Abstract
Introduction
The 1-year PROspective sarilumab (preFILled syringe/pen) multinational, obsErvational (PROFILE) study evaluated the real-world effectiveness and safety of sarilumab in patients with moderate-to-severe rheumatoid arthritis (RA).
Methods
Safety endpoints included adverse events (AEs) and lab abnormalities. Effectiveness endpoints included the ACR core set. The primary endpoint was the change from baseline in Clinical Disease Activity Index (CDAI). All statistics are descriptive and p values were nominal.
Results
In total, 595 patients were treated, of whom 223 (37.5%) received sarilumab monotherapy and 372 (62.5%) received combination therapy. Upon initiation of sarilumab, an improvement in the mean (SD) CDAI score was observed at week 24 [11.4 (10.3)] and was maintained through week 52 [10.0 (10.5)], resulting in a mean [SD] reduction of −14.9 (12.7) and −14.4 (12.9), respectively. There were consistent improvements in disease activity that were similar for patients on monotherapy vs. combination therapy. An increase in the proportion of patients achieving remission and low disease activity was reported. By week 52, both groups had improved physical function and quality of life. There were no new safety signals. The proportions of any patients reporting a treatment-emergent adverse event (TEAE) or serious treatment-emergent AE (SAE) was 66.2% and 5.9%, respectively, and were similar between both treatment groups. Overall, 15.6% of patients discontinued sarilumab treatment due to TEAEs. The most commonly reported TEAE of interest was neutropenia (14.1%).
Conclusions
In this 1-year, observational real-world study, sarilumab therapy resulted in improved clinical outcomes. The safety profile was consistent with that observed in sarilumab randomized clinical trials.
This study was entered on the German website (Paul Ehrlich Institute) on January 11, 2018, with NIS No.: 423.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-024-00715-9.
Keywords: Antirheumatic agents, Patient-reported outcome measures, Quality of life, Real-world, Rheumatoid arthritis, Sarilumab
Key Summary Points
| Why carry out this study? |
| Sarilumab is approved for the treatment of adult patients with rheumatoid arthritis (RA). However, there is a need for longitudinal real-world observational studies that can confirm the information provided by randomized controlled trials. |
| The PROspective sarilumab (preFILled syringe/pen) multinational observational (PROFILE) study evaluated the real-world effectiveness and safety of sarilumab as monotherapy or in combination with csDMARDs in patients with moderate-to-severe RA in routine clinical practice. |
| What was learned from the study? |
| This observational study confirmed the safety and effectiveness of sarilumab in adult patients with RA in routine clinical practice. |
| Improvements in clinical outcomes were observed through week 52 for patients on either sarilumab mono- or combination therapy. |
| The safety profile of sarilumab was consistent with that observed in randomized clinical trials, and no new safety signals emerged. |
Introduction
Clinical remission is considered as the main therapeutic target for rheumatoid arthritis (RA) management, but low disease activity (LDA) is the best possible alternative [1]. Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) are commonly recommended as the first-line treatment for RA [1]. A combination of csDMARDs, such as methotrexate (MTX), with biologic disease-modifying antirheumatic drugs (bDMARDs) or targeted-synthetic DMARDs (tsDMARDs) is recommended as the most effective approach for the treatment of patients with RA refractive to csDMARDs alone [1]. Alternatively, tumor necrosis factor (TNF) inhibitors and treatments targeting other pathways (interleukin-6 receptor [IL-6R] blockers, Janus-kinase [JAK]-inhibitors, T-cell co-stimulation, and antiCD20 antibodies) are also considered in the treatment of RA [2].
Interleukin-6 plays an important role in the pathogenesis of RA by regulating chronic inflammation that underlies both local and systemic clinical symptoms of RA via cell signaling modulated by membrane-bound and soluble IL-6R [3, 4]. Sarilumab is a human monoclonal antibody that inhibits the binding of IL-6 to soluble and membrane-bound IL-6R-α and is approved for the treatment of adult patients with RA as monotherapy or in combination with csDMARDs [5, 6].
Although the efficacy and safety of sarilumab are established in clinical trial settings, the results may not be generalizable to real-world clinical practice owing to the strict inclusion and exclusion criteria used for the randomized controlled trials (RCTs). Therefore, there is a need for longitudinal real-world observational studies that can confirm the information provided by RCTs [7]. The PROFILE (PROspective sarilumab [preFILled syringe/pen] multinational observational) study evaluated the real-world effectiveness and safety of sarilumab with or without csDMARDs in patients with moderate-to-severe RA in routine clinical practice.
Methods
Study Design and Patients
PROFILE was an open-label, single-arm, multinational, observational, prospective, non-interventional 52-week study in adult patients with moderate-to-severe RA. The non-interventional nature of the study was ensured by enrolling patients for whom the treating physician had decided to initiate treatment independently of the study (Supplementary Material Fig. S1). Data were collected every 12 weeks after treatment initiation until week 52 (study end). If a patient prematurely discontinued sarilumab, they were asked to continue in the study unless a new bDMARD or tsDMARD was initiated. The study was conducted across 74 sites in eight countries, namely the USA, Canada, Belgium, Germany, the Netherlands, Israel, France, and Italy.
The study protocol was approved by the institutional review boards/ethics committees (IRBs/ECs), and each enrolled patient provided written informed consent. The study was conducted in accordance with the principles laid by the 18th World Medical Assembly (Helsinki, 1964) and all subsequent amendments.
Patient Population
Adult patients (≥ 18 years of age) with a diagnosis of RA who agreed to sign a written informed consent and fulfilled the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria [8] were included in the study if the treating physician had decided to initiate sarilumab before patient inclusion and independently for the purpose of the study or if they had received sarilumab treatment up to 4 weeks before study enrollment or within 8 weeks after the study enrollment.
Treatment
The prescription of sarilumab or any other therapy was under the sole responsibility of the investigator alone. Sarilumab dosing was 200 mg once every 2 weeks (q2w) subcutaneously administered in the abdomen, thigh, or upper arm with dose reduction per the label recommendation. Concomitant use of any b/tsDMARDs with sarilumab was prohibited during the study. If a switch to any b/tsDMARD was necessary, treatment with sarilumab was discontinued. Changes in csDMARDs treatment were allowed.
