Abstract
Introduction
Guselkumab, a human monoclonal antibody targeting the p19 subunit of interleukin-23 (IL-23), has shown efficacy in psoriasis and psoriatic arthritis. However, long-term real-world data on its effectiveness in patients with inadequate response to ustekinumab are limited. This study investigates guselkumab’s long-term effectiveness and safety in patients with psoriasis with partial response to ustekinumab.
Methods
We performed a retrospective multicentric study analyzing data of patients with psoriasis from seven Italian hospitals between January 2021 and May 2024. The study included 169 patients who switched from ustekinumab to guselkumab. Primary endpoints were Psoriasis Area and Severity Index (PASI) 75, PASI 90, PASI 100, and absolute PASI ≤ 2. Site-specific Physician Global Assessment (PGA) scores were also collected for difficult-to-treat areas.
Results
The study included 169 patients. After 3 years of treatment, PASI 75, PASI 90 and PASI 100 were achieved by 88.4%, 55.8%, and 32.6% of patients, respectively. Site-specific PGA showed significant improvements, especially in the scalp and genital areas. After 3 years of treatment, no significant impact of higher body mass index (BMI) or cardiometabolic comorbidities on guselkumab effectiveness was detected. No severe adverse events were reported during the study period.
Conclusions
In our study, guselkumab provided significant long-term effectiveness and safety in patients partially responsive to ustekinumab, improving both PASI score and site-specific PGA and confirming its potential use for patients with psoriasis switching from ustekinumab.
Keywords: Psoriasis, Anti-IL-12/23, Anti-IL-23, Guselkumab, Biologics
Key Summary Points
| The effectiveness and safety of guselkumab have been assessed in both clinical trials and real life, but data on patients with inadequate response to ustekinumab are still limited. |
| We performed a multicentric study to assess the effectiveness and safety of guselkumab in patients with psoriasis with partial response to ustekinumab in a real-life setting. |
| We observed excellent results in terms of both relative Psoriasis Area and Severity Index ((PASI) 90 and PASI 100) and absolute PASI ≤ 2 with better response rates compared with the NAVIGATE trial. |
| The results achieved at each time point confirmed the effectiveness of guselkumab in difficult-to-treat areas such as scalp, genitals, nails, palmoplantar surfaces, and lower limbs. |
| After 3 years of treatment, body mass index (BMI) and presence of cardiometabolic comorbidities did not show any significant impact on guselkumab effectiveness. |
Introduction
Plaque psoriasis is a chronic immune-mediated skin disease affecting up to 2–3% of the general population worldwide [1]. In recent years, the development of monoclonal antibodies has completely revolutionized the treatment of moderate-to-severe forms [2].
Guselkumab is a human monoclonal antibody targeting the p19 subunit of interleukin-23 (IL-23), which is involved in regulating immune responses and inflammation [3]. By binding to IL-23, guselkumab interferes with the signaling pathways of various immune-mediated conditions, such as psoriasis and psoriatic arthritis [3].
Guselkumab has also been shown to be effective in patients with inadequate response to the IL-12/23 inhibitor ustekinumab, both in registered clinical trials (RCTs) and in real-life evidence [3–5]. Furthermore, data from rheumatological RCTs highlight the better outcomes of guselkumab compared with ustekinumab in psoriatic arthritis (PsA) [6].
However, real-world data on long-term follow-up after switching patients with psoriasis from ustekinumab to guselkumab are still limited.
We performed a 156-week multicentric study in patients affected by plaque-type psoriasis who were partial responders to ustekinumab in real-life clinical practice.
Methods
We performed a noninterventional retrospective multicentric study by analyzing the psoriasis database records of seven Italian hospitals located in Lombardy between January 2021 and May 2024. All patients were treated with guselkumab following the Italian adaptation of the EuroGuiDerm Guideline to manage chronic plaque psoriasis [7].
