Abstract
Introduction
Biologic treatments have made complete skin clearance in moderate to severe plaque psoriasis a real possibility. Although clinical trials demonstrated the superiority of bimekizumab over secukinumab, adalimumab, and ustekinumab, direct comparisons with other biologics are not available. This systematic literature review (SLR) and network meta-analysis (NMA) aimed to evaluate the 1-year efficacy and safety of bimekizumab versus other biologic systemic therapies for moderate to severe plaque psoriasis.
Methods
We conducted an SLR to retrieve published randomised controlled trials (RCTs) in patients with moderate to severe plaque psoriasis. We searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews and PsycINFO on 13 January 2022. Two NMA types were used to analyse the long-term achievement of 100% improvement from baseline in Psoriasis Area and Severity Index (PASI 100): (1) NMA of cumulative clinical benefits, based on the area under the curve, from week 0 to 52; (2) multinomial NMA at weeks 44‒60. Binomial NMA was used to evaluate long-term serious adverse events (SAEs).
Results
The SLR identified 38 RCTs, of which 19 were included in the NMA. Bimekizumab 320 mg administered every 4 weeks to week 16 then every 8 weeks (Q4W/Q8W) showed a greater cumulative average number of days of PASI 100 response compared with all other biologics. These differences were statistically significant versus all biologics, except risankizumab 150 mg. The multinomial NMA demonstrated that interleukin (IL)-17 and IL-23 inhibitors were the most efficacious treatments. No significant differences were found in long-term occurrence of SAEs.
Conclusion
Bimekizumab 320 mg Q4W/Q8W was superior to most other treatments in maintaining complete skin clearance during the first year of treatment. It demonstrated a greater cumulative average number of days with completely clear skin while displaying a comparable safety profile compared with all other biologics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01302-0.
Keywords: Adalimumab, Bimekizumab, Guselkumab, Long-term efficacy, Long-term safety, Network meta-analysis, Psoriasis, Risankizumab, Secukinumab, Ustekinumab
Key Summary Points
| Biologic therapies have revolutionised the management of moderate to severe plaque psoriasis, offering the possibility of achieving and maintaining completely clear skin. |
| While short-term efficacy and safety of biologics have been extensively studied, data reporting long-term comparative efficacy and safety is limited, necessitating further investigation. |
| This systematic literature review (SLR) identified randomised controlled trials (RCT) that evaluated the long-term comparative efficacy and safety of bimekizumab and other biologic therapies, which were considered for inclusion in the network meta-analyses (NMA). |
| The NMA evaluated the comparative efficacy among biologics in achieving complete skin clearance (100% improvement from baseline in Psoriasis Area and Severity Index [PASI 100]) as well as the comparative safety, particularly, serious adverse events (SAEs). |
| Bimekizumab 320 mg administered every 4 weeks to week 16 then every 8 weeks during the maintenance period (Q4W/Q8W) was superior to the majority of other biologics in maintaining complete skin clearance, with no significant differences in the occurrence of SAEs during the first year of treatment. |
Introduction
Plaque psoriasis is a chronic, immune-mediated dermatological condition characterised by the presence of well-demarcated, erythematous and itchy skin plaques. Its impact extends beyond physical manifestations, often impairing the psychological and social well-being of patients [1]. In Europe and North America, plaque psoriasis is relatively prevalent, with reported prevalence rates ranging from 1.5% to 3% [2, 3]. Notably, 20% of patients present with the moderate to severe form of plaque psoriasis, which is often defined as involving at least 10% of body surface area or affecting crucial body parts such as the hands, feet, facial, or genital areas [4].
Biologic therapies have had a transformational impact on the management of plaque psoriasis, expanding the available effective treatment options [5]. As a result, in recent years, attaining completely clear skin has become a real possibility for patients [6]. Approved biologic therapies target crucial cytokines involved in the pathogenesis of psoriasis, including tumour necrosis factor (TNF)-α and interleukin (IL)-12/23, IL-17 and IL-23 [7]. The distinct roles the different cytokines play in the pathophysiologic mechanisms underlying psoriasis have led to varying efficacy profiles among different biologics [5]. Bimekizumab, a novel, humanised monoclonal IgG1 antibody, is characterised by its simultaneous dual inhibition of IL-17F in addition to IL-17A, offering rapid and sustained clinical improvement for patients with moderate to severe psoriasis [8–10].
The Psoriasis Area and Severity Index (PASI) is the most commonly used instrument to evaluate psoriasis disease severity [11]. Achieving 100% improvement from baseline in PASI (PASI 100), indicating full resolution of lesions and completely clear skin, has become more clinically relevant given the recent advancements with biologic therapies [12, 13]. Complete or almost complete skin clearance in patients with plaque psoriasis has been shown to significantly improve their quality of life, as evidenced by reductions in signs and symptoms of the disease such as pain, itching, redness and scaling, and a decrease in overall disease severity [14]. Additionally, it is important to examine not only the ultimate treatment effects but also the cumulative benefit over time of treatments for psoriasis. The cumulative clinical benefit can be examined using the area under the curve (AUC) method, which enables assessment of both the speed of onset as well as the durability of treatment effect throughout the follow-up period [15].