Effectiveness Endpoints and Assessments
Effectiveness endpoints included the ACR core set and patient reported outcomes (PROs). The primary effectiveness endpoint was the change from baseline in the Clinical Disease Activity Index (CDAI) at weeks 24 and 52. The secondary effectiveness endpoints were change from baseline in the CDAI at weeks 12 and 36, and in the Simplified Disease Activity Index (SDAI) at weeks 12, 24, 36, and 52; the proportion of patients at weeks 12, 24, 36, and 52 achieving CDAI remission (≤ 2.8); CDAI LDA (≤ 10.0) response; disease activity score (DAS)28 (for both C‐reactive protein [CRP] and erythrocyte sedimentation rate [ESR], separately) < 2.6 and < 3.2. Additionally, the Routine Assessment of Patient Index Data 3 (RAPID3) was calculated post hoc using the following equation:
Other secondary endpoints included changes in physical function as assessed by the Health Assessment Questionnaire—Disability Index (HAQ-DI) and quality of life (QoL) endpoints including morning stiffness-visual analog scale (VAS) (scale: 0 mm [no problem] to 100 mm [major problem]), pain-VAS (scale: 0 [no pain] to 100 mm [severe pain]), Patient Global Assessment (PtGA)-VAS (scale from 0 to 100 mm; higher score representing a higher disease activity), and fatigue using the 13-item Functional Assessment of Chronic Illness Therapy (FACIT-F-13) questionnaire (score: each item from 0 [not at all] to 4 [very much], with 52 as the highest possible score and a higher score representing a better QoL) and were assessed from baseline to weeks 12, 24, 36, and 52. Exploratory endpoints included reporting of extra-articular manifestations through week 52.
Safety Assessments
Safety assessments included incidence of treatment-emergent adverse events (TEAEs), serious treatment-emergent AEs (SAEs), AEs of special interest (AESI), and specific abnormalities in laboratory test results. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 24.0.
Statistical Analysis
A sample size of 1000 patients was planned to provide adequate precision of estimation in terms of 95% confidence interval (CI) for the primary and secondary effectiveness endpoints and a 95% confidence to detect any AE with an incidence of ≥ 0.3% (one patient out of 333). Effectiveness and safety analyses were conducted in all enrolled patients who had received ≥ 1 dose of sarilumab for RA (ITT population).
Patient demographics and baseline characteristics, effectiveness (primary and secondary), and safety endpoints were summarized with descriptive statistics overall and by the initial treatment regimen. For each effectiveness endpoint, either a clinical assessment or PRO, the results were presented as follows:
Responders in ITT Population
The proportion of patients in the following categories were evaluated in the ITT population.
Data Available and Responders (Observed Responders)
Patients in whom the endpoints were assessed and classified as observed responders based on the endpoint, such as achieving remission, LDA response, or minimal clinically important difference (MCID) response.
Data Available and Non-responders (Observed Non-responders)
Patients in whom the endpoints were assessed and were classified as observed non-responders.
Missing Data and Stayed on Treatment
Patients who stayed in the study and remained on sarilumab, but their endpoint measurements were not assessed (missing).
Missing Data and Discontinued Sarilumab
Patients who discontinued sarilumab and consequently their endpoint measurements were not assessed (missing).
To adjust for missing values, we estimated the proportions of responders in the ITT population with the following imputation for patients with missing data.
Missing Data and Stayed on Sarilumab (Imputed as Observed)
The response rate of patients with missing data who stayed on sarilumab treatment was estimated utilizing the same response rate as observed in the patients with available data using the following formula:
Rmissing-data/stayed-in-treatment = Robserved = (Nobserved-responders)/(Nobserved-responders + Nobserved-non-responders)
where R represents the response rate and N represents the number of patients in the cohort referenced per the subscript.
Missing Data and Discontinued Sarilumab (Imputed as Non-responders)
Patients with missing data who discontinued sarilumab treatment were imputed as non-responders.
With the imputation for the missing data above, the estimated response rate in the ITT population was calculated using the following formula:
Roverall = (Nobserved-responders + Nmissing-data/stayed-in-treatment*Robserved)/Noverall
where Noverall = Nobserved + Nmissing-data/stayed-in-treatment + Ndiscontinued-treatment
Observed Score Change from Baseline
The observed mean (SD) score change from baseline was calculated as follows: Only patients with the endpoint measurement were included in the analysis; patients with missing data, due to either sarilumab discontinuation or no assessment despite staying on the study treatment, were not included.
The changes from baseline in continuous effectiveness measures were analyzed using a mixed-effect model for repeated measures (MMRM) approach for a comparison between monotherapy and combination therapy, as well as for a comparison among the subgroups. Of note, the MMRM analysis assumed that the data were missing at random (MAR).
A sensitivity analysis was performed in which missing data in the post-baseline period were imputed by the worst value available for each patient. If only baseline values were available, the missing post-baseline values were imputed as the baseline value, which resulted in zero change from baseline. Patients without baseline assessments were regarded as missing completely at random and excluded from the analysis. After imputation, the changes from baseline in continuous effectiveness measures were analyzed using the same MMRM approach for the comparison of monotherapy with combination therapy.
As PROFILE was an observational study, all comparisons were for exploratory purposes only; no statistical significance was claimed, and the nominal p values were reported. A post hoc analysis was performed in which the p values adjusted for a false discovery rate were calculated per the Benjamini–Hochberg procedure for all the subgroup comparisons in CDAI changes from baseline [9].
Results
Patient Disposition and Baseline Characteristics
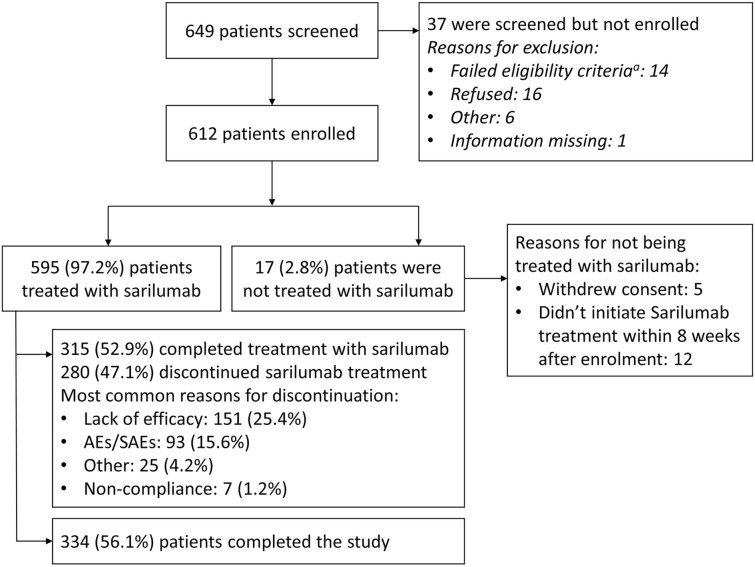
Due to the COVID-19 pandemic, patient recruitment slowed, and the enrollment stopped before reaching the planned 1000 patients. Of the 649 patients screened, 612 patients were enrolled by May 15, 2020, and met all eligibility criteria to participate in the study (Fig. 1).
Fig. 1.
Patient disposition. AEs adverse events, SAEs serious adverse events. aA patient might have two or multiple failed eligibility criteria
In total, 595 patients received at least one dose of sarilumab and were included in the analyses, with 223 (37.5%) patients receiving sarilumab monotherapy and 372 (62.5%) patients receiving combination therapy; 315 (52.9%) completed treatment with sarilumab and 280 (47.1%) discontinued treatment early. The most commonly observed reasons for treatment discontinuation were lack of efficacy (151 [25.4%]), AEs/SAEs (93 [15.7%]), and others (25 [4.2%]).
