Abstract
Background:
Economic evaluations in healthcare are designed to inform decisions by the estimation of cost and effect trade-off of two or more interventions. We aimed to evaluate the standards of systematic reviews on health economic evaluation studies using the CHEERS (Consolidated Health Economic Evaluation Report Standards) tool.
Methods:
We searched the PubMed database with keywords CHEERS and its complete form in combination with keywords related to cost or economic evaluation without language and time limits until November 17, 2021. The CHEERS tool was then used to include systematic reviews.
Results:
Overall, 32 systematic reviews, included 610 primary studies were included. Of the 32 included studies, only 1 study (3.1%) had poor quality, 5 studies (15.6%) had good quality, remaining studies had very good and excellent quality.
Conclusion:
Some studies still have problems in expressing the standards. The necessity of standards for reporting economic evaluation studies in the field of health is very serious, and Cheers is one of the most important tools.
Keywords: Health economics, Cost-effectiveness, Quality assessment
Introduction
Due to the lack of required healthcare resources, cost-effective choices are necessary. Decision makers use economic evaluations to effectively allocate resources so that they choose the best service or intervention by identifying, measuring, valuing, and comparing the cost and results of various services (1). Therefore, access to sufficient, accurate, and reliable information is essential for decision-making that can lead to economic and health effectiveness (2). The use of economic evaluation research is increasing, but methodological errors and their usefulness for healthcare decisions affect their validity (1). Therefore, qualitative evaluation of economic evaluation studies is very necessary before applying the results.
Systematic reviews of health economic evaluation studies can provide policymakers, physicians, patients, and other decision-makers with useful information for counseling and decision-making. They can identify the scope and quality of existing studies, conditions promoting the effectiveness and efficiency of the intervention under evaluation, and understand the impact of key parameters on the general outcome (3).
One of the tools used for qualitative review of economic evaluation studies is Consolidated Health Economic Evaluation Reporting Standards (CHEERS). In 2013, CHEERS statement (which was recommended as minimum information that is needed for reporting economic evaluations) was released and in this way, the previous guidelines for health economic evaluation were made available to researchers as a single reporting guideline (4).
Therefore, we aimed to summarize the standards of the conducted systematic review studies on economic evaluation of various diseases.
Methods
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline.
Criteria for considering studies for this review
This systematic literature review was conducted to identify other systematic review studies that have been critically appraised by researchers using CHEERS tools in the PubMed database. We included all studies that were free to access and the quality of the study was assessed with the mentioned tool without any restriction in language or time of publication. Studies in which 23 instrument items were not reviewed for each study, and studies in which wrong references were given to some of the reviewed studies, which caused the year of the study to be unclear, were excluded from the study.
Search strategy
Based on the inclusion criteria, we searched the PubMed database with keywords CHEERS and its open form i.e., Consolidated health economic evaluation reporting standard in combination with keywords related to cost or economic evaluation with this search strategy: ((Consolidated Health Economic Evaluation Reporting Standards) OR (CHEERS) AND (ffrft[Filter])) AND ((Costs and Cost Analysis[Mesh Terms]) OR (cost) AND (ffrft[Filter])) Filters: Free full text, without language and time limits until November 17, 2021. All studies imported into EndNote ×20.