A systematic literature review (SLR) and network meta-analysis (NMA) published in 2022 examining the short-term efficacy of biologic therapies for psoriasis showed that IL inhibitors were the most effective, with bimekizumab demonstrating superiority over all other tested biologics in achieving PASI 100 within 10–16 weeks of treatment initiation [16]. However, since psoriasis is a chronic condition requiring lifelong treatment, it is crucial for comparative efficacy analyses to also evaluate the long-term benefit–risk profiles of available biologics.
While comparative short-term efficacy and safety among biologics have been extensively investigated using randomised controlled trials (RCTs) and indirect treatment comparisons [17], data assessing their long-term efficacy and safety remains limited. For instance, only two trials (BE RADIANT and BE VIVID) investigated the long-term efficacy and safety of bimekizumab versus other biologics (secukinumab and ustekinumab, respectively) [9, 18, 19]. Direct long-term comparisons versus other biologics are otherwise lacking. Therefore, we conducted an SLR and NMA, including a cumulative benefit analysis, to compare the long-term efficacy, in terms of complete skin clearance (PASI 100), and safety of bimekizumab versus different systemic biologics in patients with moderate to severe psoriasis.
Methods
SLR
An SLR was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Collaboration guidelines [20, 21] to identify RCTs that evaluated the long-term efficacy and safety of approved biologic (licensed dosage forms and strengths) and non-biologic therapies for the treatment of moderate to severe plaque psoriasis. Searches were restricted to English language studies conducted in humans and published from database inception to 13 January 2022. Searches were first conducted in March 2019 and were updated on 1 July 2020 and 13 January 2022. Searches were performed in Embase, Ovid, MEDLINE, PsycINFO, the Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews. The search strategies for each database are detailed in Supplementary Tables 1–4.
Detailed information on the SLR methods has been previously published [17]. Records were screened on the basis of the specific eligibility criteria outlined in the population, interventions, comparators, outcomes, study design and time framework (Supplementary Table 5). Eligibility was restricted to RCTs investigating long-term efficacy, assessed via percentage improvement from baseline in PASI, and safety, assessed via serious adverse events (SAEs), during the maintenance period (44‒60 weeks post-randomisation). Two reviewers independently screened the titles, abstracts, and full texts of the articles. Any discrepancies were resolved through consensus or by consulting a senior reviewer.
Standardised data extraction forms were used to extract the relevant patient characteristics, study characteristics and outcome data. The Cochrane Risk of Bias Assessment Tool v2.0 was used to assess the risk of bias in the studies [22]. For each study, an overall quality score was provided that was based on how well the publication met the quality criteria for study randomisation, concealment of treatment allocation, baseline differences, blinding of patients and assessors, imbalances in withdrawals, completeness of outcome reporting and use of intention-to-treat analyses.
NMA
NMA methods have been previously described [17]. Prior to conducting the NMA, a rigorous feasibility assessment of the assumptions, i.e. consistency and similarity, in each network was undertaken. This involved exploring the distribution of the potential effect modifiers, such as disease duration, baseline PASI scores, prior biologic therapy use and presence of comorbidities across the different trials connected to the network. As a result of variation in study designs over their maintenance periods, additional considerations related to the homogeneity of study designs were closely examined. On the basis of the feasibility assessment, our base-case scenario included trials that evaluated long-term efficacy and safety outcomes for patients who were initially randomised to an active treatment and remained in that treatment group through the maintenance period, irrespective of their response at the end of the initial period. Open-label extension studies of placebo-controlled trials were also included in a sensitivity analysis. In this scenario, placebo response rates at the end of the initial periods were carried through to the end of the maintenance periods, with the assumption that the placebo response would not vary substantially throughout the maintenance periods [23]. This approach allowed for indirect comparisons among treatments that were connected to the network only via placebo arms, and for the adjustment of baseline risk via placebo response rates. Trials that utilised a responder-enrichment design, in which only responders were re-randomised following the initial period, were excluded from the analysis.
Two NMA types were carried out to analyse long-term achievement of PASI 100: (1) NMA of cumulative clinical benefits over the course of treatment from week 0 to 52; and (2) multinomial NMA at weeks 44‒60. Furthermore, binomial NMA was used to compare the difference between treatments in long-term SAEs.
NMA of Cumulative Clinical Benefits from Week 0 to 52
The NMA of difference in AUC between the active treatments was conducted using the technique proposed for the NMA of mean differences in the National Institute for Health and Care Excellence (NICE) Technical Support Document 2 [24]. This analysis was carried out in the base-case scenario, i.e. studies with at least two active treatment arms in which patients received the same active intervention from randomisation through the initial and maintenance periods, irrespective of response at the end of the initial period. The sensitivity analysis including placebo-controlled trials was not available for the analysis of cumulative clinical benefits, given the required assumption for carrying forward the placebo response rate.
The AUC and its variance were calculated for PASI 100 response rates using all available data points between weeks 0 and 48‒52. When needed, values were estimated from published figures using the BormiSoft DigitizeIt software [25]. Only analyses reporting data using non-responder imputation were included. Results from trials with follow-up only to week 48 were extrapolated, assuming that the response rate at week 52 was the same as at week 48. This extrapolation was performed in three trials: BE RADIANT (bimekizumab versus secukinumab), ECLIPSE (guselkumab versus secukinumab) and VOYAGE 1 (guselkumab versus adalimumab) [18, 26, 27].