All eligible patients were switched to guselkumab owing to primary or secondary ineffectiveness of ustekinumab. According to the guideline, inadequate response to ustekinumab was defined as Dermatology Life Quality Index (DLQI) ≥ 5 and/or Psoriasis Area and Severity Index (PASI) ≥ 10 or PASI < 10 with one or more difficult-to-treat affected areas such as palms/soles, genitalia, face/scalp, or nails. Guselkumab was administered following the summary of product characteristics (100 mg at weeks 0 and 4 and then every 8 weeks) [8].
Patient demographics, comorbidities, previous biologic treatments, disease characteristics, and PASI score at baseline, week 16, week 36, week 52, week 104, and week 156 were retrieved from the electronic medical records of the different hospital internal databases. At each timepoint, we calculated the percentages of patients achieving an improvement of 75%, 90%, and 100% (PASI 75, PASI 90, and PASI 100, respectively) in PASI score, compared with baseline. An additional endpoint was the percentage of patients achieving absolute PASI ≤ 2, in accordance with the Italian adaptation of the EuroGuiDerm guidelines [7]. Moreover, in patients with difficult-to-treat areas involvement, a site-specific Physician Global Assessment (PGA) was assessed at each timepoint. To evaluate the effectiveness in each difficult-to-treat area, we assessed the percentage of patients who achieved site-specific PGA of 0 or 1 (clear or almost clear). According to a recent study by Bardazzi et al., we also considered the legs as a difficult-to-treat area [9].
Continuous parameters are reported using mean and standard deviation (SD), while categorical values are reported as absolute number and percentage. In addition, the percentage of patients achieving absolute PASI ≤ 2, PASI 90, and PASI 100 rates with guselkumab was examined in relation to body mass index (BMI) class (BMI ≥ 30 and BMI < 30) and the presence of at least one cardiometabolic comorbidities (CMD) such as arterial hypertension, type 2 diabetes mellitus, hypercholesterolemia, obesity, and cardiovascular event. To assess the statistical difference between these categories, we used the chi-squared test, if the distribution was normal, and Fisher’s exact test, when it was not. Statistical significance was defined as p value ≤ 0.05.
At each timepoint, we evaluated the occurrence of any adverse events (AEs), including serious AEs and AEs leading to discontinuation.
Owing to this study’s retrospective design, not all patients attended every follow-up visit. Consequently, any data for missed follow-up visits were considered absent.
The study was exempted from institutional review board as the protocol did not deviate from standard clinical practice. All patients received ixekizumab as part of routine clinical practice in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Results
One hundred sixty-nine patients previously treated with ustekinumab and then switched to guselkumab were enrolled in this study from seven different Italian Dermatology units. The mean age of the participants was 53.52 years (SD 13.46 years), with male patients accounting for 117 (69.2%) of the sample. The mean BMI was 26.89 (SD 4.96), and 31 patients (18.3%) were obese (BMI ≥ 30). At least one comorbidity was present in 71 patients (42%), with 62 patients (36.7%) having cardiometabolic comorbidities. A diagnosis of concomitant psoriatic arthritis (PsA) was made in 30 patients (17.8%), and 2 patients presented latent tuberculosis infection. Analysis of previous treatments revealed that all 169 patients had experienced treatment failure with ustekinumab, and 56 of them (33.1%) had been exposed to at least two different biologics. Among anti-tumor necrosis factor (TNF) medications, adalimumab had been prescribed to 26 patients (15.4%), etanercept to 25 patients (14.8%), and infliximab to 6 patients (3.6%). In terms of anti-IL-17 drugs, 6 patients (3.6%) were treated with secukinumab while 3 patients (1.8%) received ixekizumab. The residual mean PASI score before starting guselkumab was 9.22 (SD 5.17). Complete demographic characteristics are presented in Table 1.
Table 1.
