Abstract
Introduction
Despite advancements in the treatment of psoriasis (PsO), there are few head-to-head studies assessing comparative effectiveness of the newest therapies approved to treat PsO. Our objective was to assess the comparative clinical effectiveness of risankizumab and deucravacitinib in patients with moderate-to-severe PsO.
Methods
This placebo-anchored matching-adjusted indirect comparison (MAIC) analysis utilized data from UltIMMa-1/2 risankizumab and POETYK PSO-1/2 deucravacitinib trials. Individual patient data from UltiMMA-1/2 were weighted via propensity score to match POETYK PSO-1/2 published summary data. Rate differences between risankizumab and deucravacitinib were assessed for Psoriasis Area and Severity Index (PASI) 75/90/100, the Static Physician Global Assessment (sPGA = 0 or 0/1), and the Dermatology Life Quality Index (DLQI) 0/1.
Results
At 16 weeks, risankizumab-treated patients demonstrated statistically significantly higher rates of skin clearance and greater improvement in quality of life (QoL) compared to those treated with deucravacitinib. Across all outcomes, risankizumab demonstrated a lower number needed to treat compared to deucravacitinib. Limitations are potential bias due to unobserved/unmeasurable differences and limited generalizability of the results.
Conclusions
This indirect comparison demonstrates that risankizumab has higher rates of skin clearance and greater improvements in QoL than deucravacitinib. This study will help inform healthcare providers in their treatment and management strategy of PsO.
Keywords: Deucravacitinib, Matching-adjusted indirect comparison, Plaque psoriasis, Risankizumab
Key Summary Points
| Why carry out this study? |
| Data on the comparative effectiveness of risankizumab and deucravacitinib, two therapies approved for the treatment of moderate to severe psoriasis, are lacking. |
| This analysis used matching-adjusted indirect comparison methodology to compare risankizumab and deucravacitinib to better understand the effectiveness of these two treatments in patients with moderate to severe psoriasis. |
| What was learned from the study? |
| Patients treated with risankizumab had significantly higher rates of skin clearance, as well as significantly improved patient reported quality of life, compared to those treated with deucravacitinib. |
| This analysis demonstrated that, compared with deucravacitinib, patients with moderate to severe psoriasis had significantly greater improvements with risankizumab. These data may help inform healthcare providers in their treatment and management strategy for psoriasis. |
Introduction
Plaque psoriasis (PsO) is an immune-mediated, chronic, inflammatory skin disease characterized by dry, itchy plaques [1]. Approximately 7.5 million adults in the USA, and 29.5 million adults worldwide, have PsO [2–4]. Patients with PsO not only experience psychosocial impacts of the disease but have also reported that the plaques negatively impact their health-related quality of life (HRQoL) [5].
Those with moderate-to-severe PsO (> 3–5% of body surface area [% BSA]) often require continuous and lifelong treatment to maintain disease control [6, 7]. Such treatments may include phototherapy and biologics, such as tumor necrosis factor alpha inhibitors, interleukin-12/23 inhibitors, interleukin-17 inhibitors, and interleukin-23 inhibitors, which are generally administered subcutaneously [4]. Recent advancements in PsO treatment have led to the 2022 approval of deucravacitinib, an oral, first-in-class, allosteric tyrosine kinase 2 inhibitor for treatment of adults with moderate-to-severe PsO [8]. Studies have shown that deucravacitinib has demonstrated superior efficacy compared with placebo and apremilast, a phosphodiesterase 4 inhibitor [9, 10]. Risankizumab, a subcutaneous interleukin-23 inhibitor approved for the treatment of PsO in 2019 [11], has also been shown to be well tolerated and to have improved efficacy compared to apremilast (a phosphodiesterase 4 inhibitor) [12], adalimumab (a tumor necrosis factor inhibitor) [13], secukinumab (an interleukin-17 inhibitor) [14], and ustekinumab (an interleukin-23 inhibitor) [15].