The total AUC for PASI 100 response was determined using the trapezoidal rule, and the results were normalised as a percentage of maximum possible AUC. Calculation of variance of AUC assumed that the correlation structure of the proportion of responders between timepoints was similar for different compounds. A correlation matrix for the proportion of responders at different weeks was obtained using individual patient-level data from the studies BE RADIANT, BE VIVID and BE SURE [9, 18, 19]. BE RADIANT, BE VIVID and BE SURE data were included for bimekizumab; bimekizumab-treated patients were dosed 320 mg every 4 weeks throughout (Q4W/Q4W) or switched to every 8-week dosing from week 16 onwards (Q4W/Q8W). On-label doses of comparators were included.
All analyses of AUC response were run with fixed effects (FE) and random effects (RE) modelling. The analysis was conducted using R v4.0.1, using the BUGSnet package. The code was adapted from the MetaInsight tool v3.1.4 [28].
Results are expressed as the mean number of cumulative days for which patients achieved PASI 100 (with 95% credible intervals [CrIs]) and as the mean differences in these values (with 95% CrIs) for all comparisons. Results that were statistically significant refer to mean differences with 95% CrIs that did not include 0.
Multinomial and Binomial NMA at Weeks 44‒60
PASI 100 outcomes among the assessed biologics at approximately 1 year of treatment (as opposed to cumulative outcomes over the first year) were estimated using the Bayesian NMA approach. In order to leverage information across studies where other PASI thresholds were reported (PASI 50, PASI 75, and PASI 90), multinomial likelihood (probit link) NMA models were employed. The differences in PASI 100 findings are presented here.
The multinomial NMA of PASI 100 was carried out both in the base-case scenario defined above and the sensitivity analysis scenario (including placebo-controlled trials). The binomial NMA of SAEs was carried out only in the base-case scenario, given the required assumption for carrying forward the placebo response rate.
The PASI NMA models were conducted via multinomial ‘REZ’ models, which allowed the relative treatment effect to vary across the different PASI thresholds, as previously described by Fahrbach et al. [29] and Armstrong et al. [17]. Both standard and placebo-adjusted models were run. For the placebo-adjusted sensitivity analysis, baseline-adjusted models were also conducted, modelling the relationship between placebo rates and relative effects versus placebo. All analyses were run with FE and RE modelling. Final model selection was based on clinical input and evaluation of the goodness of fit of the different analytic models using the posterior mean residual deviance and deviance information criteria (DIC).
The posterior distributions of the response rates were summarised using the median and 95% CrIs. These estimates were obtained following the same methodological approaches described in the NMA by Armstrong et al. [17]. Posterior samples of risk ratios (RRs) were obtained using the response/event probabilities of treatments and were summarised as above to obtain the estimates and corresponding CrIs of RRs at different PASI thresholds.
Network inconsistency was assessed using an unrelated mean-effect model (UME), as recommended in technical support documents by NICE [30] and as previously reported in the short-term NMA for bimekizumab [17].
An FE binomial NMA model was conducted to evaluate SAEs in the base-case scenario. Odds ratios (OR) and corresponding CrIs were obtained from each of the binomial models.
Results that were statistically significant refer to ORs or RRs with 95% CrIs that did not include 1.0. Bayesian NMA of multinomial models were conducted in JAGS v4.3.0, and binomial NMA were conducted in OpenBUGS v3.2.3 [31, 32].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
SLR
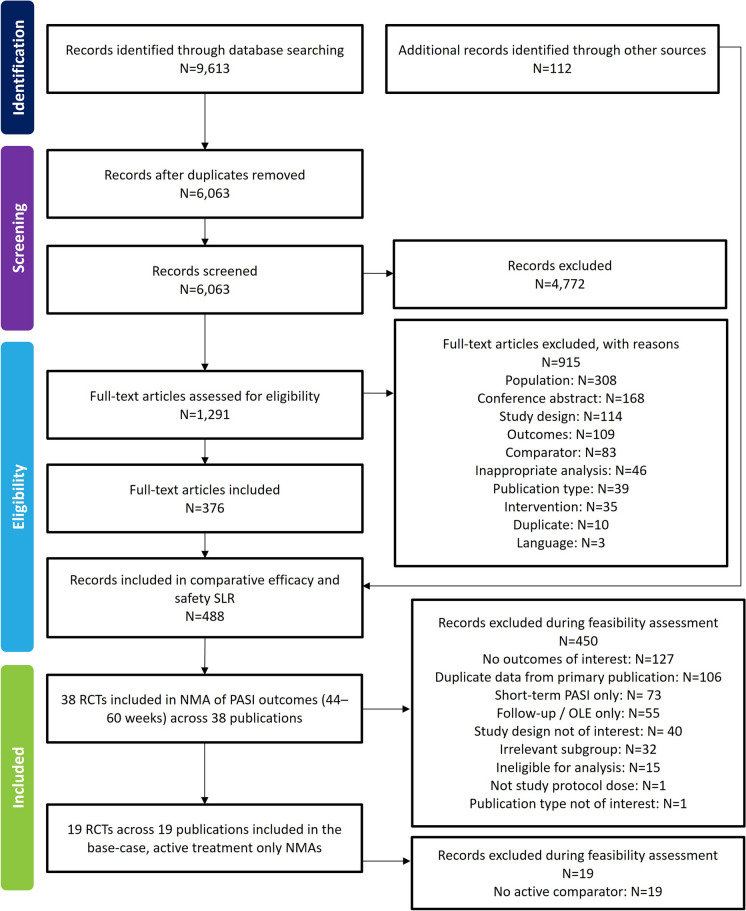
The searches yielded 9613 unique publications from the electronic literature databases and 112 from other sources, including conference proceedings and data on file provided by UCB (clinical study reports). After screening, 38 trials met the inclusion criteria for the long-term clinical efficacy and/or safety NMA. Nineteen trials were eligible for inclusion in the base-case scenario of both the cumulative clinical benefit and multinomial NMAs. Figure 1 summarises the flow of included studies in the SLR and NMA.
