ABSTRACT
Backround
Anterior gradient 2 (AGR2) is a resident endoplasmic reticulum (ER) protein with a vital role in embryonal development, mucus maturation, tissue regeneration, and wound healing.
Methods
To determine the prevalence and clinical significance of AGR2 expression in cancer, a tissue microarray containing 14,966 tumors from 134 different tumor types and subtypes as well as 608 samples of 76 different normal tissue types was analyzed by immunohistochemistry (IHC).
Results
AGR2 positivity was found in 103 of 134 tumor categories, and 83 tumor categories contained at least one strongly positive case. AGR2 expression was most frequently seen in tumors of the female genital tract, particularly adenocarcinomas (up to 100%), various breast cancer subtypes (57.1%–100%), urothelial carcinoma (74.6%–100%), adenocarcinomas of the upper and lower gastrointestinal tract (93.6%–99.6%), and pancreaticobiliary cancers (65.2%–98.2%). AGR2 positivity was slightly less common in squamous cell carcinomas (46.4%–77.3%) and mainly absent in mesenchymal and lymphoid tumors. While AGR2 expression was only weak or absent in the normal thyroid, it was moderate to strong in 46.0% of adenomas, 52.8% of follicular carcinomas, and 81.8% of papillary carcinomas of the thyroid. High AGR2 expression was strongly linked to poor ISUP (p < 0.0001), Fuhrman (p < 0.0001), and Thoenes (p < 0.0001) grades as well as advanced pT stage (p = 0.0035) in clear cell renal cell carcinoma (ccRCC). Low AGR2 expression was associated with high BRE grade in breast cancer (p = 0.0049), nodal metastasis (p = 0.0275) and RAS mutation (p = 0.0136) in colorectal cancer, nodal metastasis (p = 0.0482) in endometrioid endometrial carcinoma, high grade in noninvasive urothelial carcinoma (p = 0.0003), and invasive tumor growth in urothelial carcinoma (p < 0.0001).
Conclusions
It is concluded that AGR2 expression occurs in a broad range of different tumor entities and that AGR2 assessment may serve as a diagnostic aid for the distinction of thyroidal neoplasms and as a prognostic marker in various cancer types.
1. Introduction
Anterior gradient 2, AGR2, is a resident endoplasmic reticulum (ER) protein with a role in oxidative protein folding in the endoplasmic reticulum [1]. The protein is also termed Xenopus Anterior gradient 2 (XAG2) or secreted cement gland protein XAG‐2 homolog because it was initially discovered in the African frog Xenopus laevis, where it plays a role in embryonic development and cement gland differentiation [1, 2]. AGR2 is not restricted to the ER, has protein disulfide isomerase (PDI) activity, and exerts physiological roles in embryonal development, mucus maturation, tissue regeneration, and wound healing [1, 3, 4, 5, 6]. Its mucus stabilizing role is critical for maintaining epithelial barrier function in the intestine, where AGR2 forms a heterodisulfide bond with cysteine residues of MUC2 [4, 7]. Disturbed AGR2 function causes intestinal pathology due to a disrupted mucin maturation [4, 7, 8]. AGR2 knockout mice show a loss of intestinal mucus and develop ileitis and colitis [8]. A critical role of AGR2 in gastrointestinal health is also supported by the observation of a human enteropathy caused by a loss‐of‐function variant of AGR2 which is associated with a disturbed processing of mucins, increased ER stress, and goblet cell loss [7].
Altered expression of AGR2 has been implicated with several signal transduction pathways and cell functions related to cancer initiation, progression, and metastasis [9, 10, 11, 12, 13]. For example, high AGR2 levels have been linked to downregulation of the p53 response [14], increased cell migration [13], and cell transformation in cancer cell lines [15] although others have observed repression of cell growth and proliferation in case of high AGR2 levels [16, 17]. In breast and prostate cancer, AGR2 expression has been linked to estrogen receptor [17, 18, 19] and androgen receptor regulation [20, 21]. Studies evaluating larger cohorts of cancers by immunohistochemistry (IHC) have identified associations between high AGR2 expression and unfavorable tumor phenotype or poor prognosis in adenocarcinoma of the lung [10], gastric adenocarcinoma [22], and adenocarcinoma of the prostate [23]. However, the number of studies analyzing AGR2 in cancer is still limited. In 34 studies using IHC, only 26 different tumor entities have so far been evaluated (Medline, May 2024). Moreover, in 12 cancer entities where multiple studies have been executed, the obtained results were sometimes considerably discrepant. For example, the range of reported AGR2‐positive cases ranged from 56.8% to 100% in adenocarcinoma of the lung [10, 24], from 57.0% to 95.4% in prostate cancer [21, 23], and from 19.0% to 46.1% in serous carcinoma of the ovary [13, 25]. Such conflicting results could be caused by the use of different antibodies, immunostaining protocols, and criteria to define positivity.