Physicians should assess the comparative effectiveness of newly approved treatments to appropriately manage their patients; however, given the ever-changing landscape and the large number of treatments, it is not feasible to conduct head-to-head trials to compare all available therapies. In such instances, an indirect comparison can provide insight into the comparative effectiveness of two treatments. While there are many methods to compare treatments indirectly, the concern of biases is always present when comparing different populations against each other [16, 17]. For example, network meta-analyses are common forms of indirect treatment comparisons but are especially vulnerable to the influence of differing population characteristics [18]. A matching-adjusted indirect comparison (MAIC) minimizes this risk of bias by utilizing individual patient data from one trial and reweighing the data in a way that matches the average patient characteristics for the other trial of interest, allowing for outcomes to be compared between equivalent trial populations [19–21]. In this analysis, we use MAIC methodology to compare risankizumab and deucravacitinib to better understand the effectiveness of these two treatments in patients with moderate-to-severe PsO.
Methods
Study Design and Data Source
This comparative effectiveness study is based on an MAIC of data from four phase 3 clinical trials, two risankizumab trials (UltIMMa-1 and UltIMMa-2) [15] and two deucravacitinib trials (POETYK PSO-1 and POETYK PSO-2) [9, 10], which were conducted among patients with moderate-to-severe PsO.
For the population of interest from the UltIMMa-1 and UltIMMa-2 trials, patients were ≥ 18 years of age with moderate-to-severe PsO who were candidates for systemic therapy or phototherapy and were candidates for ustekinumab treatment [15]. Similarly, for the deucravacitinib trials, patients were ≥ 18 years of age with moderate-to-severe PsO for ≥ 6 months [9, 10]. For both datasets, patients were allowed to have prior biologic therapy exposure, but were required to have discontinued those therapies prior to study start. For all trials included in this analysis, moderate-to-severe PsO was defined as body surface area (BSA) involvement ≥ 10%, Static Physician’s Global Assessment [sPGA] ≥ 3, and Psoriasis Area and Severity Index (PASI) ≥ 12 [9, 15].
All four trials compared their respective study drugs to placebo at week 16; therefore, placebo was used as an anchor in the MAIC analyses. Individual patient data from UltIMMa-1 and UltIMMa-2 were pooled for the risankizumab and placebo groups and were compared with pooled published data for the deucravacitinib and placebo groups from POETYK PSO-1 and POETYK PSO-2 [9, 10, 13]. While the anchored MAIC should control both effect modifiers and prognostic factors, not all baseline information for effect modifiers and prognostic factors was available; thus at 52 weeks, MAICs were unanchored, as patients in the placebo groups switched to different treatments at the end of the double-blind treatment (week 16) and the common comparator (placebo) was no longer available. Furthermore, the POETYK PSO-2 study did not report any outcomes at 52 weeks; thus, for the 52-week MAIC, data from UltiMMA 1 and 2 were only matched with POETYK PSO-1.
Outcomes
Baseline demographic and clinical characteristics were assessed before and after matching. Outcomes assessed at 16 weeks included the proportion of patients achieving PASI 75, 90, and 100, defined as achievement of ≥ 75%, 90%, and 100% reduction from baseline PASI score, respectively; the proportion of patients achieving clear/almost clear skin or clear skin (i.e., sPGA = 0/1 and sPGA = 0, respectively); and the proportion of patients reporting little to no effect of PsO on their HRQoL (i.e., DLQI 0/1). At 52 weeks, the proportion of patients achieving PASI 75 and sPGA 0/1 were determined (other outcomes were not reported in POETYK PSO-1 and POETYK PSO-2 trials) [9, 10]. Rate differences are reported for risankizumab versus placebo, deucravacitinib versus placebo, and risankizumab versus deucravacitinib. Numbers needed to treat (NNTs) for risankizumab versus deucravacitinib were also assessed.
Ethics
This study, and each clinical trial included in the analysis, was conducted in accordance with the ethical principles that have their origin in the current Declaration of Helsinki and was consistent with International Conference on Harmonization Good Clinical Practice, Good Epidemiology Practices, and applicable regulatory requirements [9, 10, 13]. This analysis utilized de-identified data from published clinical trial data; thus no ethics committee approval was required. However, each individual trial included in this analysis was approved by independent ethics committees or institutional review boards at each study site and all patients provided written informed consent before enrolling in each clinical trial [9, 10, 13].