Fig. 1.
PRISMA flow diagram. NMA network meta-analysis, OLE open-label extension, PASI Psoriasis Area and Severity Index, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT randomised controlled trial, SLR systematic literature review
Among the 38 included trials, 30 were phase 3, four were phase 2, two were phase 2/3 and two were phase 4. Sample sizes ranged from 62 to 1465 patients, with the majority of studies including at least 100 patients. The mean age of participants ranged from 38 to 53 years of age. Patients had moderate to severe plaque psoriasis for an average of 11–21 years. The proportion of patients with comorbid psoriatic arthritis (PsA) ranged from 3.1% to 35%. The most studied treatments were secukinumab and ustekinumab, assessed in 17 and 9 trials, respectively (Supplementary Table 6).
A total of 28 trials were considered to have a low risk of bias, six were rated as having some concerns, and two (CARIMA, ELCIPSE) [26, 33] had a high risk of bias. When concerns with risk of bias were identified, the main drivers were missing outcome data or the randomisation process. Two trials were only published in abstract form; hence, there were insufficient data to evaluate the risk of bias of these trials [34, 35]. A summary of the risk of bias assessment for each trial is presented in Supplementary Table 7.
NMA
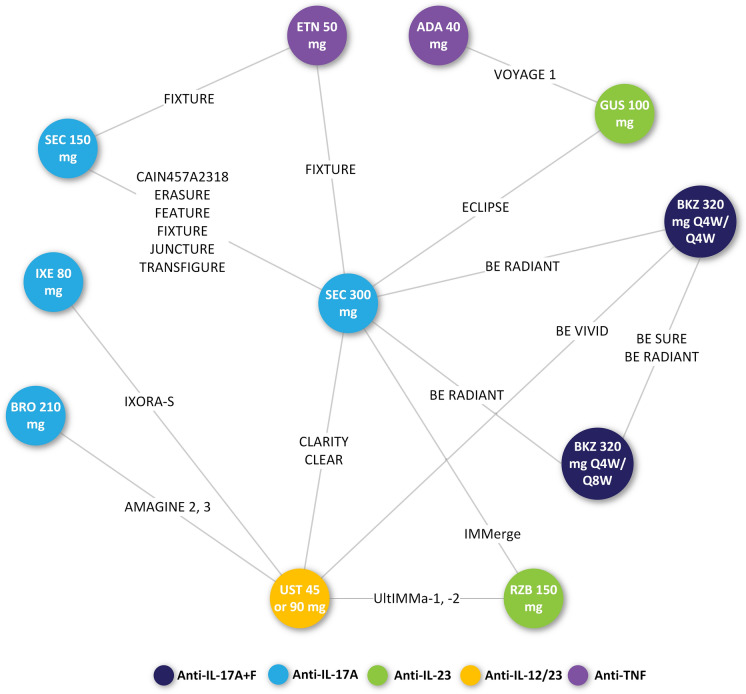
The network diagram of the trials included in the base-case analysis of both the cumulative clinical benefit and multinomial NMA for PASI is presented in Fig. 2. Supplementary Fig. 1 displays the network of trials included in the NMA of SAEs and Supplementary Fig. 2 shows the network of trials included in the sensitivity analysis of the multinomial PASI NMA.
Fig. 2.
Network diagram for trials reporting PASI outcomes (base-case, active treatment only). Number of studies included = 19. ADA adalimumab, BKZ bimekizumab, BRO brodalumab, ETN etanercept, GUS guselkumab, IL interleukin, IXE ixekizumab, PASI Psoriasis Area and Severity Index, Q4W every 4 weeks, Q8W every 8 weeks, RZB risankizumab, SEC secukinumab, TNF tumour necrosis factor, UST ustekinumab
PASI 100: NMA of Cumulative Clinical Benefits at Weeks 0–52
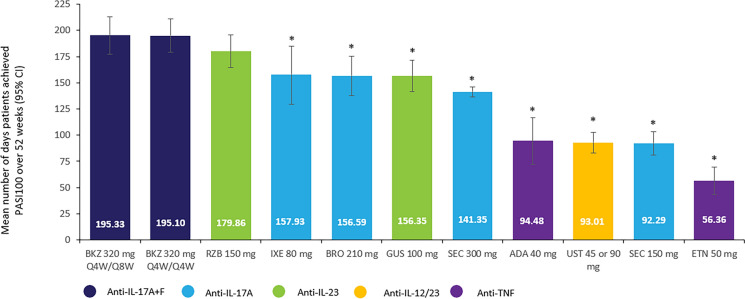
The best-fitting model for the base-case analytic scenario was the FE model. Bimekizumab 320 mg Q4W/Q8W showed a greater cumulative average number of days of PASI 100 response over the first 52 weeks of treatment compared with all other biologics (Fig. 3). These differences were statistically significant versus all biologics. In particular, the mean difference in number of days with PASI 100 response for bimekizumab was significantly higher compared with brodalumab 210 mg (− 38.68; 95% CrI − 63.34, − 14.10), ixekizumab 80 mg (− 37.74; 95% CrI − 70.43, − 5.88), and guselkumab 100 mg (− 38.78; 95% CrI − 61.93, − 15.95). A non-significant numerical benefit was observed versus risankizumab 150 mg (mean difference in number of days for bimekizumab versus risankizumab, − 15.31; 95% CrI − 38.29, 7.39) (Table 1).