To better understand the prevalence and potential clinical significance of AGR2 expression in cancer, a comprehensive study analyzing a large number of neoplastic and nonneoplastic tissues under highly standardized conditions is needed. Therefore, AGR2 expression was analyzed in more than 14,000 tumor tissue samples from 134 different tumor types and subtypes as well as 76 nonneoplastic tissue categories by immunohistochemistry (IHC) in a tissue microarray (TMA) format in this study.
2. Materials and Methods
2.1. Tissue Microarrays (TMAs)
The normal tissue TMA was composed of eight samples from eight different donors for each of 76 different normal tissue types (608 samples on one slide). The cancer TMAs contained a total of 14,966 primary tumors from 134 tumor types and subtypes. Detailed histopathological data on grade, pT, or pN were available from subsets of colorectal cancer (n = 2351), endometrioid endometrial carcinoma (n = 182), clear cell (n = 1224) and papillary renal cell carcinoma (n = 310), serous (n = 369) and endometrioid carcinoma of the ovary (n = 40), thyroid carcinoma (n = 518), urothelial carcinoma (n = 829), as well as pancreatic carcinomas (n = 598). Clinical follow‐up data were available from 789 patients with ccRCC and from 177 patients with pRCC with a median follow‐up time of 48.0 and 50.5 months (range 1–250 and 1–247) and 254 patients with urothelial carcinoma with a median follow‐up time of 14.0 months (range 1–77). The composition of both normal and cancer TMAs is described in detail in the results section. All samples were from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. The TMA manufacturing process was described earlier in detail [26, 27]. In brief, one tissue spot (diameter: 0.6 mm) per patient was used. The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF‐049/09). All work has been carried out in compliance with the Helsinki Declaration.
2.2. Immunohistochemistry (IHC)
Freshly cut TMA sections were immunostained on 1 day and in one experiment. Slides were deparaffinized with xylol, rehydrated through a graded alcohol series and exposed to heat‐induced antigen retrieval for 5 min in an autoclave at 121°C in pH 7.8 Tris‐EDTA‐Citrat (TEC) puffer. Endogenous peroxidase activity was blocked with Dako REAL Peroxidase‐Blocking Solution (Agilent Technologies, Santa Clara, CA, USA; #S2023) for 10 min. Primary antibody‐specific against AGR2 protein (rabbit recombinant, HMV‐325, ardoci GmbH, Hamburg, Germany) was applied at 37°C for 60 min at a dilution of 1:150. For the purpose of antibody validation, the normal tissue TMA was also analyzed by the rabbit recombinant AGR2 antibody EPR3278 (ab76473, Abcam Limited, Cambridge CB2 0AX, GB) at a dilution of 1:1800 and an otherwise identical protocol. Bound antibody was then visualized using the Dako REAL EnVision Detection System Peroxidase/DAB+, Rabbit/Mouse kit (Agilent Technologies, Santa Clara, CA, USA; #K5007) according to the manufacturer's directions. The sections were counterstained with hemalaun. For tumor tissues, the percentage of AGR2‐positive tumor cells was estimated, and the staining intensity was semi‐quantitatively recorded (0, 1+, 2+, 3+). For statistical analyses, the staining results were categorized into four groups as follows: negative: no staining at all, weak staining: staining intensity of 1+ in ≤ 70% or staining intensity of 2+ in ≤ 30% of tumor cells, moderate staining: staining intensity of 1+ in > 70%, staining intensity of 2+ in > 30% but in ≤ 70% or staining intensity of 3+ in ≤ 30% of tumor cells, strong staining: staining intensity of 2+ in > 70% or staining intensity of 3+ in > 30% of tumor cells. The evaluation was performed by an experienced pathologist (SB).