Demographic characteristics at baseline of our patients
| Number of patients | 169 |
| Mean (SD) | |
| Age (years) | 53.52 (13.46) |
| BMI | 26.89 (4.96) |
| Residual PASI after ustekinumab | 9.22 (5.17) |
| N (%) | |
| Male | 117 (69.2) |
| Obese | 31 (18.3) |
| PsA | 30 (17.8) |
| ≥ 1 comorbidity | 71 (42) |
| CMD | 62 (36.7) |
| Previous exposure to ustekinumab | 169 (100) |
| Previous exposure to ≥ 2 biologics | 56 (33.1) |
| Previous biologic treatments | |
| Adalimumab | 26 (15.4) |
| Etanercept | 25 (14.8) |
| Infliximab | 6 (3.6) |
| Secukinumab | 6 (3.6) |
| Ixekizumab | 3 (1.8) |
PASI Psoriasis Area and Severity Index, BMI body mass index, PsA psoriatic arthritis, CMD cardiometabolic disease, SD standard deviation
We assessed the involvement of difficult-to-treat areas (scalp, genitalia, palms/soles, and nails). Eighty-eight patients (52.1%) had the involvement of at least one difficult-to-treat area at baseline. The scalp was the most frequently affected one, being involved in 58 patients (34.3%), followed by genitalia in 19 patients (11.2%), palmoplantar psoriasis in 16 patients (9.47%), and nails in 11 patients (6.51%). Before treatment with guselkumab, the lower limbs were involved in 108 patients (63.9%), the upper limbs in 88 patients (52.1%), the trunk in 58 patients (34.3%), the back in 12 patients (7.1%) and the face in 9 patients (5.3%) (Fig. 1).
Fig. 1.
Percentage of patients with involvement of specific residual areas
In our cohort, 53 patients (31.4%) had legs involved as a residual area prior to switching to guselkumab.
After 16 weeks of treatment with guselkumab, 92 patients (56.8%) achieved PASI 75, 57 patients (35.2%) achieved PASI 90, and 50 patients (30.9%) achieved complete skin clearance (PASI 100). One hundred three patients (63.6%) reached absolute PASI ≤ 2 at the same timepoint. At week 36, 114 patients (75%) achieved PASI 75, 74 patients (48.7%) achieved PASI 90, 61 patients (40.1%) achieved PASI 100, and 123 patients (80.9%) achieved absolute PASI ≤ 2. After 1 year of the treatment, PASI 75, PASI 90, PASI 100, and PASI ≤ 2 was reached by 86.9%, 61.3%, 41.8%, and 89.8% of the patients, respectively. At week 104, 83.3% of the patients reached PASI 75, 60.3% achieved PASI 90, and 48.70% reached PASI 100. PASI ≤ 2 was achieved by 85.90% of the patients at the same timepoint. After 3 years of treatment, the percentages of PASI 75, PASI 90, PASI 100, and PASI ≤ 2 were 88.40%, 55.80%, 32.60%, and 81.40%, respectively. Figure 2 shows the percentage of patients reaching these endpoints at weeks 52, 104, and 156.
Fig. 2.
Percentage of patients achieving PASI 75, PASI 90, PASI 100, and PASI ≤ 2 at weeks 16, 36, 52, 104, and 156. PASI Psoriasis Area and Severity Index, ns not significant. *p < 0.05; **p < 0.01; ***p < 0.001
Concerning lower limbs, 65% of the patients achieved PGA0/1 at week 16, while it was reached by 78%, 84%, 85%, and 86% at week 36, 52, 104, and 156, respectively.