Selection of studies
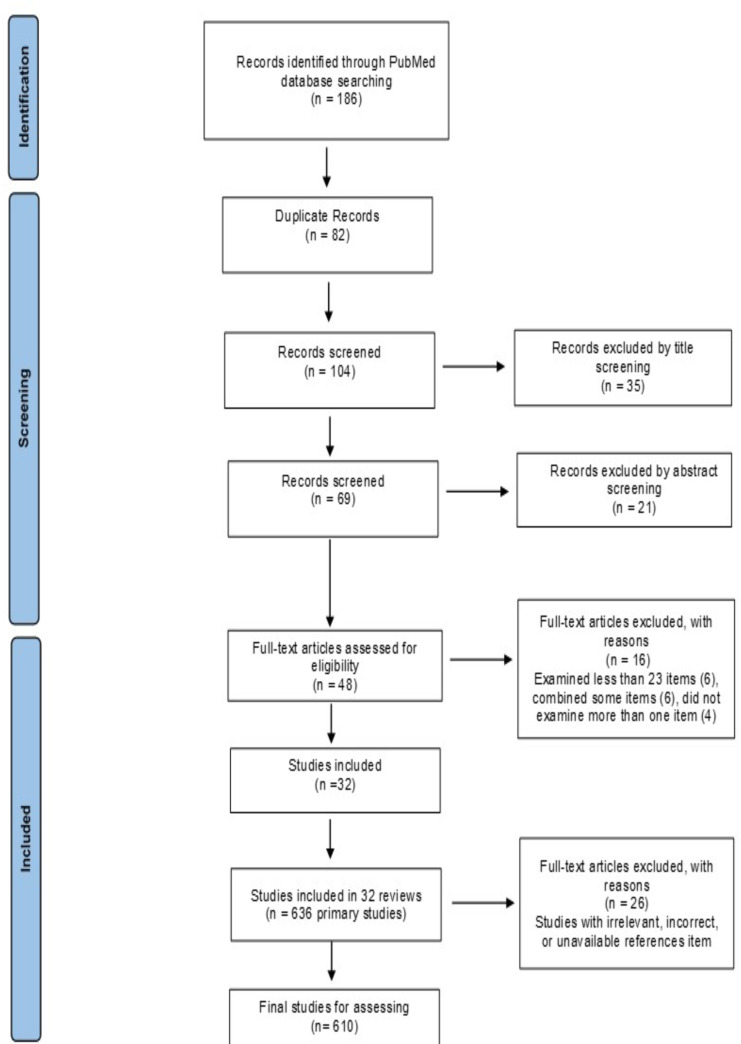
As shown in Fig. 1 (the PRISMA Flow diagram) which indicates the process of identifying, reviewing, and selecting articles, at first, 186 studies were obtained. All duplicate records (n= 82) and 102 records remained for further review. We reviewed the titles and abstracts of remaining review articles. Finally, 32 studies met the inclusion criteria for this review study.
Fig. 1:
The PRISMA flow diagram illustrating article selection and elimination
Quality assessment
Methodological quality of reviews was assessed using quality criteria adapted from De Vet, De Ridder and De Wit (5) and based on the Quality Assessment Tool for Reviews (6). This criterion is an 8-point criterion (0–8). If every criterion was met by the review, it received a score of one and otherwise a score of zero. According to the agreement between the research team, if the total score of the criteria for each review was less than 6, it was excluded from the study. Therefore, 2 other studies were excluded from the reviews and 32 studies leaved. These 32 review studies included 636 primary studies, 26 duplicated primary studies were excluded, and finally 610 studies were included.
Data extraction and analysis strategy
Data extraction included specific details about the number of included studies, the average quality score of the included studies, the quality of the included studies (based on the average quality score), the period of the included studies, the country/region where the primary studies were conducted, and the scope of the study (Table 1).