2.3. Statistics
Statistical calculations were performed with JMP17 software (SAS, Cary, NC, USA). Contingency tables and the chi‐squared test were performed to search for associations between AGR2 staining and tumor phenotype. The log‐rank test was applied to detect significant differences between groups.
3. Results
3.1. Technical Issues
A total of 12,434 (83.1%) of 14,966 tumor samples were interpretable in our TMA analysis. Noninterpretable samples demonstrated a lack of unequivocal tumor cells or a lack of entire tissue spots. A sufficient number of samples (≥ 4) of each normal tissue type was evaluated.
3.2. AGR2 Immunostaining in Normal Tissues
A strong, predominantly cytoplasmic AGR2 staining occurred in various epithelial cell types such as from the colorectum, stomach (except parietal cells), gallbladder, pancreas (intercalated ducts and excretory ducts), seminal vesicle, urothelium, respiratory epithelium, lung (a large subset of pneumocytes), breast (a subset of luminal cells), endocervix, endometrium, and the fallopian tube. A somewhat less intense AGR2 staining also occurred in glandular (especially mucinous) and excretory duct cells of salivary glands, Brunner glands of the duodenum, a small subset of tubuli/collecting ducts of the kidney, a subset of epithelial cells in the epididymis, and a subset of epithelial cells of the adenohypophysis. Staining was variable ranging from absent to strong in pancreatic acinar cells as well as in acinar and basal cells of the prostate. AGR2 staining was mostly absent in squamous epithelial cells except in the tonsil, a fraction of cells of corpuscles of Hassall's, and the inner (Huxley) layers of hair follicles of the skin. In the thyroid, AGR2 staining was mostly absent although a weak to moderate staining occasionally was seen. Representative images are shown in Figure 1. All cell types found to be positive by HMV‐325 were also confirmed as AGR2 positive by EPR3278 (Figure S1). AGR2 staining was always absent in hepatocytes, testis, ovary, placenta, adrenal gland, parathyroid, brain, mesenchymal tissues, and in hematolymphatic cells.
FIGURE 1.
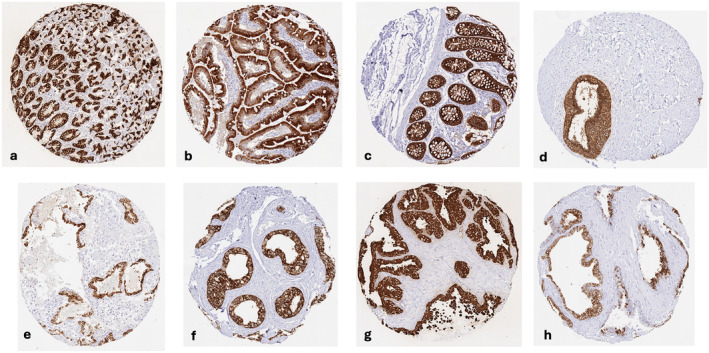
AGR2 immunostaining of normal tissues. The panels show a strong cytoplasmic AGR2 immunostaining of all epithelial cells of the stomach except parietal cells (a), as well as of all epithelial cells of the duodenal mucosa (b), the colorectal mucosa (c), the endocervical epithelium (d), the endometrium of the pregnant uterus (e), the cauda epididymis (f), and the seminal vesicle (g). Most epithelial cells of a prostate sample are also AGR2 positive (h).
