pnas.89.10.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Györke S., Palade P. Role of local Ca2+ domains in activation of Ca(2+)-induced Ca2+ release in crayfish muscle fibers. Am J Physiol. 1993 Jun;264(6 Pt 1):C1505–C1512. doi: 10.1152/ajpcell.1993.264.6.C1505. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Microscopic spiral waves reveal positive feedback in subcellular calcium signaling. Biophys J. 1993 Dec;65(6):2272–2276. doi: 10.1016/S0006-3495(93)81316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993 May;14(5):359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J Physiol. 1994 Feb 1;474(3):439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Ryanodine prolongs Ca-currents while suppressing contraction in rat ventricular muscle cells. Br J Pharmacol. 1984 Jan;81(1):13–15. doi: 10.1111/j.1476-5381.1984.tb10735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Real-time confocal microscopy and calcium measurements in heart muscle cells: towards the development of a fluorescence microscope with high temporal and spatial resolution. Cell Calcium. 1990 Feb-Mar;11(2-3):121–130. doi: 10.1016/0143-4160(90)90065-3. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Voltage-independent calcium release in heart muscle. Science. 1990 Oct 26;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- O'Neill S. C., Mill J. G., Eisner D. A. Local activation of contraction in isolated rat ventricular myocytes. Am J Physiol. 1990 Jun;258(6 Pt 1):C1165–C1168. doi: 10.1152/ajpcell.1990.258.6.C1165. [DOI] [PubMed] [Google Scholar]
- Rose W. C., Balke C. W., Wier W. G., Marban E. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. J Physiol. 1992 Oct;456:267–284. doi: 10.1113/jphysiol.1992.sp019336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford A. W., O'Neill S. C., Eisner D. A. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflugers Arch. 1993 Oct;425(1-2):181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Egan T. M., López-López J. R., Balke C. W. Local control of excitation-contraction coupling in rat heart cells. J Physiol. 1994 Feb 1;474(3):463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]