Fig. 3.
Cumulative number of days with PASI 100 from week 0 to 52. *Differences in the mean number of days with PASI 100 were statistically significant versus BKZ 320 mg Q4W/Q8W. Model: FE NMA. ADA adalimumab, BKZ bimekizumab, BRO brodalumab, CI confidence interval, ETN etanercept, FE fixed effects, GUS guselkumab, IL interleukin, IXE ixekizumab, NMA network meta-analysis, PASI 100 achievement of 100% improvement from baseline in Psoriasis Area and Severity Index, Q4W every 4 weeks, Q8W every 8 weeks, RZB risankizumab, SEC secukinumab, TNF tumour necrosis factor, UST ustekinumab
Table 1.
Findings of the NMA of cumulative clinical benefits
| Treatment | Mean difference (95% CrI) in number of days with PASI 100 for BKZ 320 mg Q4W/Q8W versus comparatorsa |
|---|---|
| Bimekizumab 320 mg Q4W/Q8Wb | Reference |
| Bimekizumab 320 mg Q4W/Q4W | − 0.10 (− 16.88, 16.50) |
| Brodalumab 210 mg | − 38.68 (− 63.38, − 14.10) |
| Risankizumab 150 mg | − 15.31 (− 38.29, 7.39) |
| Ixekizumab 80 mg | − 37.74 (− 70.43, − 5.88) |
| Guselkumab 100 mg | − 38.78 (− 61.93, − 15.95) |
| Secukinumab 300 mg | − 53.81 (− 71.33, − 36.61) |
| Ustekinumab 45 or 90 mg | − 102.14 (− 121.05, − 83.46) |
| Secukinumab 150 mg | − 102.90 (− 123.78, − 82.34) |
| Adalimumab 40 mg | − 100.68 (− 129.30, − 72.31) |
| Etanercept 50 mg | − 138.80 (− 160.85, − 116.84) |
BKZ bimekizumab, CrI credible interval, NMA network meta-analysis, PASI 100 achievement of 100% improvement from baseline in Psoriasis Area and Severity Index, Q4W every 4 weeks, Q8W every 8 weeks
aNMA of cumulative clinical benefits from weeks 0–52. Model: fixed effects NMA
bBimekizumab-treated patients were dosed 320 mg every 4 weeks through week 16 and then switched to every 8 weeks maintenance dosing (Q4W/Q8W)
PASI 100: Multinomial NMA at Weeks 44‒60
The best-fitting model for the base-case analytic scenario was the FE model. However, given the small differences in the DIC and on the basis of clinical recommendations, the RE model was selected for reporting. The similarity of residual deviances as well as the leverages between inconsistency (i.e. UME) and consistency models across studies for PASI 75 and PASI 90, which were more commonly reported than PASI 100, indicated no notable inconsistency in the base-case scenario.
The NMA demonstrated that IL-17 and IL-23 inhibitors, including bimekizumab 320 mg Q4W/Q4W, bimekizumab 320 mg Q4W/Q8W, risankizumab 150 mg, ixekizumab 80 mg, brodalumab 210 mg, and guselkumab 100 mg, were the most efficacious treatments in the network. However, there were no statistical differences between bimekizumab 320 mg Q4W/Q8W and each of the aforementioned treatments (Supplementary Table 8).
At weeks 44–60 post-randomisation, bimekizumab 320 mg Q4W/Q8W was significantly more efficacious in achieving PASI 100 than adalimumab 40 mg (RR 2.34; 95% CrI 1.66, 3.14), etanercept 50 mg (RR 5.09; 95% CrI 3.19, 8.94), secukinumab 150 mg (RR 2.52; 95% CrI 1.92, 3.42) or 300 mg (RR 1.52; 95% CrI 1.27, 1.83), and ustekinumab 45 or 90 mg (RR 1.99; 95% CrI 1.56, 2.55). Differences versus other biologics including risankizumab 150 mg, guselkumab 100 mg, brodalumab 210 mg, and ixekizumab 80 mg were not statistically significant (Supplementary Table 8).
The results remained consistent in the sensitivity analysis, in which the placebo response rates at the end of the initial period were carried over to the end of the maintenance period (Supplementary Fig. 3, Supplementary Table 9).