The mean (SD) age of the treated population was 57.6 (11.8) years. Three-quarters of the treated population were female (n = 448, 75.3%), and most patients were Caucasian (n = 503, 84.5%). Overall, two-thirds of patients were overweight (defined as body mass index [BMI] ≥ 25 and < 30 kg/m2: n = 173/548, 31.6%) or obese (BMI ≥ 30 kg/m2: 198/548, 36.1%).
Most baseline demographic and disease characteristics were similar among patients from both treatment groups except for patients on monotherapy who had a slightly longer mean duration of disease than patients on combination therapy (11.2 vs. 9.3 years; Table 1). The mean disease activity scores, including CDAI, SDAI, swollen joint count (SJC) 28, tender joint count (TJC) 28, DAS28-CRP, and DAS28-ESR, at baseline were similar between patients on sarilumab as monotherapy or combination therapy.
Table 1.
Baseline demographics and disease characteristics
| Monotherapy (N = 223) | Combination therapy (N = 372) | All treated (N = 595) | |
|---|---|---|---|
| Age, mean (SD), years | 57.9 (12.6) | 57.5 (11.4) | 57.6 (11.8) |
| Female, n (%) | 178 (79.8) | 270 (72.6) | 448 (75.3) |
| Caucasians, n (%) | 198 (88.8) | 305 (82.0) | 503 (84.5) |
| N = 212 | N = 353 | N = 565 | |
| Weight, mean (SD), kg | 77.7 (19.3) | 81.6 (18.6) | 80.1 (18.9) |
| N = 205 | N = 343 | N = 548 | |
| BMI, mean (SD), kg/m2 | 27.9 (6.4) | 29.2 (6.8) | 28.7 (6.7) |
| BMI group (kg/m2) | |||
| < 25, n (%) | 70 (34.1) | 107 (31.2) | 177 (32.3) |
| ≥ 25 and < 30, n (%) | 69 (33.7) | 104 (30.3) | 173 (31.6) |
| ≥ 30, n (%) | 66 (32.2) | 132 (38.5) | 198 (36.1) |
| Missing, n (%) | 18 | 29 | 47 |
| N = 218 | N = 367 | N = 585 | |
| Duration of RA, mean (SD), years | 11.2 (10.6) | 9.3 (8.6) | 10.0 (9.4) |
| RA severity (at study start)a | N = 222 | N = 372 | N = 594 |
| Moderate, n (%) | 157 (70.7) | 259 (69.6) | 416 (70.0) |
| Severe, n (%) | 65 (29.3) | 113 (30.4) | 178 (30.0) |
| CDAI, mean (SD) | N = 201 | N = 332 | N = 533 |
| 26.0 (13.0) | 27.2 (13.6) | 26.7 (13.4) | |
| SDAI, mean (SD) | N = 164 | N = 292 | N = 456 |
| 27.4 (13.8) | 28.2 (13.7) | 27.9 (13.7) | |
| SJC (28), mean (SD) | N = 218 | N = 365 | N = 583 |
| 5.9 (5.3) | 6.6 (5.8) | 6.3 (5.6) | |
| TJC (28), mean (SD) | N = 218 | N = 365 | N = 583 |
| 8.8 (7.0) | 9.2 (7.1) | 9.1 (7.1) | |
| PtGA (mm), mean (SD) | N = 207 | N = 343 | N = 550 |
| 60.4 (23.6) | 57.0 (24.9) | 58.3 (24.5) | |
| HAQ-DI score (0–3), mean (SD) | N = 208 | N = 346 | N = 554 |
| 1.5 (0.7) | 1.4 (0.7) | 1.4 (0.7) | |
| DAS28-CRP, mean (SD) | N = 168 | N = 297 | N = 465 |
| 4.6 (1.3) | 4.7 (1.2) | 4.7 (1.2) | |
| DAS28-ESR, mean (SD) | N = 149 | N = 252 | N = 401 |
| 4.8 (1.5) | 4.9 (1.3) | 4.9 (1.4) | |
| Medications | |||
| Prior RA medicationsb, n (%) | 217 (97.3) | 371 (99.7) | 588 (98.8) |
| csDMARDs, n (%) | 178 (79.8) | 369 (99.2) | 547 (91.9) |
| bDMARDs, n (%) | 133 (59.6) | 198 (53.2) | 331 (55.6) |
| tsDMARDs, n (%) | 48 (21.5) | 47 (12.6) | 95 (16.0) |
| Number of prior b/ts DMARDs | |||
| 0, n (%) | 81 (36.3) | 168 (45.2) | 249 (41.8) |
| 1, n (%) | 46 (20.6) | 84 (22.6) | 130 (21.8) |
| 2, n (%) | 43 (19.3) | 62 (16.7) | 105 (17.6) |
| ≥ 3, n (%) | 53 (23.8) | 58 (15.6) | 111 (18.7) |
| Prior RA medications ongoing at baseline and continuedc, n (%) | 127 (57.0) | 368 (98.9) | 495 (83.2) |
| csDMARDs, n (%) | 0 (0) | 365 (98.1) | 365 (61.3) |
| Methotrexate, n (%) | 0 (0) | 279 (75.0) | 279 (46.9) |
| NSAIDs, n (%) | 52 (23.3) | 118 (31.7) | 170 (28.6) |
| Others, n (%) | 43 (19.3) | 133 (35.8) | 176 (29.6) |
| Prednisone equivalent daily dose at baseline, mgd | |||
| n (%) | 73 (32.7) | 154 (41.4) | 227 (38.2) |
| Mean (SD) | 9.0 (6.9) | 8.5 (9.9) | 8.6 (9.0) |
| Concomitant RA medications started after sarilumab initiation, n (%) | 61 (27.4) | 123 (33.1) | 184 (30.9) |
| csDMARDs, n (%) | 10 (4.5) | 54 (14.5) | 64 (10.8) |
| NSAIDs, n (%) | 10 (4.5) | 18 (4.8) | 28 (4.7) |
| Corticosteroids, n (%) | 51 (22.9) | 86 (23.1) | 137 (23.0) |
| Others, n (%) | 9 (4.0) | 16 (4.3) | 25 (4.2) |
bDMARDs biologic DMARD, BMI body mass index, CDAI Clinical Disease Activity Index, CRP C-reactive protein, csDMARDs conventional synthetic DMARD, DAS28 disease activity score in 28 joints, DMARD disease-modifying anti-rheumatic drug, ESR erythrocyte sedimentation rate, HAQ-DI Health Assessment Questionnaire—Disability Index, N number of patients, n number of patients, NSAID nonsteroidal anti-inflammatory drug, PtGA Patient Global Assessment, RA rheumatoid arthritis, SD standard deviation, SDAI Simplified Disease Activity Index, SJC swollen joint count, TJC tender joint count, tsDMARD targeted synthetic DMARD, VAS visual analog scale
aAs per the investigator’s judgement
bPrior RA medications that had been discontinued before sarilumab initiation or were ongoing at the time of sarilumab initiation
cPrior RA medications that were ongoing at baseline and continued after sarilumab initiation
dPrednisone equivalent daily dose at baseline = (total prednisone equivalent doses in 4 weeks prior to sarilumab initiation [mg, not including the date of sarilumab initiation])/28
In total, 588 (98.8%) patients were treated with RA medications that had been discontinued before or were ongoing at the time of sarilumab initiation. There were 331 (55.6%) patients previously treated with bDMARDs (monotherapy group: 133 [59.6%]; combination therapy group: 198 [53.2%]) and 95 (16.0%) patients had received tsDMARDs (monotherapy group: 48 [21.5%]; combination therapy group: 47 [12.6%]). A higher proportion of patients on sarilumab monotherapy were previously on ≥ 3 b/tsDMARDs before sarilumab initiation compared with the combination therapy group (23.8 vs. 15.6%). Overall, about one-third of patients (monotherapy: 61 [27.4%]; combination therapy: 123 [33.1%]) received additional concomitant medication for RA after initiation of sarilumab (Table 1).