Data Analysis
Placebo-anchored MAICs were used to compare outcomes between risankizumab and deucravacitinib, in which the individual patient data from the risankizumab trials were weighted via propensity scores from a logistic regression model to match the published summary data for the deucravacitinib trials. Suitable MAICs were identified by a visual examination for skew and outliers in the distribution of the assigned weights. Data were matched on the basis of baseline age, sex, race, body mass index, prior exposure to biologic therapy (yes/no), sPGA (score = 3 or 4), PASI (mean score), % BSA (mean), DLQI (mean), and diagnosis of psoriatic arthritis (yes/no). Matching variables were determined as per the National Institute for Health Care Excellence and European Network for Health Technology Assessment MAIC methodology guidelines [22, 23]. After matching and identifying suitable MAICs, we calculated weighted outcomes from matched individual patient data from the risankizumab trial and compared with published summary results from the deucravacitinib trial. The standard error for risankizumab was calculated by robust sandwich estimator since it allows estimated weights to be subject to sampling uncertainty, records were not independent. Rate differences were compared between risankizumab and deucravacitinib for both week 16 (anchored via placebo) and week 52 (unanchored) outcomes. p values were calculated from z-score test.
At week 16, differences in the proportion of patients achieving PASI 75/90/100, sPGA = 0, sPGA = 0/1, and DLQI = 0/1 between risankizumab and its anchor group (risankizumab − placebo), deucravacitinib and its anchor group (deucravacitinib − placebo), and between risankizumab and deucravacitinib [(risankizumab − placebo) − (deucravacitinib − placebo)] were calculated with corresponding standard error and p values. Unanchored MAIC was performed at week 52, and differences in the proportion of patients achieving PASI 75 and sPGA 0/1 between risankizumab and deucravacitinib (risankizumab − deucravacitinib) were calculated.
NNTs were calculated as the inverse of the difference in proportions of patients achieving each outcome between risankizumab and deucravacitinib. Data analyses were performed using SAS Studio 9.4 (Cary, NC) and RStudio 2021.09.0 (Boston, MA).
Results
Baseline Demographic and Clinical Characteristics
This analysis included 598 patients in the risankizumab group and 200 patients in the associated placebo group, and 843 patients in the deucravacitinib group and 421 in the associated placebo group.
Before matching, baseline characteristics were comparable across cohorts (Table 1). The most notable difference between risankizumab and deucravacitinib groups was that over 95% of participants reported nail PsO in the risankizumab cohorts, whereas < 47% reported nail PsO in the deucravacitinib studies. After individual patient data from UltIMMa-1/2 were matched to the POETYK PSO-1/2 population, baseline characteristics were well balanced (Table 1). The average age was approximately 47 years, two-thirds of the population was male, and the majority were White. Approximately 35% had prior exposure to biologic therapy. Most patients (78.9%–81.9%) had a sPGA score of 3, with a mean PASI score of approximately 21, a mean % BSA of 25.3%–26.4%, and a mean DLQI score of approximately 12. Up to 20% of patients had psoriatic arthritis; however, as a result of skewed distribution and extreme weighing during the matching process, scalp and nail PsO were excluded from the effect modifiers for matching.
Table 1.