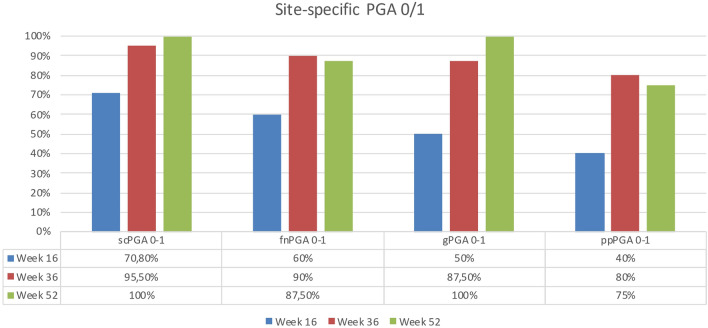
At week 16, site-specific PGA0/1 was achieved by 70.8% of patients with scalp involvement, 60% of patients with fingernail involvement, 50% of patients with genital involvement, and 40% of patients with palmoplantar psoriasis. At week 36, 95.5% of the patients with scalp involvement reached PGA0/1; the same endpoint was achieved by 90% of patients with nail involvement, 87.5% of the subjects with genital localization, and 80% of patients with the involvement of palms or soles. After 1 year of treatment, scPGA0/1 was achieved by all the analyzed patients (100%) as well for gPGA (100%). At the same timepoint, 87.5% of patients with nail involvement reached fnPGA0/1, and 75% of the patients with palmoplantar psoriasis achieved ppPGA0/1 (Fig. 3).
Fig. 3.
Percentage of patients achieving site-specific PGA0/1 at weeks 16, 36, and 52. PGA Psoriasis Global Assessment
In this study, we also analyzed the influence of cardiometabolic comorbidities on the impact of the achievement of PASI 90, PASI 100, and PASI ≤ 2. Specifically, at weeks 16 and 52, patients with and without CMD achieved similar outcomes in terms of PASI 90, PASI 100, and PASI ≤ 2. Interestingly, after 2 years of treatment with guselkumab, a higher percentage of patients with CMD reached PASI 90 compared with those without CMD (81% versus 52.6%, p = 0.023). There were no differences in PASI 100 and PASI ≤ 2 between the two groups at each timepoint. After 3 years, no differences were observed between the two groups for any of the endpoints (Fig. 4a).
Fig. 4.
Percentage of patients with and without CMD achieving PASI 90, PASI 100, and PASI ≤ 2 at weeks 16, 52, 104, and 156 (a). Percentage of patients with BMI ≥ 30 and BMI < 30 achieving PASI 90, PASI 100, and PASI ≤ 2 at weeks 16, 52, 104, and 156 (b). PASI Psoriasis Area and Severity Index, CMD cardiometabolic disease, BMI body mass index, ns not significant. *p < 0.05; **p < 0.01; ***p < 0.001
In our cohort, we analyzed PASI 90, PASI 100, and PASI ≤ 2 responses in patients with BMI ≥ 30 (obese) and patients with BMI < 30 (not obese), and we observed no significant differences at week 16 between the two groups for all the outcomes. At week 52, patients with BMI ≥ 30 had a lower probability of achieving PASI 100 and PASI ≤ 2 compared with those not obese (29.2% versus 65.4%, p = 0.002; 87.5% versus 98.7%, p = 0.013, respectively), though no significant difference was observed for PASI 90 response. After 2 and 3 years of treatment, no differences were observed between the two groups regarding any of the endpoints (Fig. 4b).
Only two patients (1.2%) discontinued the treatment for secondary ineffectiveness. Regarding the safety profile of guselkumab (Table 2), we did not observe severe AEs or AEs leading to discontinuation. The most common AE was upper respiratory tract infection (2 patients), followed by headache (2 patients).
Table 2.
Adverse events in our cohort of patients
| Adverse events | N (% on total population) |
|---|---|
| Upper respiratory tract infection | 2 (1.2%) |
| Headache | 2 (1.2%) |
| Reaction at injection site | 1 (0.6%) |
| Total | 5 (3%) |
| Severe AE | 0 (0%) |
| AE leading to discontinuation | 0 (0%) |
AE adverse events
Discussion
Our study represents one of the largest and longest real-world experiences with guselkumab in patients with inadequate response to ustekinumab.
In our cohort of patients, we observed the relapse/persistence of psoriatic plaques on lower limbs, particularly in pretibial areas, very frequently. This might be due to the higher presence of tissue-resident memory T cells (TRMs) in these areas [10].