Sarilumab Exposure
The total cumulative exposure to sarilumab during the study period was 443.4 years among a total of 595 patients, of which 165.9 and 277.4 patient-years were accumulated for monotherapy and combination therapy groups, respectively (Supplementary Material Table S1). The mean (SD) sarilumab treatment duration was 38.9 (20.0) weeks and was comparable between monotherapy and combination therapy (38.8 [21.3] vs. 38.9 [19.2], respectively). The proportion of patients persistent with sarilumab therapy for the duration of the study was 52.9% (n = 315), while 24.4% (n = 145) switched to another bDMARD or tsDMARD. The rate of sarilumab treatment discontinuation was relatively steady through the first 18 weeks and then decreased slightly for both the treatment groups (Supplementary Material Fig. S2).
Effectiveness Outcomes
CDAI
CDAI Remission (CDAI ≤ 2.8)
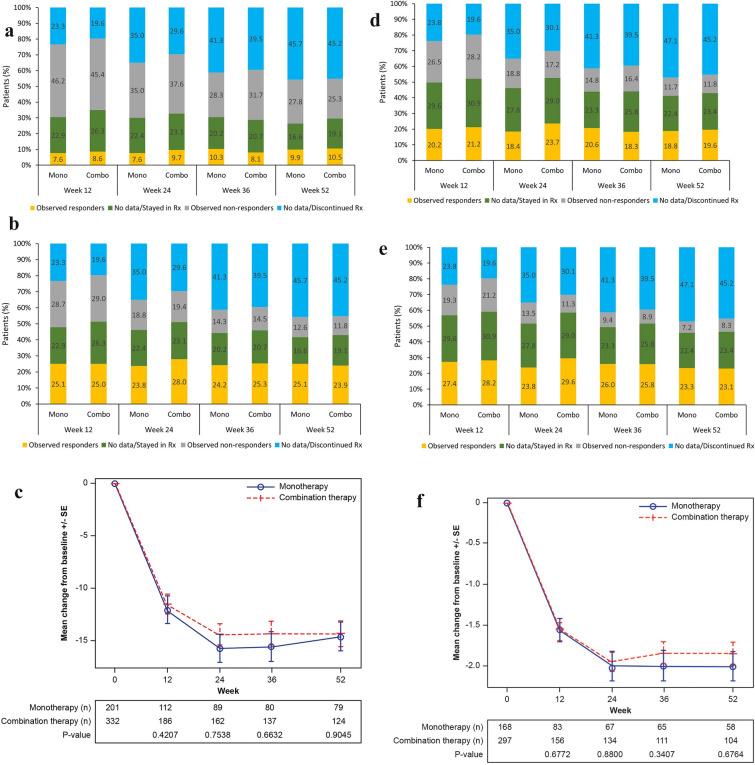
In the ITT population, 8.2% of patients were observed responders at week 12 and 10.3% at week 52; 25.0% of patients stayed on sarilumab treatment without a CDAI assessment (missing data) at week 12 and 18.2% at week 52. The proportion of patients in each of these two categories were stable across the study visits (Fig. 2a).
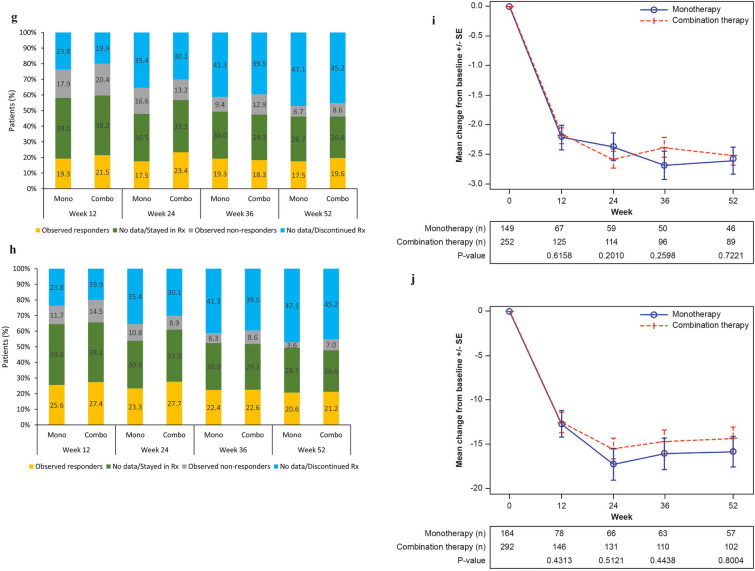
Fig. 2.
Observed remission/LDA and missing data in ITT population, and observed mean change from baseline in patients with data available. a CDAI remission (CDAI ≤ 2.8) observed and missing data. b CDAI LDA (CDAI ≤ 10) observed and missing data. c Observed CDAI mean score change from baseline. d DAS28-CRP remission (DAS28-CRP < 2.6) observed and missing data. e DAS28-CRP LDA (DAS28-CRP < 3.2) observed and missing data. f Observed DAS28-CRP mean score change from baseline. g DAS28-ESR remission (DAS28-ESR < 2.6) observed and missing data. h DAS28-ESR LDA (DAS28-ESR < 3.2) observed and missing data. i Observed DAS28-ESR mean score change from baseline. j Observed SDAI mean score change from baseline. CDAI Clinical Disease Activity Index, CRP C-reactive protein, DAS28 disease activity score in 28 joints, ESR erythrocyte sedimentation rate, LDA low disease activity, MMRM mixed-effect model for repeated measures, Rx treatment, SDAI Simplified Disease Activity Index, SE, standard error. In each figure of the observed mean change from baseline, the mean and SE at each visit were calculated based on the observed data at the visit; the p values for a comparison between monotherapy and combination therapy were calculated using an MMRM approach that included the initial treatment regimen, visit, and initial treatment regimen-by-visit interaction as fixed effects and the baseline value as a covariate
The proportion of patients who were observed as non-remission decreased from 45.7% at week 12 to 26.2% at week 52. However, the proportion of patients who discontinued sarilumab treatment increased from 21.0% at week 12 to 45.4% at week 52. This change was consistent with the scenario that the non-responders tended to discontinue treatment (Fig. 2a).