Baseline demographic and clinical characteristics before and after matching
| Characteristica | Before matching | After matching | ||||||
|---|---|---|---|---|---|---|---|---|
| UltIMMa-1/2 | POETYK PSO-1/2 | UltIMMa-1/2 | POETYK PSO-1/2 | |||||
| PBO N = 200 |
RZB 150 mg QD N = 598 |
PBO N = 421 |
DEU 6 mg QD N = 843 |
PBO N = 200 |
RZB 150 mg QD N = 598 |
PBO N = 421 |
DEU 6 mg QD N = 843 |
|
| Age [years], mean | 47.8 | 47.3 | 47.5 | 46.5 | 47.5 | 46.5 | 47.5 | 46.5 |
| BMI [kg/m2], mean | 30.2 | 30.5 | 30.3 | 30.5 | 30.3 | 30.5 | 30.3 | 30.5 |
| Male,% | 73.0 | 69.4 | 69.8 | 67.1 | 69.8 | 67.1 | 69.8 | 67.1 |
| White,% | 79.0 | 76.1 | 85.5 | 87.9 | 85.5 | 87.9 | 85.5 | 87.9 |
| Any previous biologic therapy,% | 41.0 | 37.1 | 34.7 | 35.0 | 34.7 | 35.0 | 34.7 | 35.0 |
| sPGA score,b % 3 (moderate) | 81.5 | 80.9 | 81.9 | 78.9 | 81.9 | 78.9 | 81.9 | 78.9 |
| PASI, mean | 19.7 | 20.6 | 20.9 | 21.1 | 20.9 | 21.1 | 20.9 | 21.1 |
| % BSA, mean | 25.9 | 26.2 | 25.3 | 26.4 | 25.3 | 26.4 | 25.3 | 26.4 |
| DLQI,b mean | 12.6 | 13.2 | 11.6 | 11.9 | 11.6 | 11.9 | 11.6 | 11.9 |
| Psoriasis-related history, % | ||||||||
| Scalpc | 98.5 | 98.2 | 90.5 | 88.3 | – | – | – | – |
| Nailsc | 98.5 | 96.8 | 46.3 | 43.3 | – | – | – | – |
| Psoriatic arthritisd | 34.0 | 20.2 | 16.9 | 19.5 | 16.9 | 19.5 | 16.9 | 19.5 |
BMI body mass index, BSA body surface area, DEU Deucravacitinib, DLQI Dermatology Life Quality Index, PASI Psoriasis Area and Severity Index, PBO placebo, QD once daily, RZB Risankizumab, SD standard deviation, sPGA Static Physician’s Global Assessment
aMatching was based on mean values from deucravacitinib publications; thus standard deviations and confidence intervals cannot be calculated
bThe total scoring range for PASI is 0–72 and the scoring range for DLQI is 0–30; higher scores indicate worse disease severity or quality of life, respectively
cScalp and nails were excluded from effect modifier as a result of skewed distribution and extreme weighting in matching process
dDiagnosed or suspected
Clinical and Patient-Reported Outcomes
Outcomes at 16 Weeks
After 16 weeks of treatment, 90.9% of risankizumab-treated patients versus 55.2% of deucravacitinib-treated patients achieved PASI 75, which corresponds to a rate difference (%, standard error [SE]) of 39.9% (0.034) between treatment groups (p < 0.001); < 11% of placebo-treated patients achieved PASI 75 (Fig. 1a). Similarly, 76.8% and 44.2% of risankizumab-treated patients achieved PASI 90 and PASI 100, respectively, compared with 30.4% and 11.7% of deucravacitinib-treated patients; < 4% and < 2% of placebo-treated patients in both treatment groups achieved these outcomes, respectively. This resulted in significant rate differences of 46.8% (0.030) and 31.8% (0.029) between risankizumab and deucravacitinib for PASI 90 and PASI 100 (both p < 0.001), respectively. When compared to their respective placebos, NNTs were lower for risankizumab than for deucravacitinib (Table 2). When comparing risankizumab to deucravacitinib, NNTs were 2.6, 2.2, and 3.2, respectively, for PASI 75, PASI 90, and PASI 100.
Fig. 1.
a, b Rate difference of clinical and patient-reported outcomes at 16 weeks (after matching). *p < 0.001. DLQI Dermatology Life Quality Index, PASI Psoriasis Area and Severity Index, QD once daily, SE standard error, sPGA Static Physician’s Global Assessment
Table 2.