In a clinical trial, Mehta et al. demonstrated that guselkumab could potentially reduce TRMs maintaining regulatory T cells compared with patients treated with secukinumab [11]. In our study, the promising results achieved in patients with involvement of lower limbs confirm the preliminary findings on guselkumab effectiveness in reducing this subset of inflammatory cells [11].
Our real-world study demonstrated higher clinical responses to guselkumab compared with the NAVIGATE trial data [3]. At week 52, 61.3% of our patients achieved PASI 90, compared with 51.1% in the NAVIGATE study, and 48.9% of our patients reached PASI 100 versus 20% in the clinical trial. These differences could be attributed to the strict inclusion criteria of the NAVIGATE trial [3].
Our results in the first year of treatment are in line with those observed in another multicenter real-life study [5]. After the first year of treatment with guselkumab, the authors found higher PASI 100 rates than those observed in our study (67.6% versus 48.7% and 81.3% versus 32.60% after 2 and 3 years of treatment, respectively) [5].
Our study supports a patient-oriented strategy for the treatment of moderate-to-severe psoriasis, showing effectiveness even in patients who have previously switched to another biological drug. These results are in line with a real-life study aimed at demonstrating the efficacy of anti-interleukin drugs in patients with psoriasis who had previously failed on adalimumab [12].
Regarding the impact of BMI on the achievement of PASI 90, PASI 100, and PASI ≤ 2, our analysis showed significant differences in terms of PASI ≤ 2 and PASI 100 at week 52, with higher percentages of response in patients with BMI < 30. However, no other statistically significant differences were found in the long term in terms of PASI 90, PASI 100, and PASI ≤ 2, showing that guselkumab also performs well in obese patients. In a recent analysis of pooled data from the VOYAGE-1 and VOYAGE-2 trials, the authors found that patients who were less obese, with a lower baseline PGA score, and a lower body weight had a higher probability of being super-responders to guselkumab [13].
Regarding the involvement of difficult-to-treat areas, we observed a high percentage of patients who achieve site-specific PGA of 0 or 1 at every timepoint. These results support data from two clinical trials that demonstrated a better efficacy of guselkumab compared with adalimumab at weeks 16 and 24 [14]. Similarly, our data support the effectiveness of guselkumab and other IL-23 inhibitors in plaque psoriasis with the involvement of difficult sites, as observed in other real-world experiences [15, 16].
In our study, the presence of CMD did not particularly impact the effectiveness of guselkumab at all the timepoints. These findings confirm the data from clinical trials and other real-life studies on anti-IL-23 effectiveness and safety in patients with CMD [17–19].
In our study, we did not observe severe safety findings throughout the study period, as learned from clinical trials [20]. The safety of guselkumab was also confirmed in patients with chronic infection, such as latent TB, as no reactivation was reported throughout the study period, supporting the results of a previous real-life study about IL-23 inhibitors [21].
This real-world experience has some limitations, the first being its retrospective design, which prevents the retrieval of missing data, the smaller sample size at week 156, the absence of a randomized controlled setting, and the heterogeneity of clinical evaluations from different clinicians. Additionally, the number of reported AEs was likely underestimated, as it is uncommon for patients to mention mild side effects during routine clinical practice. Despite these limitations, our study represents one of the largest and longest real-world experiences with guselkumab in patients with plaque psoriasis with an inadequate response to ustekinumab.
Conclusions
Our study shows that guselkumab achieves significant results in terms of relative and absolute PASI up to week 156 in patients with partial response to ustekinumab. Furthermore, our data suggest its effectiveness also in difficult-to-treat areas, including the lower limbs, which were frequently involved before the switch from ustekinumab to guselkumab.
Regarding safety, no significant AEs were reported throughout the study period.
However, larger and longer studies would be helpful to confirm our results.
Acknowledgements
We thank the participants of the study.