With the specified imputation, the estimated CDAI remission rate in the ITT population was 12.1% at week 12 and 15.4% at week 52. The proportions were stable across the study visits. The estimated remission rates were similar between the two cohorts of monotherapy and combination therapy (Fig. 2a and Supplementary Material Fig. S3).
CDAI LDA (CDAI ≤ 10.0)
In the ITT population, 25.0% and 24.4% of patients were observed achieving CDAI LDA at week 12 and week 52, respectively, and the proportions were stable across the study visits. With the specified imputation, the estimated CDAI LDA rate was 36.7% at week 12 and 36.5% at week 52, and the proportions were stable across the study visits. These proportions were similar between the cohorts of monotherapy and combination therapy (Fig. 2b and Supplementary Material Fig. S3).
CDAI Change from Baseline over 52 weeks
Among patients (n = 533/595) with available scores in the ITT population, the mean (SD) CDAI score was 26.7 (13.4) at baseline. Upon initiation of sarilumab, an improvement in the mean (SD) CDAI score (14.4 [12.5]) was observed at week 12 with a mean (SD) reduction of −11.7 (13.6). The observed mean (SD) CDAI score decreased to 11.4 (10.3) at week 24, 10.9 (11.0) at week 36, and 10.0 (10.5) at week 52, resulting in a mean (SD) reduction of −14.9 (12.7), −14.7 (13.0), and −14.4 (12.9), respectively from baseline.
Similarly, a reduction in the CDAI at week 12 was also observed for sarilumab either as monotherapy or combination therapy (mean [SD] change from baseline: −12.1 [14.0] vs. −11.5 [13.3], respectively); further an additional moderate reduction was observed at week 24 (−15.7 [12.9] vs. −14.4 [12.6]) and was observed through week 52 (−14.6 [12.4] vs. −14.3 [13.3], respectively; Fig. 2c).
Under the assumption of data MAR, the data were analyzed based on MMRM model, and the results showed a data pattern similar to Fig. 2c.
The sensitivity analysis showed a pattern of CDAI change from baseline similar to the observed CDAI, except for a smaller mean reduction from baseline. This was expected because of imputation by the worst value, especially with a sizable number of patients being imputed with zero reduction from baseline due to no post-baseline assessments. Additionally, the mean reduction from baseline was stable from weeks 12–52 because of the additional moderate reduction at weeks 24–52, which was offset by additional missing data being imputed by the worst value due to treatment discontinuation. The mean CDAI changes from baseline were similar across monotherapy and combination therapy (data not shown).
A pattern similar to that of CDAI was observed for all other effectiveness endpoints (DAS-28 CRP, DAS-28 ESR, and SDAI) (Fig. 2d-j and Supplementary Material Fig. S3).
Routine Assessment of Patient Index Data 3 and PRO scores
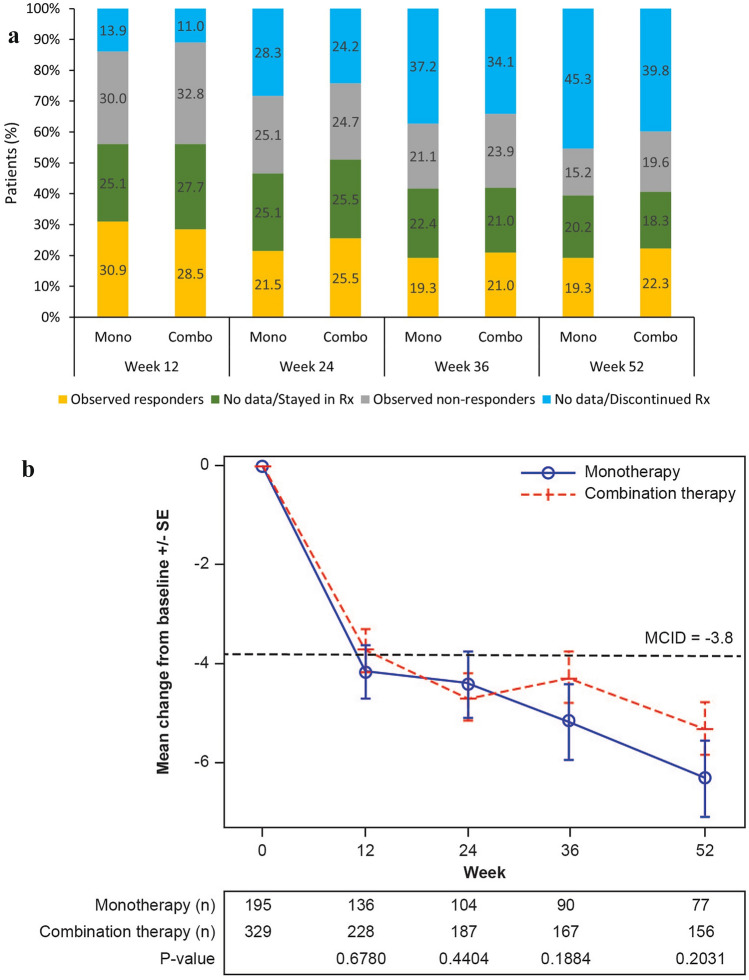
In the ITT population, the observed proportion of patients achieving the MCID of RAPID3 was 29.4% at week 12 and 21.2% at week 52. The estimated proportions achieving the MCID of RAPID3 with the specified imputation were 42.3% at week 12 and 31.4% at week 52. A consistent improvement in the as observed population and a slight decrease in patients achieving MCID over time were reported for RAPID3 (Fig. 3a-b and Supplementary Material Fig. S4j).
Fig. 3.
Observed RAPID3 MCID response and missing data in ITT population, and observed mean change from baseline in patients with data available. a RAPID3 change from baseline ≤ −3.8 (MCID) observed and missing data. b Observed RAPID3 mean score change from baseline. MMRM mixed-effect model for repeated measures, RAPID3 routine assessment of patient index data 3, Rx treatment, SE standard error. The mean and SE at each visit were calculated based on the observed data at the visit; the p values for a comparison between monotherapy and combination therapy were calculated using an MMRM approach that included the initial treatment regimen, visit, and initial treatment regimen-by-visit interaction as fixed effects and the baseline value as a covariate
A similar pattern was observed for all other PROs (HAQ-DI, FACIT-Fatigue, Pain-VAS, and morning stiffness; Supplementary Material Fig. S4a–k).
Subgroup Analyses: Clinical Disease Activity Index Change from Baseline by Subgroups
When adjusted for baseline values, noticeable differences (p < 0.05) in the CDAI mean change from baseline was observed through week 52 between countries and among patients with/without prior tsDMARDs (Supplementary Material Fig. S5). Also, a noticeable difference (p < 0.05) in the CDAI mean change from baseline was observed among patients with/without prior bDMARDs and on the number of prior bDMARDs through week 36 and with/without prior tumor necrosis factor inhibitor (TNFi) and in the number of prior TNFis through week 24.