Table 1:
Number and details of the included studies
| Ref. | Original studies (N) | Average cheers score of included studies (%) | Quality status of the included studies | The time frame of the included studies | Country/region | Scope of study | Quality score |
|---|---|---|---|---|---|---|---|
| Ma, et al (7) 2016 | 32 | 77.3 | Very good | 2003–2014 | China | Pharmacoeconomic | 8 |
| Zakiyah, et al (8) 2016 | 9 | 73.5 | Very good | 2006–2013 | Low- and Middle-Income Countries | Family Planning Interventions | 8 |
| Banke-Thomas, et al (9) 2017 | 5 | 90.5 | Excellent | 2002–2011 | Nigeria, Indonesia, Kenya and Tanzania, Mozambique and Zambia | emergency obstetric care training | 8 |
| Gillespie, et al (10) 2017 | 5 | 68.3 | Good | 2000–2016 | Great Britain, Spain, Japan and two studies from Australia | intraoperative interventions to prevent surgical-site infection | 7 |
| Hope, et al (11) 2017 | 14 | 86.7 | Excellent | 2005–2014 | All of the world | population-based sodium reduction interventions | 8 |
| Ibrahim, et al (12) 2017 | 5 | 91.7 | Excellent | 2009–2017 | Western countries | Antimicrobial Stewardship Programs | 8 |
| Iribarren, et al (13) 2017 | 30 | 78.6 | Very good | 2005–2016 | 19 countries, most of which were conducted in upper and upper-middle income countries | MHealth | 8 |
| Melendez-Torres, et al (14) 2017 | 19 | 81.6 | Very good | 2005–2016 | a lot of countries | Drugs | 8 |
| Velentzis, et al (15) 2017 | 5 | 58.3 | Good | 2005–2009 | 2 USA, UK, Finland, Sweden | Menopausal hormone therapy | 7 |
| Wong, et al (16) 2017 | 9 | 89.8 | Excellent | 2001–2014 | Hong Kong | vaccination programs | 8 |
| Dritsaki, et al (17) 2018 | 4 | 98.2 | Excellent | 2011–2015 | 2USA, UK, CANADA | managing Dupuytren’s disease | 8 |
| Grochtdreis, et al (18) 2018 | 15 | 78.1 | Very good | 2004–2018 | UK, US, Austria, Australia, Belgium, Canada, Switzerland, Germany, France, Italy, the Netherlands, New Zealand, Portugal, Sweden | castration-resistant prostate cancer | 7 |
| Jiang, et al (19) 2019 | 14 | 79.2 | Very good | 2011–2018 | Australia, UK, Spain, USA, | Digital Health Interventions on the Management of Cardiovascular Diseases | 8 |
| Ling, et al (20) 2019 | 15 | 58.1 | Good | 2007–2017 | Uganda, Kenya, Afghanistan, Ethiopia, Tanzania, Senegal, Congo, Brazil, Ghana, Sub-Saharan, Nigeria | malaria rapid diagnostic tests | 6 |
| Mendivil, et al (21) 2019 | 33 | 76.5 | Very good | 2012–2018 | Most studies were conducted in Europe (36,4%), followed by United States (24,2%) and Asia (24,2%) | Screening strategies for early detection of colorectal cancer | 7 |
| Sultana, et al (22) 2019 | 19 | 61.3 | Good | 1998–2017 | Germany, USA, France, Malaysia, Scotland, Multicountry (mainly North America), Multicountry (17 countries in Europe, Latin America and South Africa), Belgium, Hong Kong, UK, Spain, Malawi, Netherlands | community acquired pneumonia management strategies | 6 |
| Anopa, et al (23) 2020 | 16 | 79.5 | Very good | 1986–2017 | United States, United Kingdom, Canada, Sweden, Australia, USSR, Chile, Finland, Taiwan, Uzbekistan | Primary prevention of tooth decay in preschool childrenaged 2 to 5 years | 8 |
| dela Perrelle, et al (24) 2020 | 8 | 74.3 | Very good | 1999–2017 | 3The Netherlands,4 United States of America, Niger | Quality Improvement Collaboratives in healthcare | 8 |
| Hao, et al (25) 2020 | 21 | 80.6 | Very good | 2011–2018 | India, South Africa, Uganda, Russia, Eastern Europe, Uganda, USA, Tanzania, Hongkong, Malawi, Brail, Mozambique, Ethiopia | Xpert in detecting Mycobacterium tuberculosis | 7 |
| Niyomsri, et al (26) 2020 | 14 | 90.2 | Excellent | 2002–2017 | Sweden, UK, US, The Netherlands, Canada, | Spinal Cord Stimulation | 8 |
| Ten Ham, et al (27) 2020 | 12 | 82.7 | Very good | 2013–2019 | US, France, | Gene Therapies and Their Application | 8 |
| Woods, et al (28) 2020 | 19 | 78.5 | Very good | 1997–2019 | Peru, Australia, Canada, US, UK, Germany, Netherlands, Sweden, Colombia, Slovakia, China | diabetic foot ulcer infections | 8 |
| Qiu, et al (29) 2021 | 13 | 85.7 | Excellent | 2007–2019 | Belgium, USA, UK, Hong Kong, Southeast Asia, Singapore, Germany, Japan, Italy | Aprepitant in Preventing Chemotherapy-Induced Nausea and Vomiting | 8 |