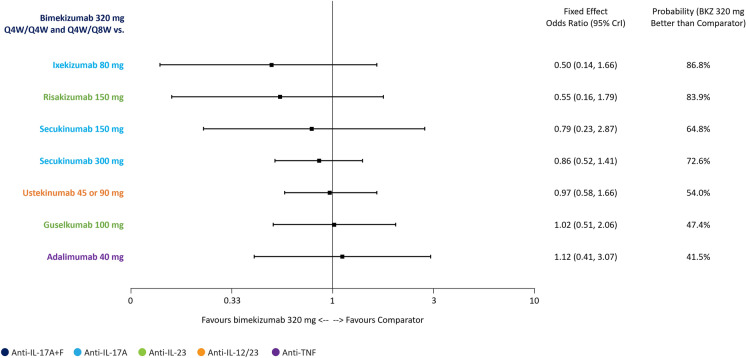
SAEs: Binomial NMA at Weeks 44‒60
At the level of SAEs, none of the treatments were statistically different from bimekizumab 320 mg Q4W/Q4W and Q4W/Q8W. Nonetheless, ORs numerically favoured bimekizumab 320 mg Q4W/Q4W and Q4W/Q8W versus ixekizumab 80 mg, risankizumab 150 mg and secukinumab 150 mg and 300 mg. In contrast, ORs numerically favoured adalimumab 40 mg compared to bimekizumab 320 mg Q4W/Q4W and Q4W/Q8W (Fig. 4).
Fig. 4.
Binomial NMA at weeks 44‒60: odds of experiencing SAE with bimekizumab 320 mg Q4W/Q4W and Q4W/Q8W compared with other treatments. Model: FE NMA. BKZ bimekizumab, CrI credible interval, FE fixed effects, IL interleukin, NMA network meta-analysis, Q4W every 4 weeks, Q8W every 8 weeks, TNF tumour necrosis factor, SAE serious adverse event
Discussion
This SLR and subsequent NMA demonstrated that bimekizumab 320 mg Q4W/Q8W led to a greater cumulative average number of days with a PASI 100 response compared with all other biologics among patients with moderate to severe psoriasis over the first 48–52 weeks of treatment. At weeks 44–60, bimekizumab along with other IL-17 and IL-23 inhibitors, including risankizumab 150 mg, ixekizumab 80 mg, brodalumab 210 mg and guselkumab 100 mg were the most efficacious treatments in PASI 100 achievement.
The assessment of cumulative clinical benefit via AUC analysis accounted for both the speed of onset and maintenance of treatment response. During the first year of treatment, the AUC analysis demonstrated that bimekizumab 320 mg Q4W/Q8W ranked highest for the cumulative average number of days with PASI 100 achievement, with statistically significant differences versus most other biologics. Bimekizumab 320 mg Q4W/Q4W came second, while risankizumab and ixekizumab ranked third and fourth, respectively, in terms of the number of days with PASI 100 response. These risankizumab and ixekizumab findings are supported by a recently published NMA of cumulative clinical benefit of biologics in plaque psoriasis, crucially, that used a cut-off date of September 2020 excluding bimekizumab trials from the analysis. The study found that ixekizumab and risankizumab showed the greatest cumulative average number of days compared with other biologics, with respect to PASI 100 over 1 year [36]. However, it is noteworthy that the latter analysis included placebo-controlled trials, where patients in the placebo arms are typically followed for only up to 12 to 16 weeks; thus, imputation using last observation carried forward to week 52 in placebo arms was undertaken, which is arguably a strong assumption.
The comparative efficacy of the different interventions evaluated by the modified multinomial NMA showed a superiority of IL inhibitors. This NMA employed an enhancement to the standard multinomial analysis model, allowing for different rankings of treatments across the different PASI thresholds [17]. The findings of this NMA demonstrated that IL inhibitors, including bimekizumab 320 mg Q4W/Q8W, brodalumab 210 mg, risankizumab 150 mg, ixekizumab 80 mg, guselkumab 100 mg, and the high dose of secukinumab (i.e. 300 mg), were the most efficacious treatments in achieving PASI 100 as assessed in the long-term networks. In this NMA, significantly greater proportions of patients achieved long-term PASI 100 with bimekizumab 320 mg Q4W/Q8W compared with TNF inhibitors, including adalimumab, etanercept and infliximab, in addition to some other biologics assessed (excluding guselkumab 100 mg, ixekizumab 80 mg, risankizumab 150 mg and brodalumab 210 mg). These findings were consistent when placebo-controlled trials were included in the analysis. These results were comparable to a recently published NMA, which also found IL inhibitors to be significantly more effective compared with other interventions in achieving long-term PASI 100 [37]. However, the aforementioned SLR, conducted by Yasmeen et al. [37], also did not include the bimekizumab trials, as the search cut-off date was September 2019.
Considering the superiority of bimekizumab in PASI 100 achievement versus other biologics within 10–16 weeks of treatment [17] and the significantly better AUC findings in achieving PASI 100, it appears that bimekizumab is associated with more rapid responses versus most biologics, with similar long-term response to the closest comparators. This study demonstrates the promising potential of bimekizumab and other biologic therapies in achieving sustained complete remission, thus expanding the therapeutic landscape of efficacious treatments in moderate to severe plaque psoriasis to long-term treatment.
For safety outcomes, there were no significant differences in the long-term occurrence of SAEs between bimekizumab 320 mg Q4W/Q4W and Q4W/Q8W versus other tested biologic treatments. Our findings align with those reported by Shear et al. [38] who conducted an NMA of seven trials evaluating six different biologic therapies (risankizumab, adalimumab, ustekinumab, guselkumab, ixekizumab and secukinumab) that showed a lack of significant differences in long-term SAEs between assessed biologics [38].
