Abstract
This is a summary of the original article ‘Ten-year safety and clinical benefit from open label etanercept treatment in children and young adults with juvenile idiopathic arthritis’. Juvenile idiopathic arthritis (JIA) usually appears before the age of 16. JIA causes pain, swelling, and stiffness in the joints. People with JIA receive treatment for several years until the disease goes into prolonged remission. Therefore, the long-term safety of these treatments is an important topic. Etanercept is a treatment for JIA, which acts on the body’s immune system to reduce arthritis. This summary of research article describes safety and how well etanercept works in children with JIA taking it for up to 10 years.
Summary of Research
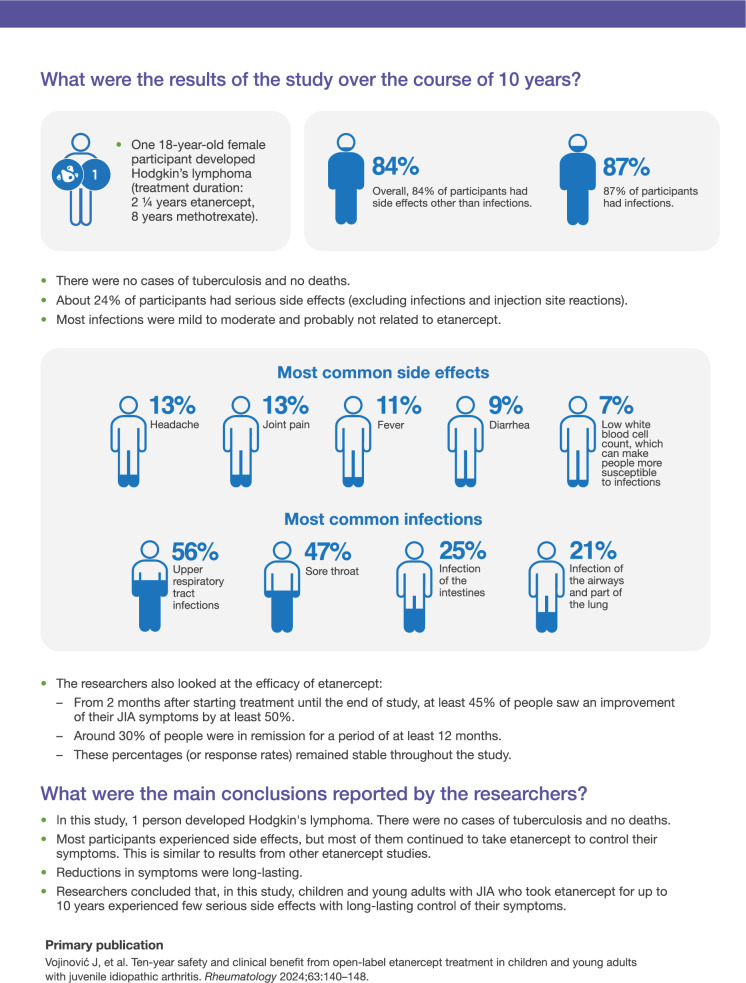
This is a summary of a peer-reviewed article previously published in the journal Rheumatology: Vojinović J, Foeldvari I, Dehoorne J, et al. Ten-year safety and clinical benefit from open-label etanercept treatment in children and young adults with juvenile idiopathic arthritis. Rheumatology (Oxford). 2024;63(1):140–148. [1]. Reproduced by permission of Oxford University Press on behalf of the British Society for Rheumatology (Fig. 1).
Fig. 1.
Summary of research
Acknowledgements
For acknowledgements, please see the original publication.
Medical Writing and Editorial Assistance
The first draft of this summary was written by Andrea Schauenburg, PhD, CMPP, of Engage Scientific Solutions under the supervision of the PRINTO officers (NR and AM), and was funded by Pfizer.
Author Contributions
The trial was designed jointly by PRINTO investigators (NR, AM) and the sponsor. Data were collected by the PRINTO investigators. The sponsor was involved in the overall management of the trial, data collection, and data analysis. All authors reviewed and approved the final document.
Funding
The CLIPPER and CLIPPER2 studies were sponsored by Pfizer. The journal’s Rapid Service Fee for this article was funded by Pfizer.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Declarations
Conflict of Interest
Please see the original article for full author disclosures. Edmund Arthur is now an employee of Johnon & Johnson and Bonnie Vlahos is now retired.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Please see the referenced article for ethics relating to the original study.
Reference
- 1.Vojinović J, Foeldvari I, Dehoorne J, et al. Ten-year safety and clinical benefit from open-label etanercept treatment in children and young adults with juvenile idiopathic arthritis. Rheumatology. 2024;63:140–148. 10.1093/rheumatology/kead183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.