| Rezapour, et al (30) 2021 | 26 | 76 | Very good | 2020 | A lot of countries (such as؛ China, Israel, us, Uganda …) | programs against COVID-19 | 8 |
| Schwander, et al (31) 2021 | 4 | 81.2 | Very good | 2007–2014 | UK | Health Economic Obesity Models | 8 |
| Stawowczyk, et al (32) 2018 | 15 | 88.1 | Excellent | 2008–2017 | Canada, the UK and Poland were mostly performed | Biological drugs compared to (conventional, surgery, drugs, etc.) in Ulcerative Colitis | 8 |
| sanyal, et al (33) 2019 | 20 | 84.1 | Very good | 2007–2018 | UK, Spain, France, Netherlands, Belgium, Italy, Canada, USA, Brazil | Community-based services by pharmacists | 7 |
| Avanceña, et al (34) 2021 | 54 | 90.8 | Excellent | 2010–2019 | From all continent | Health Interventions | 8 |
| El Alili, et al (35) 2017 | 45 | 43 | low | 2000–2017 | All Countries | Obstetrics and Gynecology | 6 |
| Ding, et al (36) 2020 | 22 | 96.2 | Excellent | 2010–2019 | countries all over the world, with ten from the USA, seven studies from China (one from Hong Kong), two studies from Canada, one each from Australia, France, Switzerland | immune checkpoint inhibitors for treatment of non-small cell lung cancer | 8 |
| Werner, et al (37) 2020 | 39 | 63.2 | Good | 1983–2019 | low and middle-income countries | emergency care interventions | 7 |
| Galekop, et al (38) 2021 | 49 | 76.3 | Very good | 1987–2017 | Sweden, Switzerland, Australia, UK, USA, Canada | Interventions with a personalized nutrition item in adults | 7 |
Given that each of the 32 studies encompassed multiple primary investigations, a total of 610 studies were meticulously examined by two independent researchers, relying on the evaluations assigned by the original study authors. The objective was to extract the conclusions and synthesize the overarching findings of the studies into a set of 24 items, which were subsequently entered into Excel-2013 software. A score of 1 was allocated to studies that fully adhered to the established criteria, while a score of 0.5 was assigned to studies that partially complied with the standards, and no score was designated for studies that failed to meet the criteria. In instances where the application or non-application of the standard was ambiguous, such studies were categorized as non-applicable, thus excluding the respective item from scoring, with the score for the corresponding item redistributed among the remaining items.
The included primary studies were divided in to 4 categories based on the quality score. So that the quality score above 85 was classified as excellent, 70–85 as very good, 55–70 as good, and below 55 as poor (11, 22). Therefore, more than 70% of the studies were in very good or excellent quality category, and only 15% of the primary studies were in low-quality category.
Results
Description of the included studies
As shown in Table 1, of the 32 included studies, only 1 study (3.1%) was poor quality (35), 5 studies (15.6%) were good quality (15) (20) (22) (10, 37), and remaining studies had very good and excellent quality.
All 32 secondary studies were published after 2013, include 610 primary economic evaluation studies that were conducted during 1986–2020. 33.2% of the studies had an excellent quality score (score from 85–100), 35.4% of studies had a very good quality (score from 70–85), 17.5% of studies have a good quality (score from 55–70) and only 14% of the studies had obtained a score below 55, that is, weak (the score obtained by adjusting the non-applicable items).
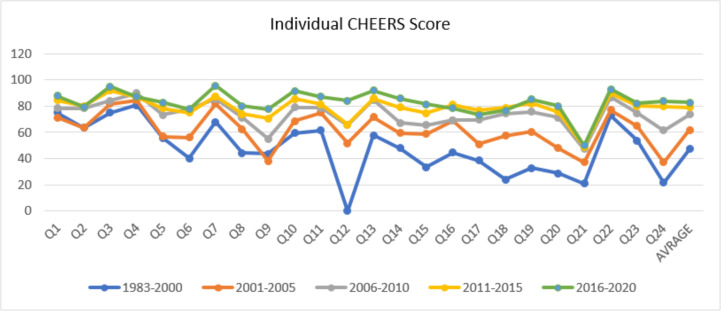
As shown in Fig. 2, the item 4 has the highest score in the times of 1983–2010. After that, in the period of 2015–2011, the item 3 with 92% and in the period of 2016–2020, the item 22 with 92.8% had the highest percentage among other items. In the period of 2000–1883, item 12 ‘Measurement and valuation of preference-based outcomes’ (0%), in the period of 2001–2005, item 24 ‘Conflicts of interest’ (37.2%), and from 2006 to 2020, item 21 ‘Characterizing heterogeneity’ had the lowest percentage. In general, percentages have improved in all items despite some fluctuations over time.