Author Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Mario Valenti, Luciano Ibba and Ruggero Cascio Ingurgio. Data collection was performed by Mario Valenti, Luciano Ibba and Ruggero Cascio Ingurgio, Andrea Carugno, Marco Campoli, Carlo G. Carrera, Francesca M. Gaiani, Nicola Zerbinati, Angelo V. Marzano, Davide Strippoli and Federica Mola. All authors commented on previous versions of the manuscript. Supervision: Alessandra Narcisi, Antonio Costanzo and Piergiorgio Malagoli. All authors read and approved the final manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Mario Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, UCB-Pharma, Difa-Cooper, Almirall, AbbVie and Boehringer Ingelheim. Luciano Ibba has been a consultant for Almirall. Piergiorgio Malagoli has been a speaker for AbbVie, Lilly, Novartis, Janssen-Cilag, Celgene, Leopharma, and Almirall. Andrea Carugno has been speaker and/or consultant for Almirall, Amgen, Abbvie, Boehringer-Ingelheim, Eli-Lilly, Leopharma, Jansse-Cilag, Novartis, UCB Pharma. Francesca M. Gaiani acted as a speaker or consultant for Novartis, Abbvie, Eli Lilly, Celgene, LeoPharma, and Almirall. Carlo G. Carrera has served as a board participant or speaker for Abbvie, Lilly, Janssen, Novartis, Celgene, Almirall, and Leopharma. Angelo V. Marzano reports consultancy/advisory boards disease-relevant honoraria from AbbVie, Boehringer-Ingelheim, Novartis, Pfizer, Sanofi and UCB. Antonio Costanzo has been a consultant and/or speaker for Abb-Vie, Almirall, Amgen, Janssen, Leo Pharma, Eli Lilly, Galderma, Boehringer, Novartis, Pfizer, Sandoz, and UCB. Alessandra Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. Davide Strippoli has been a consultant and/or speaker for Abbvie, Celgene, Janssen, Eli Lilly, Novartis, Pfizer. Marco Campoli, Nicola Zerbinati, Ruggero Cascio Ingurgio and Federica Mola, have nothing to declare.
Ethical Approval
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received ixekizumab as part of routine clinical practice in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
References
- 1.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397(10281):1301–15. 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- 2.Shear NH, Betts KA, Soliman AM, et al. Comparative safety and benefit-risk profile of biologics and oral treatment for moderate-to-severe plaque psoriasis: a network meta-analysis of clinical trial data. J Am Acad Dermatol. 2021;85(3):572–81. 10.1016/j.jaad.2021.02.057. [DOI] [PubMed] [Google Scholar]
- 3.Langley RG, Tsai TF, Flavin S, Song M, Randazzo B, Wasfi Y, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114–23. [DOI] [PubMed] [Google Scholar]
- 4.Gargiulo L, Ibba L, Malagoli P, Angileri RG, Bardazzi F, Bernardini N, et al. Real-life effectiveness and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: a 104-week multicenter retrospective study—IL PSO (ITALIAN LANDSCAPE PSORIASIS). J Eur Acad Dermatol Venereol. 2023;37:1017–27. [DOI] [PubMed] [Google Scholar]
- 5.Megna M, Balato A, Caccavale S, Cacciapuoti S, Calabrese G, Di Brizzi EV, Di Costanzo L, Manzo R, Marino V, Puca RV, Romano F, Sarno O, di Luzio GS, Lembo S. Real-life effectiveness and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: a 3-year multicenter study. J Clin Med. 2024;13(9):2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thilakarathne P, Schubert A, Peterson S, Noel W, Patel BP, Hassan F. Comparing efficacy of guselkumab versus ustekinumab in patients with psoriatic arthritis: an adjusted comparison using individual patient data from the DISCOVER and PSUMMIT trials. Rheumatol Ther. 2024;11(2):457–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisondi P, Fargnoli MC, Amerio P, Argenziano G, Bardazzi F, Bianchi L, et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital J Dermatol Venerol. 2022;157(Suppl. 1 to No. 1):1–78. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency. Tremfya (guselkumab): summary of product characteristics. 2017[cited 2024 June 1]. https://www.ema.europa.eu/en/medicines/human/EPAR/tremfya.