3.3. AGR2 Immunostaining in Neoplastic Tissues
A cytoplasmic AGR2 immunostaining was observed in 7514 (60.4%) of 12,434 analyzable tumors including 732 (5.9%) with weak, 874 (7.0%) with moderate, and 5908 (47.5%) with strong staining intensity. We did not see any membranous or extracellular staining of AGR2. Representative images are shown in Figure 2. At least an occasional weak AGR2 positivity was detected in 103 of 134 tumor types and tumor subtypes and 83 entities included at least one case with strong AGR2 positivity (Table 1). AGR2 expression was most frequently seen in tumors of the female genital tract, particularly adenocarcinoma of the cervix (100%) and mucinous carcinoma of the ovary (100%), various subtypes of breast cancer (57.1%–100%), urothelial carcinoma (74.6%–100%), adenocarcinoma of the upper and lower gastrointestinal tract (93.6%–99.6%), as well as in pancreatico‐biliary cancers (65.2%–98.2%). AGR2 positivity was slightly less common in neuroendocrine neoplasms (20.0%–100%) and in squamous cell carcinoma (46.4%–77.3%). AGR2 staining was only rarely seen in adrenocortical tumors (4.3%–9.3%) and in renal cell carcinoma (7.8%–26.5%), and mainly absent in melanoma, seminoma, embryonal carcinoma, adrenocortical neoplasms, tumors of the hematopoietic and lymphoid tissues, as well as mesenchymal tumors. A graphical representation of a ranking order of AGR2 positive and strongly positive cancers is given in Figure 3. The relationship between AGR2 expression and tumor phenotype in different cancer types is summarized in Table 2. High AGR2 expression was strongly linked to poor ISUP (p < 0.0001), Fuhrman (p < 0.0001), and Thoenes (p < 0.0001) grades as well as advanced pT stage (p = 0.0035) in clear cell renal cell carcinoma (ccRCC). AGR2 staining was associated with shortened recurrence‐free survival (p = 0.0275) in ccRCC. That AGR2 staining was unrelated to overall survival in ccRCC (p = 0.1826), may be due to the low number of positive cases (Figure S2). A reduced AGR2 expression was significantly associated with high BRE grade in breast cancer (p = 0.0049), nodal metastasis (p = 0.0275) and RAS mutation (p = 0.0136) in colorectal cancer, nodal metastasis (p = 0.0482) in endometrioid endometrial carcinoma, high grade in noninvasive urothelial carcinoma (p = 0.0003), and with invasive tumor growth in urothelial carcinoma (p < 0.0001). In urothelial carcinoma, AGR2 staining was unrelated to overall and recurrence‐free survival (p > 0.1). AGR2 immunostaining was unrelated to parameters of tumor aggressiveness in pancreatic, ovarian, and thyroidal cancer as well as papillary renal cell carcinoma although tumors with lower AGR2 expression often tended to exhibit more unfavorable tumor features.
FIGURE 2.
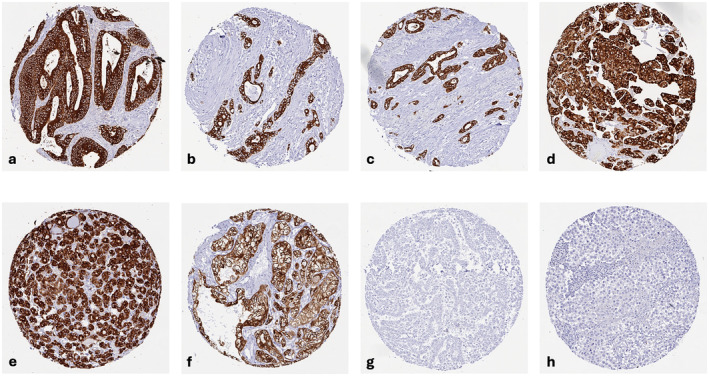
AGR2 immunostaining in cancer. AGR2 staining was cytoplasmic. The panels show a strong AGR2 positivity in cancer cells of adenocarcinoma of colon (a), gastric adenocarcinoma (b), adenocarcinoma of the pancreas (c), urothelial carcinoma (d), follicular thyroid carcinoma (e) and clear cell renal cell carcinoma (f), AGR2 immunostaining is absent in mesothelioma (g), and seminoma (h).
TABLE 1.
AGR2 immunostaining in human tumors.