Fig 2:
Average score of 24 items and their trend
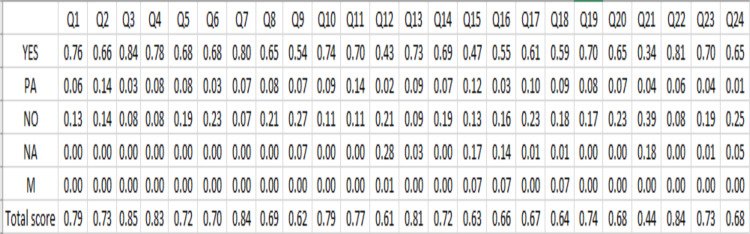
According to Fig. 3, a comprehensive examination reveals that items 21 ‘Characterizing heterogeneity’, 9 ‘Discount rate’, 15 ‘Choice of model’, and 18 ‘Study parameters’ exhibited the lowest quality evaluations, with mean scores of 43.6%, 62%, 63.2%, and 64.5%, respectively. In contrast, items 3 ‘Background and objectives’, 7 ‘Comparators’, 22 ‘Study findings, limitations, generalizability, and current knowledge’, 4 ‘Target population and subgroups’, and 13 ‘Estimating resources and costs’ achieved the highest quality evaluations, with average scores of 83.91%, 85.2%, 83.9%, 82.6%, and 80.8%, respectively. The overall mean score for the 24 items is calculated to be 72.7%, whereas the average score for the CHEERS framework across all studies is determined to be 76.6%. This observed discrepancy could be attributed to the exclusion of non-applicable items. In addition, items 2 ‘Abstract’ (79.3%), 7 ‘Comparators’ (77.8%), 9 ‘Discount rate’ (77.7%), 16 ‘Assumptions’ (78.2%), 17 ‘Analytical methods’ (73.3%), and 18 ‘Study parameters’ (76.7%) were in range 70–80 %, mean while other items were more than 80%.
Fig. 3:
Quality score of all items
Furthermore, each item was assigned a score on a 5-point scale, where complete adherence to the item is denoted as YES with a corresponding score of 1(1), partial adherence as PA with a score of 0.5, non- adherence as NO with a score of 0, non-applicable instances are marked as NA, and items that were not evaluated or were overlooked by the secondary researchers are represented as M (missing) (4, 11, 22, 39). In instances where items are deemed non-applicable and remain unexamined by secondary researchers, the score attributed to such items was proportionately allocated among the remaining items.
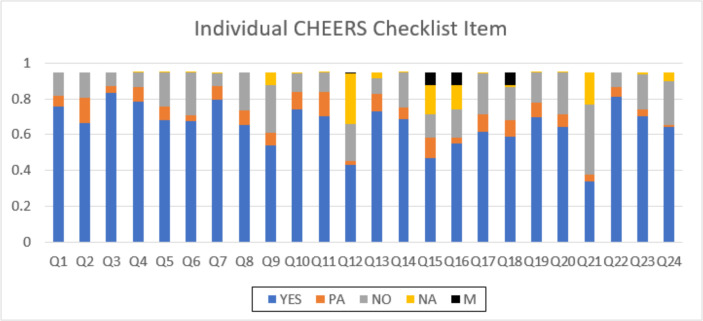
As shown in Fig. 4, the most frequent items that were not reported in the articles implied item 21 ‘Characterizing heterogeneity’ (39% no compliant), item 9 ‘Discount rate’ (26% no compliant), and item 24 ‘Conflicts of interest’ (31% no compliant) and item 20 ‘Characterizing uncertainty’. The most common missing items were item 15, ‘Choice of model’, item 16 ’Assumptions’, and item 18, ‘Study parameters’ with almost 0/06 %.