Despite the notable strengths of our review in adhering to a rigorous methodology and identifying a comprehensive evidence base of studies evaluating the long-term efficacy and safety of biologics in plaque psoriasis, our study had some limitations. The inclusion of the IMMERGE trial, an open-label study, in our analyses may have potentially introduced some bias. Nevertheless, it is important to note that excluding this trial could have resulted in a higher number of days difference with PASI 100 response for bimekizumab compared to risankizumab. Therefore, a more conservative approach was adopted in our analyses. Furthermore, the efficacy outcomes focused on PASI and did not investigate others, such as the Dermatology Life Quality Index, the Physician Global Assessment, and the Investigator’s Global Assessment. The inclusion of these assessment tools in the future may provide more value to the comparative efficacy profiles of the different available treatments. Our safety NMA was limited to the assessment of SAEs, which potentially limits the interpretability of the comparative long-term safety and tolerability profiles of biologics in plaque psoriasis. However, it is worth noting that the selection of SAEs as the endpoint of interest aligns with a previously conducted Cochrane SLR/NMA by Sbidian et al. [39]. Additionally, it is important to highlight that the limited evidence base, coupled with the relatively low number of events, warrants a cautious interpretation of these findings.
While no major differences in the patient characteristics were found across included trials, a degree of heterogeneity was evident in the prevalence of PsA, time since psoriasis diagnosis, and prior systemic therapy use. However, in a sensitivity analysis where a baseline-risk adjustment via placebo response rates was undertaken, results remained consistent with our base-case model.
The NMA of SAEs included a relatively small number of interventions. This is because only the base-case network of active-comparator studies was available for the SAE analysis, as the assumption required to include the placebo-controlled trials (carrying forward the placebo rate at 16 weeks) did not hold for safety outcomes.
Lastly, it is important to highlight the challenges associated with comparing outcomes over a longer duration of follow-up. For instance, variations in disease progression and unanticipated events can introduce complexities that may influence the generalisability of study findings.
Conclusion
Bimekizumab 320 mg Q4W/Q8W, a dual inhibitor of IL-17F in addition to IL-17A, was superior to the majority of other treatments in maintaining complete skin clearance during the first year of treatment. Over the course of 1 year, bimekizumab demonstrated a greater cumulative average number of days with completely clear skin compared with all other biologics. Additionally, bimekizumab was associated with a safety profile comparable with other biologic therapies during the first 44–60 weeks of treatment. However, considering the lifelong nature of therapy patients with moderate to severe psoriasis receive, the evaluation of long-term safety data in the real-world setting is required for adequate evaluation of the risk–benefit balance of all available therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing/Editorial Assistance
Medical writing and editorial assistance in the preparation of this article was provided by Hannah Floyd and Peter Seeber from Evidera and was funded by UCB Pharma. The authors also acknowledge Paulina Kazmierska from Evidera for her contribution to the systematic literature review and network meta-analysis work.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Richard B. Warren, Kerry Donnelly, Sandeep Kiri, Vanessa Taieb, Mahmoud Slim, Kyle Fahrbach, Binod Neupane, Marissa Betts, April Armstrong. Substantial contributions to study conception and design: Sandeep Kiri, Mahmoud Slim, Kyle Fahrbach, Binod Neupane, Marissa Betts; substantial contributions to the literature search and analysis and interpretation of the data: Richard B. Warren, Kerry Donnelly, Sandeep Kiri, Vanessa Taieb, Mahmoud Slim, Kyle Fahrbach, Binod Neupane, Marissa Betts, April Armstrong; drafting the article or revising it critically for important intellectual content: Richard B. Warren, Kerry Donnelly, Sandeep Kiri, Vanessa Taieb, Mahmoud Slim, Kyle Fahrbach, Binod Neupane, Marissa Betts, April Armstrong; final approval of the version of the article to be published: Richard B. Warren, Kerry Donnelly, Sandeep Kiri, Vanessa Taieb, Mahmoud Slim, Kyle Fahrbach, Binod Neupane, Marissa Betts, April Armstrong.