Numbers needed to treat
| NNT | Risankizumab 150 mg QD vs placebo | Deucravacitinib 6 mg QD vs placebo | Risankizumab 150 mg QD vs deucravacitinib 6 mg QD |
|---|---|---|---|
| 16 weeks (anchored MAIC) | |||
| PASI 75 | 1.2 | 2.3 | 2.6* |
| PASI 90 | 1.4 | 3.7 | 2.2* |
| PASI 100 | 2.4 | 9.3 | 3.2* |
| sPGA 0 | 2.4 | 6.5 | 3.8* |
| sPGA 0/1 | 1.3 | 2.4 | 2.8* |
| DLQI 0/1 | 1.6 | 3.6 | 2.8* |
| 52 weeks (unanchored MAIC)a | |||
| PASI 75 | – | – | 3.8* |
| sPGA 0/1 | – | – | 3.1* |
DLQI Dermatology Life Quality Index, NNT number needed to treat, PASI Psoriasis Area and Severity Index, QD once daily, sPGA Static Physician’s Global Assessment
*p < 0.001
aNot all baseline information for effect modifiers and prognostic factors were available; thus MAICs at 52 weeks are unanchored, as patients in the placebo groups switched to different treatments at the end of the double-blind treatment (week 16) and the common comparator (placebo) was no longer available. Furthermore, the POETYK PSO-2 study did not report any outcomes at 52 weeks; thus, for the 52-week MAIC, data from UltiMMA 1 and 2 were only matched with POETYK PSO-1
Higher proportions of risankizumab- versus deucravacitinib-treated patients achieved sPGA 0 (44.5% vs 16.4%), sPGA 0/1 (85.6% vs 51.1%), and DLQI 0/1 (70.6% vs 37.7%) after 16 weeks of treatment (Fig. 1b). This resulted in significant (p < 0.001) rate differences (SE) of 26.3% (0.030), 36.3% (0.034), and 37.0% (0.036) between risankizumab- and deucravacitinib-treated patients achieving sPGA 0, sPGA 0/1, and DLQI 0/1, respectively. Overall, < 10% of placebo-treated patients achieved sPGA 0 (range 1.0–2.8%), sPGA 0/1 (range 6.3–8.1%), or DLQI 0/1 (range 5.6–9.7%).
Overall, NNTs compared to placebo were lower for risankizumab than for deucravacitinib (Table 2). Moreover, NNTs comparing risankizumab to deucravacitinib for sPGA 0 and sPGA 0/1 were 3.8 and 2.8, respectively. For DLQI 0/1, the NNT for risankizumab compared to placebo was lower than for deucravacitinib versus placebo (1.6 vs 3.6), and the NNT comparing risankizumab to deucravacitinib was 2.8 (Table 2).
Outcomes at 52 Weeks
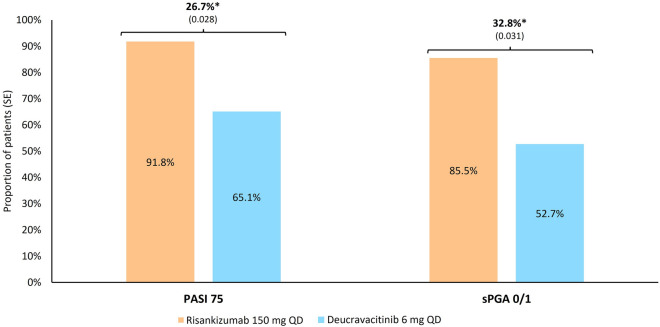
At 52 weeks, 91.8% of risankizumab-treated patients, and 65.1% of deucravacitinib-treated patients, achieved PASI 75, resulting in a rate difference (SE) of 26.7% (0.028) (Fig. 2). Similarly, 85.5% and 52.7% of risankizumab- and deucravacitinib-treated patients, respectively, achieved sPGA 0/1, resulting in a rate difference of 32.8% (0.031). The NNTs comparing risankizumab to deucravacitinib were 3.8 and 3.1 for the PASI 75 and sPGA 0/1, respectively.
Fig. 2.