In the post hoc analysis, the p values adjusted for a false discovery rate were less than 0.05 for the differences in the CDAI mean change from baseline at weeks 12, 24, and 36 between countries; at weeks 36 and 52 among patients with/without prior tsDMARDs; at weeks 12 and 24 among patients with/without prior bDMARDs, on the number of prior bDMARDs, and with/without prior TNFi; and among patients on the number of prior TNFis at week 24 (data not shown).
However, without adjustment for the CDAI baseline value, overall, the mean change from baseline in the CDAI score was similar among patients (Supplementary Material Fig. S6a–d). The incidence of sarilumab discontinuations increased with the number of prior bDMARDs (Supplementary Material Fig. S7).
Safety Outcomes
The proportions of patients reporting any TEAE (monotherapy: 65.0% [145/223]; combination therapy: 66.9% [249/372]) or treatment emergent SAEs (monotherapy: 5.4% [12/223]; combination therapy: 6.2% [23/372]) were similar between both treatment groups (Table 2). Two patients died of TEAEs during the study: one patient on monotherapy died of renal failure and another patient with cardiac and pulmonary comorbidities receiving combination therapy died of acute respiratory failure. Overall, 15.6% of patients discontinued sarilumab treatment due to TEAEs.
Table 2.
Safety
| Monotherapy (N = 223) |
Combination therapy (N = 372) |
All (N = 595) |
|
|---|---|---|---|
| Patients with any TEAEa, n (%) | 145 (65.0) | 249 (66.9) | 394 (66.2) |
| Patients with any treatment emergent SAE, n (%) | 12 (5.4) | 23 (6.2) | 35 (5.9) |
| Patients with any TEAE leading to deathb,c, n (%) | 1 (0.4) | 1 (0.3) | 2 (0.3) |
| Patients with any TEAE leading to sarilumab discontinuation, n (%) | 34 (15.2) | 59 (15.9) | 93 (15.6) |
| Adverse events of interest | |||
| Neutropenia, n (%) | 24 (10.8) | 60 (16.1) | 84 (14.1) |
| Thrombocytopenia and potential risk of bleeding, n (%) | 12 (5.4) | 27 (7.3) | 39 (6.6) |
| Serious infections, n (%) | 3 (1.3) | 5 (1.3) | 8 (1.3) |
| Herpes zoster, n (%) | 6 (2.7) | 8 (2.2) | 14 (2.4) |
| Tuberculosis, n (%) | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Hepatic disorders, including LFT elevations, n (%) | 8 (3.6) | 23 (6.2) | 31 (5.2) |
| Confirmed upper/Lower GI perforationsd, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Elevation in lipids, n (%) | 18 (8.1) | 29 (7.8) | 47 (7.9) |
| Cardiovascular adverse eventse, n (%) | 2 (0.9)e | 4 (1.1)f | 6 (1.0) |
| MACE, n (%) | 0 (0.0) | 2 (0.5) | 2 (0.3) |
| Hypersensitivity, n (%) | 19 (8.5) | 24 (6.5) | 43 (7.2) |
| Anaphylaxis, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection site reactions, n (%) | 23 (10.3) | 34 (9.1) | 57 (9.6) |
| Malignancyg, n (%) | 0 (0.0) | 2 (0.5) | 2 (0.3) |
| Malignancy excluding NMSC, n (%) | 0 (0.0) | 2 (0.5) | 2 (0.3) |
| DVT/Pulmonary embolismh, n (%) | 1 (0.4) | 1 (0.3) | 2 (0.3) |
| Pregnancyi, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Symptomatic overdosei, n (%) | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Changes from baseline at week 52 | |||
| ANC (Giga/l), mean (SD) |
N = 46 −2.9 (2.5) |
N = 105 −1.8 (2.2) |
N = 151 −2.2 (2.3) |
| Hemoglobin (g/l), mean (SD) |
N = 77 8.9 (14.0) |
N = 143 5.6 (11.1) |
N = 220 6.8 (12.3) |
| Hba1c (%), mean (SD) |
N = 10 0.02 (0.5) |
N = 26 −0.2 (0.6) |
N = 36 −0.1 (0.6) |
| ANC grade by week 52 | N = 56 | N = 125 | N = 181 |
| Grade 0: ≥ 2.0 Giga/l, n (%) | 36 (64.3) | 90 (72.0) | 126 (69.6) |
| Grade 1: ≥ 1.5 to < 2.0 Giga/l, n (%) | 12 (21.4) | 23 (18.4) | 35 (19.3) |
| Grade 2: ≥ 1.0 to < 1.5 Giga/l, n (%) | 7 (12.5) | 9 (7.2) | 16 (8.8) |
| Grade 3: ≥ 0.5 to < 1.0 Giga/l, n (%) | 1 (1.8) | 3 (2.4) | 4 (2.2) |
| Grade 4: < 0.5 Giga/l, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ALT grade by week 52 | N = 85 | N = 153 | N = 238 |
| ≤ ULN, n (%) | 71 (83.5) | 124 (81.0) | 195 (81.9) |
| > 1 to 3 × ULN, n (%) | 13 (15.3) | 27 (17.6) | 40 (16.8) |
| > 3 to 5 × ULN, n (%) | 1 (1.2) | 2 (1.3) | 3 (1.3) |
| > 5 × ULN, n (%) | 0 (0.0) | 0 (0.0) | 0 (0) |
ALT alanine transaminase, ANC absolute neutrophil count, bDMARDs biologic disease-modifying antirheumatic drugs, LFT liver function test, MACE major adverse cardiac event, N number of patients, NMSC non-melanoma skin cancer, SAE serious adverse events, SD standard deviation, TEAE treatment-emergent adverse events, TIA transient ischemic attack, tsDMARDs targeted synthetic disease-modifying antirheumatic drugs, ULN upper limit of normal
aAny AE that developed or worsened during the time from the first dose of the study treatment to the end of study or the date of last dose plus 60 days, whichever is earlier
bTEAEs leading to death: renal failure (worsening of renal failure); acute respiratory failure (acute on chronic hypoxemic respiratory failure)
cThere was one additional death due to a post-treatment AE (lung neoplasm malignant [lung cancer])
dPotential upper/lower GI perforations: anal fistula
eDistal left anterior descending artery stenosis and TIA (n = 2)
fAcute myocardial infarction, angina pectoris, myocardial infarction, and stroke (n = 4)
gMalignancy: Lung neoplasm malignant; malignant melanoma/basal cell carcinoma
hDVT/PE: Deep vein thrombosis; thrombophlebitis
iAdverse event of special interest
The most commonly reported TEAEs of interest were neutropenia (14.1%), followed by injection site reactions (9.6%), elevation in lipids (7.9%), hypersensitivity (7.2%), and thrombocytopenia and potential risk of bleeding (6.6%). In approximately half (46.7%) of the patients with neutropenia, the dose was not changed. In the other half of the patients, action taken for neutropenia included dose reduction (21.5%), drug interruption (16.3%), and drug withdrawal (14.1%). A lower proportion of patients receiving monotherapy vs. combination therapy reported neutropenia (10.8 vs. 16.1%), thrombocytopenia and potential risk of bleeding (5.4 vs. 7.3%), and hepatic disorder (3.6 vs. 6.2%) as AEs of interest. Whereas a similar proportion of patients on monotherapy vs. combination therapy reported herpes zoster infection (2.7 vs. 2.2%), elevated lipids (8.1 vs. 7.8%), hypersensitivity (8.5 vs. 6.5%), and injection site reactions (10.3 vs. 9.1%). At the end of the study (week 52), the overall observed alanine aminotransferase (ALT) elevation between 1 and 3× upper limit of normal (ULN) was 16.8% (monotherapy: 15.3% (13/85); combination therapy: 17.6% [27/153]); no ALT elevations (> 5× ULN) were observed.