| Tumor entity | On TMA (n) | AGR2 immunostaining | |||||
|---|---|---|---|---|---|---|---|
| Analyzable (n) | Negative (%) | Weak (%) | Moderate (%) | Strong (%) | |||
| Tumors of the skin | Basal cell carcinoma of the skin | 41 | 13 | 46.2 | 23.1 | 23.1 | 7.7 |
| Squamous cell carcinoma of the skin | 95 | 81 | 59.3 | 23.5 | 11.1 | 6.2 | |
| Malignant melanoma | 19 | 18 | 83.3 | 16.7 | 0.0 | 0.0 | |
| Malignant melanoma lymph node metastasis | 86 | 83 | 96.4 | 2.4 | 1.2 | 0.0 | |
| Merkel cell carcinoma | 2 | 2 | 50.0 | 0.0 | 50.0 | 0.0 | |
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 109 | 92 | 44.6 | 17.4 | 16.3 | 21.7 |
| Squamous cell carcinoma of the pharynx | 60 | 41 | 31.7 | 26.8 | 22.0 | 19.5 | |
| Oral squamous cell carcinoma (floor of the mouth) | 130 | 103 | 47.6 | 30.1 | 10.7 | 11.7 | |
| Pleomorphic adenoma of the parotid gland | 50 | 45 | 44.4 | 15.6 | 26.7 | 13.3 | |
| Warthin tumor of the parotid gland | 49 | 48 | 12.5 | 33.3 | 41.7 | 12.5 | |
| Basal cell adenoma of the salivary gland | 15 | 15 | 33.3 | 26.7 | 40.0 | 0.0 | |
| Tumors of the lung, pleura, and thymus | Adenocarcinoma of the lung | 196 | 130 | 4.6 | 3.1 | 5.4 | 86.9 |
| Squamous cell carcinoma of the lung | 80 | 44 | 22.7 | 20.5 | 15.9 | 40.9 | |
| Mesothelioma, epithelioid | 40 | 25 | 92.0 | 0.0 | 8.0 | 0.0 | |
| Mesothelioma, biphasic | 29 | 17 | 88.2 | 5.9 | 0.0 | 5.9 | |
| Thymoma | 29 | 29 | 72.4 | 10.3 | 17.2 | 0.0 | |
| Lung, neuroendocrine tumor (NET) | 29 | 24 | 45.8 | 0.0 | 33.3 | 20.8 | |
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 30 | 26 | 38.5 | 23.1 | 23.1 | 15.4 |
| Squamous cell carcinoma of the vulva | 107 | 89 | 50.6 | 29.2 | 12.4 | 7.9 | |
| Squamous cell carcinoma of the cervix | 88 | 75 | 38.7 | 13.3 | 18.7 | 29.3 | |
| Adenocarcinoma of the cervix | 23 | 23 | 0.0 | 0.0 | 4.3 | 95.7 | |
| Endometrioid endometrial carcinoma | 288 | 266 | 3.8 | 3.4 | 12.4 | 80.5 | |
| Endometrial serous carcinoma | 36 | 33 | 24.2 | 9.1 | 24.2 | 42.4 | |
| Carcinosarcoma of the uterus | 57 | 46 | 30.4 | 4.3 | 26.1 | 39.1 | |
| Endometrial carcinoma, high grade, G3 | 13 | 13 | 53.8 | 7.7 | 15.4 | 23.1 | |
| Endometrial clear cell carcinoma | 9 | 8 | 37.5 | 0.0 | 0.0 | 62.5 | |
| Endometrioid carcinoma of the ovary | 93 | 71 | 9.9 | 8.5 | 15.5 | 66.2 | |
| Serous carcinoma of the ovary | 530 | 439 | 32.8 | 19.1 | 32.1 | 15.9 | |
| Mucinous carcinoma of the ovary | 75 | 51 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Clear cell carcinoma of the ovary | 51 | 40 | 10.0 | 7.5 | 30.0 | 52.5 | |
| Carcinosarcoma of the ovary | 47 | 35 | 54.3 | 14.3 | 22.9 | 8.6 | |
| Granulosa cell tumor of the ovary | 44 | 42 | 95.2 | 2.4 | 0.0 | 2.4 | |
| Leydig cell tumor of the ovary | 4 | 4 | 75.0 | 25.0 | 0.0 | 0.0 | |
| Sertoli cell tumor of the ovary | 1 | 1 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Sertoli Leydig cell tumor of the ovary | 3 | 3 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Steroid cell tumor of the ovary | 3 | 3 | 66.7 | 33.3 | 0.0 | 0.0 | |
| Brenner tumor | 32 | 28 | 3.6 | 0.0 | 0.0 | 96.4 | |
| Tumors of the breast | Invasive breast carcinoma of no special type | 499 | 344 | 10.2 | 4.4 | 5.5 | 79.9 |