Fig. 4:
CHEERS Items met by the included studies
According to the findings, adherence with all cheers items by researchers has increased in studies since 1983. In addition, this improvement is more visible in items such as 6 ‘Study perspective’, 12 ‘Measurement and valuation of preference-based outcomes’, 18 ‘Study parameters’, and 24 ‘Conflicts of interest’.
Discussion
The aim of this study was to review systematically standards used in health economic evaluation studies with the CHEERS tool. Overall, 32 review studies met the inclusion criteria which included 610 primary studies. More than 70% of the studies had very good and excellent quality (CHEERS score above 70) and only 14.6% of the studies had low quality (CHEERS score less than 55). In Miroshnychenko et al., (40) which reviewed studies conducted in 2012–2019 the average CHEERS checklist adherence score was 63%. In addition, in Rezapour et al., which reviewed studies in 2019–2020, the CHEERS scores for more than 65% of studies most studies were good and excellent quality (30). In Nguyen et al., study, of all the included articles that were conducted in the period of 2016–1996, the CHEERS score for more than 58.8% of studies was good and excellent (2). Meanwhile, the score of item 21’Characterizing heterogeneity’ (50%) was the most poorly reported items on the CHEERS checklist and has been in the low range compared to other items in all time periods, the causality can be expressed as the assessment of sources of heterogeneity was not conducted in 40% of included economic evaluations. About item 24 ‘Conflicts of interest’, because in 24% of studies this item was not reported, quality score of that was in low range. Item 12 ‘Measurement and valuation of preference-based outcomes’ in 28% was NA (non- applicable) so its score was low than other items.
To our knowledge, this study is the first systematic review study that all the studies that were reviewed were secondary studies and no other study that reviewed secondary studies in this way was found in other databases. Although one of the constraints of this research may be attributed to the multitude of scholars who have examined the CHEERS framework across various publications, resulting in potentially divergent interpretations among different evaluators, it appears that this limitation is somewhat mitigated by the substantial sample size and the consistency observed in the item scores across the studies conducted by distinct researchers. Furthermore, it is noteworthy that the literature search was exclusively performed within the PubMed database, leading to a considerable number of articles being scrutinized; however, it is conceivable that certain relevant studies may have been overlooked, and additional researchers could expand their inquiry by accessing other databases such as Web of Science and Scopus.
It seems imperative that, similar to the investigations conducted on other methodological frameworks such as STROBE, which have been undertaken by domestic researchers, a collaborative effort involving multiple scholars should be pursued to independently examine the preliminary economic evaluation studies conducted over various years employing this particular tool; nonetheless, given the intricate nature of economic evaluations and the nascent introduction of this tool in Iran, the process of identifying several researchers proficient in its application may require an extended duration.
It is recommended that future studies involve at least two independent researchers who would delineate economic evaluation studies within a specified timeframe, geographic region (for instance, Iran), a particular category of technology (such as pharmaceutical economic evaluations), or a distinct type of economic evaluation (such as solely cost-utility analyses), and subsequently assess these investigations. The findings generated by a third researcher should then be reviewed, particularly in instances where discrepancies in evaluative scores arise between the two evaluators, necessitating a comprehensive discussion among all three researchers to reach a consensus, which would ultimately be documented as the definitive evaluation outcome.