- 9.Bardazzi F, Viviani F, Filippi F, Carpanese MA, Piraccini BM, Abbenante D. The legs: an underestimated difficult-to-treat area of psoriasis. Dermatol Ther. 2022;35(6): e15485. [DOI] [PubMed] [Google Scholar]
- 10.Dong C, Lin L, Du J. Characteristics and sources of tissue-resident memory T cells in psoriasis relapse. Curr Res Immunol. 2023;4: 100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta H, Mashiko S, Angsana J, Rubio M, Hsieh YM, Maari C, Reich K, Blauvelt A, Bissonnette R, Muñoz-Elías EJ, Sarfati M. Differential changes in inflammatory mononuclear phagocyte and T-cell profiles within psoriatic skin during treatment with guselkumab vs. secukinumab. J Invest Dermatol. 2021;141(7):1707-1718.e9. [DOI] [PubMed] [Google Scholar]
- 12.Mastorino L, Susca S, Cariti C, et al. Efficacy of anti-IL-23 and anti-IL-17 after adalimumab failure in psoriatic patients. J Eur Acad Dermatol Venereol. 2023;37(9):1848–53. 10.1111/jdv.19135. [DOI] [PubMed] [Google Scholar]
- 13.Reich K, Gordon KB, Strober B, Langley RG, Miller M, Yang YW, et al. Super-response to guselkumab treatment in patients with moderate-to-severe psoriasis: age, body weight, baseline psoriasis area and severity index, and baseline Investigator’s global assessment scores predict complete skin clearance. J Eur Acad Dermatol Venereol. 2022;36:2393–400. [DOI] [PubMed] [Google Scholar]
- 14.Foley P, Gordon K, Griffiths CEM, Wasfi Y, Randazzo B, Song M, et al. Efficacy of guselkumab compared with Adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orsini D, Gargiulo L, Ibba L, et al. Effectiveness of risankizumab in plaque psoriasis with involvement of difficult-to-treat areas: a real-world experience from two referral centers. J Dermatolog Treat. 2023;34(1):2220849. 10.1080/09546634.2023.2220849. [DOI] [PubMed] [Google Scholar]
- 16.Ibba L, Gargiulo L, Alfano A, Cascio Ingurgio R, Narcisi A, Costanzo A, Valenti M. Anti-IL-23 and anti-IL-17 drugs for the treatment of non-pustular palmoplantar psoriasis: a real-life retrospective study. J Dermatolog Treat. 2023;34(1):2199108. [DOI] [PubMed] [Google Scholar]
- 17.Mastorino L, Susca S, Megna M, Siliquini N, Quaglino P, Ortoncelli M, et al. Risankizumab shows high efficacy and maintenance in improvement of response until week 52. Dermatol Ther. 2022;35(5): e15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narcisi A, Valenti M, Gargiulo L, et al. Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: a 52-week multicenter retrospective study—IL PSO (ITALIAN LANDSCAPE PSORIASIS). J Eur Acad Dermatol Venereol. 2023;37(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drerup KA, Seemann C, Gerdes S, Mrowietz U. Effective and safe treatment of psoriatic disease with the anti-IL-23p19 biologic tildrakizumab: results of a real-world prospective cohort study in nonselected patients. Dermatology. 2022;238:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blauvelt A, Tsai TF, Langley RG, Miller M, Shen YK, You Y, et al. Consistent safety profile with up to 5 years of continuous treatment with guselkumab: pooled analyses from the phase 3 VOYAGE 1 and VOYAGE 2 trials of patients with moderate-to-severe psoriasis. J Am Acad Dermatol. 2022;86(4):827–34. [DOI] [PubMed] [Google Scholar]
- 21.Ibba L, Gargiulo L, Vignoli CA, Fiorillo G, Valenti M, Costanzo A, Narcisi A. Safety of anti-IL-23 drugs in patients with moderate-to-severe plaque psoriasis and previous tuberculosis infection: a monocentric retrospective study. J Dermatolog Treat. 2023;34(1):2241585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.