| Lobular carcinoma of the breast | 150 | 107 | 2.8 | 0.9 | 2.8 | 93.5 | |
| Medullary carcinoma of the breast | 8 | 7 | 42.9 | 14.3 | 0.0 | 42.9 | |
| Tubular carcinoma of the breast | 2 | 1 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Mucinous carcinoma of the breast | 7 | 5 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Tumors of the digestive system | Adenomatous polyp, low‐grade dysplasia | 50 | 26 | 0.0 | 0.0 | 0.0 | 100.0 |
| Adenomatous polyp, high‐grade dysplasia | 50 | 38 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Adenocarcinoma of the colon | 2483 | 2236 | 0.4 | 0.7 | 1.4 | 97.5 | |
| Gastric adenocarcinoma, diffuse type | 215 | 191 | 1.6 | 0.0 | 0.5 | 97.9 | |
| Gastric adenocarcinoma, intestinal type | 215 | 186 | 5.4 | 1.1 | 4.8 | 88.7 | |
| Gastric adenocarcinoma, mixed type | 62 | 48 | 2.1 | 2.1 | 2.1 | 93.8 | |
| Adenocarcinoma of the esophagus | 83 | 78 | 6.4 | 1.3 | 5.1 | 87.2 | |
| Squamous cell carcinoma of the esophagus | 76 | 70 | 35.7 | 17.1 | 25.7 | 21.4 | |
| Squamous cell carcinoma of the anal canal | 91 | 65 | 43.1 | 20.0 | 9.2 | 27.7 | |
| Cholangiocarcinoma | 58 | 46 | 34.8 | 4.3 | 8.7 | 52.2 | |
| Gallbladder adenocarcinoma | 51 | 46 | 4.3 | 2.2 | 2.2 | 91.3 | |
| Gallbladder Klatskin tumor | 42 | 33 | 12.1 | 0.0 | 0.0 | 87.9 | |
| Hepatocellular carcinoma | 312 | 277 | 61.4 | 6.5 | 7.2 | 24.9 | |
| Ductal adenocarcinoma of the pancreas | 659 | 435 | 1.8 | 0.5 | 2.8 | 94.9 | |
| Pancreatic/Ampullary adenocarcinoma | 98 | 69 | 4.3 | 1.4 | 1.4 | 92.8 | |
| Acinar cell carcinoma of the pancreas | 18 | 17 | 23.5 | 5.9 | 5.9 | 64.7 | |
| Gastrointestinal stromal tumor (GIST) | 62 | 60 | 98.3 | 0.0 | 1.7 | 0.0 | |
| Appendix, neuroendocrine tumor (NET) | 25 | 16 | 25.0 | 0.0 | 25.0 | 50.0 | |
| Colorectal, neuroendocrine tumor (NET) | 12 | 9 | 11.1 | 0.0 | 11.1 | 77.8 | |
| Ileum, neuroendocrine tumor (NET) | 53 | 45 | 4.4 | 2.2 | 13.3 | 80.0 | |
| Pancreas, neuroendocrine tumor (NET) | 101 | 76 | 55.3 | 7.9 | 18.4 | 18.4 | |
| Colorectal, neuroendocrine carcinoma (NEC) | 14 | 12 | 50.0 | 0.0 | 0.0 | 50.0 | |
| Ileum, neuroendocrine carcinoma (NEC) | 8 | 7 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Gallbladder, neuroendocrine carcinoma (NEC) | 4 | 4 | 75.0 | 0.0 | 0.0 | 25.0 | |
| Pancreas, neuroendocrine carcinoma (NEC) | 14 | 9 | 44.4 | 11.1 | 33.3 | 11.1 | |
| Tumors of the urinary system | Noninvasive papillary urothelial carcinoma, pTa G2 low grade | 87 | 81 | 0.0 | 0.0 | 0.0 | 100.0 |
| Noninvasive papillary urothelial carcinoma, pTa G2 high grade | 80 | 76 | 0.0 | 0.0 | 1.3 | 98.7 | |
| Noninvasive papillary urothelial carcinoma, pTa G3 | 126 | 117 | 0.0 | 3.4 | 6.0 | 90.6 | |
| Urothelial carcinoma, pT2‐4 G3 | 735 | 540 | 19.1 | 11.7 | 13.3 | 55.9 | |
| Squamous cell carcinoma of the bladder | 22 | 21 | 42.9 | 38.1 | 9.5 | 9.5 | |
| Small cell neuroendocrine carcinoma of the bladder | 5 | 5 | 80.0 | 0.0 | 0.0 | 20.0 | |
| Sarcomatoid urothelial carcinoma | 25 | 12 | 50.0 | 16.7 | 25.0 | 8.3 | |
| Urothelial carcinoma of the kidney pelvis | 62 | 59 | 25.4 | 11.9 | 13.6 | 49.2 | |
| Clear cell renal cell carcinoma | 1287 | 1048 | 92.2 | 4.5 | 1.5 | 1.8 | |