Rate difference of clinical and patient-reported outcomes at 52 weeks (after matching). *p < 0.001. PASI Psoriasis Area and Severity Index, QD once daily, sPGA Static Physician’s Global Assessment
Discussion
Despite the number of treatments available, PsO can be difficult to optimally treat, in part because of lack of understanding of the comparative effectiveness of novel treatments. While registered clinical trials (RCTs) are the gold standard for comparing different treatments, indirect comparisons offer insight when no such trials are available. Out of the variety of indirect comparisons possible [17], MAIC is a tool that minimizes the bias in differences in patient populations, reduces the impact of effect measures (e.g., age, treatment history, and concomitant disease) that may skew results, resolves differences in outcome definitions, and allows for the comparison of dosages [19, 24, 25]. MAICs have been used to compare treatments for a variety of diseases where no RCTs are available, including psoriatic arthritis [26], asthma [27], prostate cancer [28], and multiple sclerosis [29]. Recently, the results of prior MAICs have been validated by head-to-head RCTs wherein PASI 90 response rates first reported in unanchored MAICs were found to be consistent with RCTs comparing guselkumab with secukinumab and ixekizumab [30]. This finding substantiates the robustness of MAICs where direct comparisons are not yet available [30].
Our study indicates that patients with moderate-to-severe PsO who were treated with risankizumab experienced significantly higher improvement from baseline in skin clearance (i.e., PASI) and HRQoL (i.e., DLQI) measures, as well as higher rates of skin clearance (i.e., sPGA), than those who were treated with deucravacitinib. Risankizumab also consistently demonstrated a lower NNT than deucravacitinib across all outcomes. By comparing these treatments via MAIC, our results are unlikely to be due to differences in the baseline characteristics of the patients. The findings of this analysis are also consistent with that reported in a recent network meta-analysis (NMA) that compared deucravacitinib to other biologic and nonbiologic treatments for PsO. In that study, 54.1% of deucravacitinib-treated patients versus 89.6% of risankizumab-treated patients were estimated to have achieved PASI 75 within 10–16 weeks of treatment; these values increased to 65.9% and 91.6%, respectively, by 44–60 weeks [31]. When compared with other biologic and nonbiologic therapies in that study, consistently higher proportions of patients receiving risankizumab achieved PASI 75, regardless of timepoint [31]. This finding is also in line with other studies which demonstrated that, among available PsO treatments, risankizumab treatment was associated with highest PASI response rates following both induction and maintenance treatment [32, 33]. By providing more information regarding the comparative effectiveness between risankizumab and deucravacitinib, this study will allow healthcare professionals to optimize their treatment decisions for patients with moderate-to-severe PsO.
The strengths of this study include an anchor-based approach which accounts for differences in response to placebo treatment. Likewise, given the recent approval of deucravacitinib, there are no head-to-head studies assessing comparative effectiveness. Thus, this MAIC analysis allows for a robust, indirect comparison that may serve to aid treatment decisions. Limitations include a potential lack of robustness in the 52-week unanchored data due to unreported outcomes in the deucravacitinib trials. Additionally, information regarding presence of scalp and nail PsO was excluded from matching variables because of skewed distribution and extreme weighing in the matching process. It is possible that the presence of scalp and/or nail PsO may impact treatment outcomes; thus further research is needed to address this. Lastly, as with all MAICs, there may be potential bias due to unobserved or unmeasurable confounding; not all cross-study differences, such as inclusion and exclusion criteria, can be addressed by the MAIC and the generalizability of these results may be limited, as patients enrolled in clinical trials may differ from patients with moderate-to-severe PsO in the real world.
Conclusions
This MAIC evaluated the effectiveness of risankizumab versus deucravacitinib in patients with moderate-to-severe PsO, demonstrating that risankizumab had a higher rate of effectiveness after 16 weeks of treatment. The results of this study may help inform healthcare providers in their treatment decision making for bio-naïve and bio-experienced patients with PsO.
Acknowledgments
Medical Writing and Editorial Assistance
Medical writing services provided by Samantha D. Francis Stuart, PhD, of Fishawack Facilitate Ltd, part of Avalere Health, and funded by AbbVie.