Discussion
In this real-world study, sarilumab therapy resulted in improved clinical outcomes as assessed by reduced CDAI, a consistent proportion of patients achieving remission and LDA, improved physical function as evaluated by the HAQ-DI, and improved QoL with no new safety signals by week 52.
The study enrolled patients from daily clinical practice with a wide age range (23–88 years) and almost no restrictions on the previous classes of medications and concomitant medications. These data are consistent with those from the sarilumab phase 3 RCTs, which demonstrated the efficacy and safety of sarilumab as monotherapy or in combination with csDMARDs in patients with moderate to severe RA [10, 11]. Overall, and in all subgroups, a decrease in the mean CDAI score at week 12 and an improvement at weeks 24, 36, and 52 with minor differences in the treatment regimen support the effectiveness of sarilumab with or without csDMARDs.
Another phase 3 study in Japanese patients with active RA (HARUKA) reported that sarilumab either as monotherapy or in combination with non-MTX csDMARDs was equally effective and showed similar response rates, DAS28-CRP < 2.6, and improved physical function measured by HAQ-DI [12]. A post hoc analysis of MONARCH (patients from the sarilumab monotherapy arm) and MOBILITY (patients from the sarilumab plus MTX treatment arm) studies demonstrated that the least squares mean change from baseline for all assessments (CDAI, DAS28-CRP, Hb, Pain-VAS, and FACIT-F) were similar between the treatment arms [13]. Previously published studies on another IL-6R inhibitor, tocilizumab, have demonstrated a similar efficacy and safety profile when used as monotherapy, add-on, or in combination with DMARDs or MTX in patients with RA [14–17].
Although a combination of MTX and bDMARDs is recommended for better clinical outcomes, data from real-life registries report that about one-third of patients with RA take bDMARDs as monotherapy owing to intolerance or contraindication to MTX [18]. Additionally, real-world data across multiple countries indicate that biologic monotherapy is recommended by physicians or because of patients’ preference [18–21]. Taken together, monotherapy of IL-6R inhibitors (sarilumab and tocilizumab) can be clinically effective, aid in defining treatment strategies, and may improve treatment compliance in patients who are intolerant to or do not prefer MTX.
If left untreated, RA leads to functional decline, pain, impaired health-related QoL, and early mortality [22]. This necessitates a regular assessment of the impact of RA on the overall QoL and, thus, appropriate management [23]. Sarilumab therapy resulted in sustained improvements in the HAQ-DI, FACIT-fatigue, Pain-VAS, and morning stiffness scores. As the difference in mean change from baseline between the two treatment groups was minimal for all PROs, these were reflected in all clinical outcomes after sarilumab treatment translating into overall patient benefits for both treatment groups, which aligns with the results of previously published studies [10–12]. The safety and tolerability profiles of sarilumab as mono- and combination therapy were comparable. The TEAEs of interest were neutropenia and injection site reactions and were consistent with those reported in previously published phase 3 studies [10, 11]. The most commonly observed reasons for treatment discontinuation were lack of efficacy, AEs/SAEs, and other. Previous studies have reported that approximately 40–53% of patients on their first biologic and 47–55% of patients on their ≥ 2 biologics discontinued or switched to a different biologic within 1 year [24–26].
For the response analysis, missing data of patients who discontinued the study treatment were imputed as non-responders, whereas missing data from patients who stayed on study treatment were imputed based on the observed response rate of those who were assessed. Considering a patient who discontinued treatment as a non-responder is a common practice in RA clinical studies. It is reasonable to assume that the response rate of patients who stayed in the study but with missing data is similar to those patients without missing data. After imputing for missing data, similar response rates were observed in patients on mono- and combination therapy.
The limitations of the PROFILE study include the open-label design, which may potentially introduce bias because clinical assessments were not blinded and treatment was not randomized. To account for dropouts, we used an MMRM analysis for continuous variables, which was valid under the assumption of data being MAR. The results were supported by the sensitivity analysis with missing data being imputed by the worst value. Also, the 52-week follow-up may limit the overall interpretation of long-term effectiveness and safety between monotherapy and combination therapy. The study did not document reasons for discontinuation of prior treatment or bDMARDs to explore further the relationship between patient- or disease-related factors and clinical outcomes. Radiographic outcomes were not assessed. The study was not designed to report the superiority of either of the treatment regimens. With the enrollment stopped before reaching the planned 1000 patients, the sample size of 595 treated patients still had adequate precision for the summary statistics. However, the power to detect an uncommon adverse event (0.3%) was reduced to 83% from the planned 95%.
Conclusions
In this 1-year, observational, real-world study, sarilumab therapy resulted in improved clinical outcomes. Little difference was observed between the outcomes of patients treated with sarilumab as monotherapy and combination therapy. The safety profile observed in this routine clinical practice setting is consistent with that in sarilumab randomized clinical trials, and no new safety signals emerged.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing and Editorial Assistance
Medical writing support for this manuscript was provided by Ashwini Atre (former Sanofi employee), Sanjeev Kallapari and Chiranjit Ghosh of Sanofi according to the Good Publication Practice guideline. Patient recruitment, data collection, and analyses were performed by IQVIA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Alan Kivitz, Jacques Eric Gottenberg, Martin Bergman, Chunfu Qiu, Hubert van Hoogstraten, Ron de Nijs and Louis Bessette were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Study conception and design: Alan Kivitz, Jacques Eric Gottenberg, Chunfu Qiu, Hubert van Hoogstraten, Ron de Nijs, and Louis Bessette; acquisition of data: Alan Kivitz, Chunfu Qiu, Hubert van Hoogstraten, Ron de Nijs, and Louis Bessette; analysis and interpretation of data: Alan Kivitz, Jacques Eric Gottenberg, Martin Bergman, Chunfu Qiu, Hubert van Hoogstraten, Ron de Nijs, and Louis Bessette.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Sanofi, Bridgewater, NJ, USA and Regeneron.