Conclusion
Most of the reviewed studies have obtained very good grades from the reviewed standards, but some studies still have problems in expressing the standards. It seems that the necessity of standards for reporting economic evaluation studies in the field of health is very serious, and considering that CHEERS is one of the most important tools, it is necessary that the reports submitted to the Health Technology Assessment Office and other centers such as National Research Institute and Food and Drug Organization should be considered. In addition to the mentioned cases, the use of this tool is very useful for researchers who work in the field of economic evaluation to provide a standard report. Therefore, it seems necessary to hold workshops and journal clubs to introduce this tool to internal journal reviewers and researchers in this field.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
No funding to declare
Footnotes
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Stoddart GL, Torrance GW. (1987). Methods for the economic evaluation of health care programmes. ed. Oxford [Oxfordshire]; Toronto: Oxford University Press. [Google Scholar]
- 2.Nguyen HN, Ly KN, Vo QT. (2017). Assessing the quality of health economic evaluation research by cheers instrument: A critical literature review in Laos, Cambodia, and Myanmar. J App Pharm Sci, 7 (6):222–228. [Google Scholar]
- 3.Anderson R. (2010). Systematic reviews of economic evaluations: utility or futility? Health Econ, 19 (3):350–364. [DOI] [PubMed] [Google Scholar]
- 4.Husereau D, Drummond M, Petrou S, et al. (2013). Consolidated health economic evaluation reporting standards (CHEERS) statement. BJOG, 120(6):765–70. [DOI] [PubMed] [Google Scholar]
- 5.De Vet E, De Ridder D, De Wit J. (2011). Environmental correlates of physical activity and dietary behaviours among young people: a systematic review of reviews. Obes Rev, 12 (5):e130–42. [DOI] [PubMed] [Google Scholar]
- 6.Thomas H, Micucci S, Ciliska D. (2005). Effectiveness of school-based interventions in reducing adolescent risk behaviours: A systematic review of reviews. ed. Effective Public Health Practice Project (EPHPP). [Google Scholar]
- 7.Ma H, Jian W, Xu T, et al. (2016). Quality of pharmacoeconomic research in China: A systematic review. Medicine (Baltimore), 95 (41): e5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zakiyah N, van Asselt AD, Roijmans F, Postma MJ. (2016). Economic evaluation of family planning interventions in low and middle income countries; a systematic review. PLoS One, 11 (12):e0168447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banke-Thomas A, Wilson-Jones M, Madaj B, et al. (2017). Economic evaluation of emergency obstetric care training: a systematic review. BMC Pregnancy Childbirth, 17:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillespie B, Chaboyer W, Erichsen-Andersson A, et al. (2017). Economic case for intraoperative interventions to prevent surgical-site infection. Br J Surg, 104 (2):e55–e64. [DOI] [PubMed] [Google Scholar]
- 11.Hope SF, Webster J, Trieu K, et al. (2017). A systematic review of economic evaluations of population-based sodium reduction interventions. PLoS One, 12 (3):e0173600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim NH, Maruan K, Khairy HAM, et al. (2017). Economic evaluations on antimicrobial stewardship programme: a systematic review. J Pharm Pharm Sci, 20:397–406. [DOI] [PubMed] [Google Scholar]
- 13.Iribarren SJ, Cato K, Falzon L, et al. (2017). What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS One, 12 (2):e0170581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melendez-Torres G, Auguste P, Armoiry X, et al. (2017). Clinical effectiveness and cost-effectiveness of beta-interferon and glatiramer acetate for treating multiple sclerosis: systematic review and economic evaluation. Health Technol Assess, 21 (52):1–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velentzis LS, Salagame U, Canfell K. (2017). Menopausal hormone therapy: a systematic review of cost-effectiveness evaluations. BMC Health Serv Res, 17 (1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CK, Liao Q, Guo VY, et al. (2017). Cost-effectiveness analysis of vaccinations and decision makings on vaccination programmes in Hong Kong: A systematic review. Vaccine, 35 (24):3153–3161. [DOI] [PubMed] [Google Scholar]