Author Contributions
Conceptualization: Ahmed M. Soliman (lead), Manish Patel (supporting). Data curation: Ahmed M. Soliman (supporting), Siran Fang (lead), Manish Patel (supporting). Formal analysis: Ahmed M. Soliman (supporting), Siran Fang (lead), Manish Patel (supporting). Funding acquisition: (Ahmed M. Soliman lead), Manish Patel (supporting). Investigation: Ahmed M. Soliman (lead), Manish Patel (supporting). Methodology: Ahmed M. Soliman (equal), Siran Fang (equal), Manish Patel (equal). Project administration: Ahmed M. Soliman (lead), Siran Fang (supporting), Manish Patel (supporting). Resources: Ahmed M. Soliman (lead), Siran Fang (supporting), Manish Patel (supporting). Supervision: Ahmed M. Soliman (lead), Siran Fang (supporting), Manish Patel (supporting). Validation: Ahmed M. Soliman (lead), Siran Fang (supporting), Manish Patel (supporting). Visualization: Ahmed M. Soliman (supporting), Siran Fang (supporting), Manish Patel (supporting). Writing (original draft preparation): April W. Armstrong, Ahmed M. Soliman, Paolo Gisondi, Siran Fang, Manish Patel, Bruce Strober (equal). Writing (review and editing): April W. Armstrong, Ahmed M. Soliman, Paolo Gisondi, Siran Fang, Manish Patel, Bruce Strober (equal).
Funding
This study, as well as the journal’s Rapid Service Fee, was funded by AbbVie.
Declarations
Conflict of Interest
April W. Armstrong is a research investigator, scientific advisor, and/or speaker for AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, EPI, Incyte, Janssen, Leo, Lilly, Mindera Health, Nimbus, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Ahmed M. Soliman, Siran Fang, and Manish Patel are full-time employees of AbbVie and may hold AbbVie stock and/or stock options and patents. Paolo Gisondi has been a consultant and/or speaker for AbbVie, Almirall, Amgen, Janssen, Leo-pharma, Eli Lilly, Novartis, Pierre Fabre, Sandoz, Sanofi, UCB. Bruce Strober has served as a consultant (received honoraria) for AbbVie, Acelyrin, Alamar, Alumis, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Kangpu Pharmaceuticals, Bristol-Myers-Squibb, Capital One, Celltrion, CorEvitas, Dermavant, Imagenebio, Janssen, Leo, Eli Lilly, Maruho, Okura, Meiji Seika Pharma, Protagonist, Monte Carlo, Takeda, Novartis, Pfizer, UCB Pharma, Rapt, Regeneron, Sanofi-Genzyme, SG Cowen, and Union Therapeutics. He owns stock options in Connect Biopharma and Mindera Health. He has served as a speaker for AbbVie, Arcutis, Dermavant, Eli Lilly, Incyte, Janssen, Regeneron, and Sanofi-Genzyme and as a co-scientific director (consulting fee) and investigator for CorEvitas Psoriasis Registry. He also serves as editor-in-chief (honorarium) for The Journal of Psoriasis and Psoriatic Arthritis.
Ethical Approval
This study, and each clinical trial included in the analysis, was conducted in accordance with the ethical principles that have their origin in the current Declaration of Helsinki and was consistent with International Conference on Harmonization Good Clinical Practice, Good Epidemiology Practices, and applicable regulatory requirements [9, 10, 13]. This analysis utilized de-identified data from published clinical trial data; thus no ethics committee approval was required. However, each individual trial included in this analysis was approved by independent ethics committees or institutional review boards at each study site and all patients provided written informed consent before enrolling in each clinical trial [9, 10, 13].
References
- 1.Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182:840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parisi R, Iskandar IY, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020. 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945–60. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz J, Evers AW, Bundy C, Kimball AB. Getting under the skin: report from the International Psoriasis Council workshop on the role of stress in psoriasis. Front Psychol. 2016;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blauvelt A, Burge R, Gallo G, et al. A retrospective cohort analysis of treatment patterns over 1 year in patients with psoriasis treated with ixekizumab or guselkumab. Dermatol Ther. 2022;12:701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Dermatology Association. How long will i have to treat my psoriasis? 2023. https://www.aad.org/public/diseases/psoriasis/treatment/medications/how-long#:~:text=Psoriasis%20medicine%3A%20Psoriasis%20is%20often,clear%20skin%20during%20these%20periods. Accessed 7 Nov 2023.