Data Availability
Qualified researchers may request access to patient-level data and related documents [including, e.g., the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications]. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
Declarations
Conflict of Interest
Alan Kivitz: Shareholder of Pfizer, Sanofi, GlaxoSmithKline, Gilead Sciences, Inc., and Novartis; paid consultant for AbbVie, Boehringer Ingelheim, Flexion, Janssen, Pfizer, Sanofi, Regeneron, Sun Pharma Advanced Research, and Gilead Sciences, Inc.; speaker and/or a member of speakers’ bureau for Celgene, Merck, Lilly, Novartis, Pfizer, Sanofi, Flexion, and AbbVie. Jacques Eric Gottenberg: Received research grants from Bristol-Myers Squibb, Pfizer, and Roche and speaking/consulting fees from AbbVie, Bristol-Myers Squibb, Chugai, Galapagos, Gilead, Janssen, Lilly, MSD, Pfizer, Sanofi, Roche, and UCB. Martin Bergman: Consultant, advisor, and member of speakers’ bureaus for AbbVie, Amgen, BMS, Genentech/Roche, Gilead, GSK, Janssen, Merck, Novartis, Pfizer, Sandoz, and Sanofi/Regeneron; and a shareholder of Johnson and Johnson (parent company of Janssen). Chunfu Qiu and Hubert van Hoogstraten: Employees of Sanofi and may hold stock and/or stock options in the company. Ron de Nijs has nothing to disclose. Louis Bessette: Received research grants and speaker and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB.
Ethical Approval
The study protocol was approved by the institutional review boards/ethics committees (IRBs/ECs), and each enrolled patient provided written informed consent. The study was conducted in accordance with the principles laid by the 18th World Medical Assembly (Helsinki, 1964) and all subsequent amendments.
References
- 1.Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99. [DOI] [PubMed] [Google Scholar]
- 2.Mankia K, Di Matteo A, Emery P. Prevention and cure: The major unmet needs in the management of rheumatoid arthritis. J Autoimmun. 2020;110: 102399. [DOI] [PubMed] [Google Scholar]
- 3.Favalli EG. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: a comprehensive review of IL-6 inhibition for the management of rheumatoid arthritis. Rheumatol Ther. 2020;7(3):473–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2008;4(4):767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvarani C, Cantini F, Niccoli L, Macchioni P, Consonni D, Bajocchi G, et al. Acute-phase reactants and the risk of relapse/recurrence in polymyalgia rheumatica: a prospective follow-up study. Arthritis Rheum. 2005;53(1):33–8. [DOI] [PubMed] [Google Scholar]
- 6.van der Geest KS, Abdulahad WH, Rutgers A, Horst G, Bijzet J, Arends S, et al. Serum markers associated with disease activity in giant cell arteritis and polymyalgia rheumatica. Rheumatology (Oxford). 2015;54(8):1397–402. [DOI] [PubMed] [Google Scholar]
- 7.Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and Impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35(5):498–502. [DOI] [PubMed] [Google Scholar]
- 9.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 10.Fleischmann R, van Adelsberg J, Lin Y, Castelar-Pinheiro GD, Brzezicki J, Hrycaj P, et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol (Hoboken, NJ). 2017;69(2):277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genovese MC, Fleischmann R, Kivitz AJ, Rell-Bakalarska M, Martincova R, Fiore S, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol (Hoboken, NJ). 2015;67(6):1424–37. [DOI] [PubMed] [Google Scholar]
- 12.Kameda H, Wada K, Takahashi Y, Hagino O, van Hoogstraten H, Graham N, et al. Sarilumab monotherapy or in combination with non-methotrexate disease-modifying antirheumatic drugs in active rheumatoid arthritis: a Japan phase 3 trial (HARUKA). Mod Rheumatol. 2020;30(2):239–48. [DOI] [PubMed] [Google Scholar]
- 13.Burmester GR, Bykerk VP, Buch MH, Tanaka Y, Kameda H, Praestgaard A, et al. Sarilumab monotherapy versus sarilumab and methotrexate combination therapy in patients with rheumatoid arthritis. Rheumatology (Oxford). 2021. [DOI] [PMC free article] [PubMed]
- 14.Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bykerk VP, Ostor AJ, Alvaro-Gracia J, Pavelka K, Roman Ivorra JA, Graninger W, et al. Comparison of tocilizumab as monotherapy or with add-on disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and inadequate responses to previous treatments: an open-label study close to clinical practice. Clin Rheumatol. 2015;34(3):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrold LR, Reed GW, Best J, Zlotnick S, Kremer JM. Real-world comparative effectiveness of tocilizumab monotherapy vs. tumor necrosis factor inhibitors with methotrexate in patients with rheumatoid arthritis. Rheumatol Ther. 2018;5(2):507–23. [DOI] [PMC free article] [PubMed]
- 17.Pappas DA, Blachley T, Zlotnick S, Best J, Emeanuru K, Kremer JM. Methotrexate discontinuation and dose decreases after therapy with tocilizumab: results from the Corrona rheumatoid arthritis registry. Rheumatol Ther. 2020;7(2):357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emery P, Sebba A, Huizinga TW. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann Rheum Dis. 2013;72(12):1897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Backhaus M, Kaufmann J, Richter C, Wassenberg S, Roske AE, Hellmann P, et al. Comparison of tocilizumab and tumour necrosis factor inhibitors in rheumatoid arthritis: a retrospective analysis of 1603 patients managed in routine clinical practice. Clin Rheumatol. 2015;34(4):673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas DA, Reed GW, Saunders K, John A, Shewade A, Greenberg JD, et al. Characteristics associated with biologic monotherapy use in biologic-naive patients with rheumatoid arthritis in a US registry population. Rheumatol Ther. 2015;2(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detert J, Klaus P. Biologic monotherapy in the treatment of rheumatoid arthritis. Biologics. 2015;9:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlig T, Moe RH, Kvien TK. The burden of disease in rheumatoid arthritis. Pharmacoeconomics. 2014;32(9):841–51. [DOI] [PubMed] [Google Scholar]
- 23.Matcham F, Scott IC, Rayner L, Hotopf M, Kingsley GH, Norton S, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–30. [DOI] [PubMed] [Google Scholar]
- 24.Bonafede MJB, Tang DH, Harrison DJ, Stolshek BS. Compliance and cost of biologic therapies for rheumatoid arthritis. Am J Pharm Benefits. 2017;9(5):84–90. [Google Scholar]
- 25.Gu T, Mutebi A, Stolshek BS, Tan H. Cost of biologic treatment persistence or switching in rheumatoid arthritis. The American Journal of Managed Care. 2018;24(8 Spec No.):Sp338-sp45. [PubMed]
- 26.Johnston SS, McMorrow D, Farr AM, Juneau P, Ogale S. Comparison of biologic disease-modifying antirheumatic drug therapy persistence between biologics among rheumatoid arthritis patients switching from another biologic. Rheumatol Ther. 2015;2(1):59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient-level data and related documents [including, e.g., the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications]. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.