- 17.Dritsaki M, Rivero-Arias O, Gray A, et al. (2018). What do we know about managing Dupuytren’s disease cost-effectively? BMC Musculoskelet Disord, 19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grochtdreis T, König H-H, Dobruschkin A, et al. (2018). Cost-effectiveness analyses and cost analyses in castration-resistant prostate cancer: a systematic review. PLoS One, 13 (12):e0208063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X, Ming W-K, You JH. (2019). The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res, 21 (6):e13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling X-X, Jin J-J, Zhu G-D, et al. (2019). Cost-effectiveness analysis of malaria rapid diagnostic tests: a systematic review. Infect Dis Poverty, 8 (1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendivil J, Appierto M, Aceituno S, et al. (2019). Economic evaluations of screening strategies for the early detection of colorectal cancer in the average-risk population: A systematic literature review. PLoS One, 14 (12):e0227251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sultana M, Sarker AR, Ali N, et al. (2019). Economic evaluation of community acquired pneumonia management strategies: A systematic review of literature. PLoS One, 14 (10):e0224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anopa Y, Macpherson L, McIntosh E. (2020). Systematic review of economic evaluations of primary caries prevention in 2-to 5-year-old preschool children. Value Health, 23 (8):1109–1118. [DOI] [PubMed] [Google Scholar]
- 24.De La Perrelle L, Radisic G, Cations M, et al. (2020). Costs and economic evaluations of quality improvement collaboratives in healthcare: a systematic review. BMC Health Serv Res, 20:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao X, Lou H, Bai J, et al. (2020). Cost-effectiveness analysis of Xpert in detecting Mycobacterium tuberculosis: A systematic review. Int J Infect Dis, 95:98–105. [DOI] [PubMed] [Google Scholar]
- 26.Niyomsri S, Duarte RV, Eldabe S, et al. (2020). A systematic review of economic evaluations reporting the cost-effectiveness of spinal cord stimulation. Value Health, 23 (5):656–665. [DOI] [PubMed] [Google Scholar]
- 27.Ten Ham RM, Klungel OH, Leufkens HG, Frederix GW. (2020). A review of methodological considerations for economic evaluations of gene therapies and their application in literature. Value Health, 23 (9):1268–1280. [DOI] [PubMed] [Google Scholar]
- 28.Woods T-J, Tesfay F, Speck P, Kaambwa B. (2020). Economic evaluations considering costs and outcomes of diabetic foot ulcer infections: a systematic review. PLoS One, 15 (4):e0232395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu T, Men P, Sun T, Zhai S. (2021). Cost-effectiveness of aprepitant in preventing chemotherapy-induced nausea and vomiting: a systematic review of published articles. Front Public Health, 9:660514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezapour A, Souresrafil A, Peighambari MM, et al. (2021). Economic evaluation of programs against COVID-19: A systematic review. Int J Surg, 85:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwander B, Nuijten M, Evers S, Hiligsmann M. (2021). Replication of published health economic obesity models: assessment of facilitators, hurdles and reproduction success. Pharmacoeconomics, 39:433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stawowczyk E, Kawalec P. (2018). A systematic review of the cost-effectiveness of biologics for ulcerative colitis. Pharmacoeconomics, 36:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanyal C, Husereau DR. (2019). Community-based services by pharmacists: a systematic review of cost-utility analyses. Value Health, 22 (12):1450–1457. [DOI] [PubMed] [Google Scholar]
- 34.Avanceña AL, Prosser LA. (2021). Examining equity effects of health interventions in cost-effectiveness analysis: a systematic review. Value Health, 24 (1):136–143. [DOI] [PubMed] [Google Scholar]
- 35.El Alili M, van Dongen JM, Huirne JA, et al. (2017). Reporting and analysis of trial-based cost-effectiveness evaluations in obstetrics and gynaecology. Pharmacoeconomics, 35:1007–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding H, Xin W, Tong Y, et al. (2020). Cost effectiveness of immune checkpoint inhibitors for treatment of non-small cell lung cancer: a systematic review. PLoS One, 15 (9):e0238536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werner K, Risko N, Burkholder T, et al. (2020). Cost–effectiveness of emergency care interventions in low and middle-income countries: a systematic review. Bull World Health Organ, 98 (5):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galekop MM, Uyl-de Groot CA, Redekop WK. (2021). A systematic review of cost-effectiveness studies of interventions with a personalized nutrition component in adults. Value Health, 24 (3):325–335. [DOI] [PubMed] [Google Scholar]
- 39.Husereau D, Drummond M, Augustovski F, et al. (2022). Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health, 25(1):3–9. [DOI] [PubMed] [Google Scholar]
- 40.Miroshnychenko A, Uhlman K, Malone J, et al. (2021). Systematic review of reporting quality of economic evaluations in plastic surgery based on the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. J Plast Reconstr Aesthet Surg, 74 (10):2458–2466. [DOI] [PubMed] [Google Scholar]