- 8.Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29–39. [DOI] [PubMed] [Google Scholar]
- 10.Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40–51. [DOI] [PubMed] [Google Scholar]
- 11.McKeage K, Duggan S. Risankizumab: first global approval. Drugs. 2019;79:893–900. [DOI] [PubMed] [Google Scholar]
- 12.Stein Gold LF, Bagel J, Tyring SK, et al. Comparison of risankizumab and apremilast for the treatment of adults with moderate plaque psoriasis eligible for systemic therapy: results from a randomized, open-label, assessor-blinded phase IV study (IMMpulse). Br J Dermatol. 2023;189:540–52. [DOI] [PubMed] [Google Scholar]
- 13.Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576–86. [DOI] [PubMed] [Google Scholar]
- 14.Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–61. [DOI] [PubMed] [Google Scholar]
- 16.Glenny A, Altman D, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9(1–134):iii–iv. [DOI] [PubMed] [Google Scholar]
- 17.Sutton A, Ades A, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. 2008;26:753–67. [DOI] [PubMed] [Google Scholar]
- 18.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14:417–28. [DOI] [PubMed] [Google Scholar]
- 19.Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28:935–45. [DOI] [PubMed] [Google Scholar]
- 20.Torres T, Sohrt Petersen A, Ivens U, et al. Matching-adjusted indirect comparison of the efficacy at week 32 of tralokinumab and dupilumab in the treatment of moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2024;14:983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rand K, Ramos-Goni JM, Akmaz B, Sole-Feu L, Armario-Hita JC. Matching-adjusted indirect comparison of the long-term efficacy maintenance and adverse event rates of lebrikizumab versus dupilumab in moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2024;14:169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillippo D, Ades T, Dias S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. commissioned report. NICE decision support unit. 2016. https://research-information.bris.ac.uk/files/94868463/Population_adjustment_TSD_FINAL.pdf. Accessed 16 Sept 2024.
- 23.European Network for Health Technology Assessment. D4.3.1: direct and indirect comparisons. 2022. https://www.eunethta.eu/wpcontent/uploads/2022/12/EUnetHTA-21-D4.3.1-Direct-and-indirect-comparisons-v1.0.pdf. Accessed 16 Sept 2024.
- 24.Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15:940–7. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong AW, Park SH, Patel V, et al. Matching-adjusted indirect comparison of the long-term efficacy of deucravacitinib versus adalimumab for moderate to severe plaque psoriasis. Dermatol Ther. 2023. 10.1007/s13555-023-00977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirson NY, Rao S, Birnbaum HG, et al. Matching-adjusted indirect comparison of adalimumab vs etanercept and infliximab for the treatment of psoriatic arthritis. J Med Econ. 2013;16:479–89. [DOI] [PubMed] [Google Scholar]
- 27.Bourdin A, Husereau D, Molinari N, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018. 10.1183/13993003.01393-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Paller C, Hong H, et al. Comparison of treatments for nonmetastatic castration-resistant prostate cancer: matching-adjusted indirect comparison and network meta-analysis. J Natl Cancer Inst. 2022;114:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samjoo IA, Worthington E, Haltner A, et al. Matching-adjusted indirect treatment comparison of siponimod and other disease modifying treatments in secondary progressive multiple sclerosis. Curr Med Res Opin. 2020;36:1157–66. [DOI] [PubMed] [Google Scholar]
- 30.Signorovitch J, Diels J, Van Sanden S, et al. Matching-adjusted indirect comparison (MAIC) results confirmed by head-to-head trials: a case study in psoriasis. J Dermatol Treat. 2023;34:2169574. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong AW, Warren RB, Zhong Y, et al. Short-, mid-, and long-term efficacy of deucravacitinib versus biologics and nonbiologics for plaque psoriasis: a network meta-analysis. Dermatol Ther (Heidelb). 2023;13:2839–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong AW, Soliman AM, Betts KA, et al. Long-term benefit-risk profiles of treatments for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:167–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong AW, Soliman AM, Betts KA, et al. Comparative efficacy and relative ranking of biologics and oral therapies for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther (Heidelb). 2021;11:885–905. [DOI] [PMC free article] [PubMed] [Google Scholar]