Funding
Sponsorship for this study and Rapid Service Fee were funded by UCB Pharma.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Richard B. Warren declares receiving consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, GSK, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi and UCB Pharma. He also declares receiving research grants to his institution from AbbVie, Almirall, Janssen, LEO Pharma, Novartis and UCB Pharma, and honoraria from Astellas, DiCE, GSK and Union Therapeutics. Richard B. Warren is an Editor-in-Chief of Dermatology and Therapy. Richard B. Warren was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Kerry Donnelly, Sandeep Kiri, Vanessa Taieb are employees and shareholders of UCB Pharma. Mahmoud Slim, Kyle Fahrbach, Marissa Betts, Binod Neupane are employed by Evidera, a part of Thermo Fisher Scientific. April Armstrong has served as a research investigator and/or scientific advisor to AbbVie, Almirall, Arcutis, ASLAN, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, EPI, Incyte, Janssen, LEO Pharma, Nimbus, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sun Pharma, Sanofi and UCB Pharma.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;3709583:263–71. 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;1578:940–6. 10.1001/jamadermatol.2021.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369: m1590. 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;651:137–74. [DOI] [PubMed] [Google Scholar]
- 5.Brownstone ND, Hong J, Mosca M, et al. Biologic treatments of psoriasis: an update for the clinician. Biologics. 2021;15:39–51. 10.2147/btt.S252578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strober B, Ferris L, Callis Duffin K, et al. Real-world effectiveness of risankizumab in patients with moderate-to-severe psoriasis using the CorEvitas Psoriasis Registry. J Am Acad Dermatol. 2024;901:82–90. 10.1016/j.jaad.2023.08.097. [DOI] [PubMed] [Google Scholar]
- 7.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;4457130:866–73. 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 8.Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;792(277–86): e10. 10.1016/j.jaad.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;39710273:487–98. 10.1016/S0140-6736(21)00125-2. [DOI] [PubMed] [Google Scholar]
- 10.Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. 10.3389/fimmu.2020.01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. 1978;1574:238–44. 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 12.Hanover L. Is using PASI 100 realistic in a real-life setting? 2021. https://www.ajmc.com/view/is-using-pasi-100-realistic-in-a-real-life-setting-. Accessed 31 Oct 2024
- 13.Heymann WR. PASI 100 and the Perfection Paradox 2016. http://www.coppoladelecuador.com/pasi-100-and-the-perfection-paradox.html. Accessed 31 Oct 2024
- 14.Belinchon Romero I, Dauden E, Ferrandiz Foraster C, et al. PASI 100 response rates in moderate to severe psoriasis: a systematic literature review and analysis of clinical practice guidelines. J Dermatol Treat. 2022;333:1661–9. 10.1080/09546634.2021.1890683. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong AW, Feldman SR, Korman NJ, et al. Assessing the overall benefit of a medication: cumulative benefit of secukinumab over time in patients with moderate-to-severe plaque psoriasis. J Dermatol Treat. 2017;283:200–5. 10.1080/09546634.2016.1214667. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AW, Soliman AM, Betts KA, et al. Long-term benefit-risk profiles of treatments for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther (Heidelb). 2022;121:167–84. 10.1007/s13555-021-00647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;128:1777–92. 10.1007/s13555-022-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reich K, Warren RB, Lebwohl M. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021. 10.1056/NEJMoa2102383. [DOI] [PubMed] [Google Scholar]
- 19.Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;3852:130–41. 10.1056/NEJMoa2102388. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J, TJ CJ, Cumpston M, Li T, Page M, Welch V. Cochrane handbook for systematic reviews of interventions version 6.2 (updated August 2023): Cochrane; 2023.
- 21.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;101:89. 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Sawyer LM, Cornic L, Levin L, Gibbons C, Møller AH, Jemec GB. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol. 2019;332:355–66. 10.1111/jdv.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias S, Welton NJ, Sutton AJ, Ades AE. Nice DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials 2016. https://www.sheffield.ac.uk/media/34176/download?attachment. Accessed 31 Oct 2024 [PubMed]
- 25.Borman I. Digitizeit 2.5; Bormisoft: Braunschweig, Germany. 2022 https://www.digitizeit.xyz/. Accessed 31 Oct 2024
- 26.Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;39410201:831–9. 10.1016/S0140-6736(19)31773-8. [DOI] [PubMed] [Google Scholar]
- 27.Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;763:405–17. 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A. MetaInsight: an interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods. 2019;104:569–81. 10.1002/jrsm.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahrbach K, Sarri G, Phillippo DM, et al. Short-term efficacy of biologic therapies in moderate-to-severe plaque psoriasis: a systematic literature review and an enhanced multinomial network meta-analysis. Dermatol Ther (Heidelb). 2021;116:1965–98. 10.1007/s13555-021-00602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials 2014. https://www.sheffield.ac.uk/media/34181/download?attachment. Accessed 31 Oct 2024 [PubMed]
- 31.Plummer M. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. 3rd international workshop on distributed statistical computing (DSC 2003); Vienna, Austria. 2003;124.
- 32.Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Stat Med. 2009;2825:3049–67. 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- 33.von Stebut E, Reich K, Thaci D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;1395:1054–62. 10.1016/j.jid.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 34.Krueger JG, Pariser D, et al. 15354 Response to treatment with secukinumab in obese patients with moderate to severe psoriasis. J Am Acad Dermatol. 2020;83:AB38. [Google Scholar]
- 35.Papp K, Warren R, Green L, et al. Efficacy and safety of mirikizumab versus secukinumab and placebo in the treatment of moderate-to-severe psoriasis: 52-week results from OASIS-2, a multicenter, randomized, double-blind study. J Clin Aesthet Dermatol. 2021;14(Suppl. 1):S26. [Google Scholar]
- 36.Blauvelt A, Gooderham M, Griffiths CEM, et al. Cumulative clinical benefits of biologics in the treatment of patients with moderate-to-severe psoriasis over 1 year: a network meta-analysis. Dermatol Ther (Heidelb). 2022;123:727–40. 10.1007/s13555-022-00690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasmeen N, Sawyer LM, Malottki K, Levin LA, Didriksen Apol E, Jemec GB. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat. 2022;331:204–18. 10.1080/09546634.2020.1743811. [DOI] [PubMed] [Google Scholar]
- 38.Shear NH, Betts KA, Soliman AM, et al. Comparative safety and benefit-risk profile of biologics and oral treatment for moderate-to-severe plaque psoriasis: a network meta-analysis of clinical trial data. J Am Acad Dermatol. 2021;853:572–81. 10.1016/j.jaad.2021.02.057. [DOI] [PubMed] [Google Scholar]
- 39.Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2022;55:CD011535. 10.1002/14651858.CD011535.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.