| Papillary renal cell carcinoma | 368 | 275 | 73.5 | 16.4 | 6.2 | 4.0 | |
| Clear cell (tubulo) papillary renal cell carcinoma | 26 | 17 | 88.2 | 0.0 | 5.9 | 5.9 | |
| Chromophobe renal cell carcinoma | 170 | 132 | 84.1 | 11.4 | 3.0 | 1.5 | |
| Oncocytoma of the kidney | 257 | 190 | 63.2 | 21.6 | 13.7 | 1.6 | |
| Tumors of the male genital organs | Adenocarcinoma of the prostate, Gleason 3 + 3 | 83 | 83 | 3.6 | 3.6 | 8.4 | 84.3 |
| Adenocarcinoma of the prostate, Gleason 4 + 4 | 80 | 80 | 12.5 | 3.8 | 15.0 | 68.8 | |
| Adenocarcinoma of the prostate, Gleason 5 + 5 | 85 | 85 | 14.1 | 4.7 | 20.0 | 61.2 | |
| Adenocarcinoma of the prostate (recurrence) | 258 | 240 | 11.3 | 6.3 | 18.8 | 63.8 | |
| Small cell neuroendocrine carcinoma of the prostate | 2 | 2 | 0.0 | 0.0 | 50.0 | 50.0 | |
| Seminoma | 682 | 649 | 99.1 | 0.2 | 0.8 | 0.0 | |
| Embryonal carcinoma of the testis | 54 | 45 | 93.3 | 0.0 | 6.7 | 0.0 | |
| Leydig cell tumor of the testis | 31 | 30 | 90.0 | 10.0 | 0.0 | 0.0 | |
| Sertoli cell tumor of the testis | 2 | 2 | 50.0 | 50.0 | 0.0 | 0.0 | |
| Sex cord‐stromal tumor of the testis | 1 | 1 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Spermatocytic tumor of the testis | 1 | 1 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Yolk sac tumor | 53 | 41 | 80.5 | 0.0 | 17.1 | 2.4 | |
| Teratoma | 53 | 41 | 65.9 | 2.4 | 19.5 | 12.2 | |
| Squamous cell carcinoma of the penis | 92 | 69 | 53.6 | 21.7 | 8.7 | 15.9 | |
| Tumors of endocrine organs | Adenoma of the thyroid gland | 63 | 63 | 38.1 | 15.9 | 14.3 | 31.7 |
| Papillary thyroid carcinoma | 341 | 330 | 10.3 | 7.9 | 10.6 | 71.2 | |
| Follicular thyroid carcinoma | 109 | 106 | 34.0 | 13.2 | 16.0 | 36.8 | |
| Medullary thyroid carcinoma | 57 | 56 | 3.6 | 7.1 | 3.6 | 85.7 | |
| Parathyroid gland adenoma | 43 | 41 | 87.8 | 4.9 | 7.3 | 0.0 | |
| Anaplastic thyroid carcinoma | 19 | 19 | 68.4 | 10.5 | 10.5 | 10.5 | |
| Adrenal cortical adenoma | 48 | 43 | 90.7 | 9.3 | 0.0 | 0.0 | |
| Adrenal cortical carcinoma | 27 | 23 | 95.7 | 4.3 | 0.0 | 0.0 | |
| Pheochromocytoma | 51 | 48 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of hematopoietic and lymphoid tissues | Hodgkin's lymphoma | 103 | 93 | 100.0 | 0.0 | 0.0 | 0.0 |
| Small lymphocytic lymphoma, B‐cell type (B‐SLL/B‐CLL) | 50 | 39 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Diffuse large B‐cell lymphoma (DLBCL) | 113 | 101 | 98.0 | 1.0 | 1.0 | 0.0 | |
| Follicular lymphoma | 88 | 77 | 100.0 | 0.0 | 0.0 | 0.0 | |
| T‐cell non‐Hodgkin's lymphoma | 25 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Mantle cell lymphoma | 18 | 15 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Marginal zone lymphoma | 16 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Diffuse large B‐cell lymphoma (DLBCL) in the testis | 16 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Burkitt lymphoma | 5 | 2 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of soft tissue and bone | Granular cell tumor | 23 | 13 | 100.0 | 0.0 | 0.0 | 0.0 |
| Leiomyoma | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyosarcoma | 94 | 90 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Liposarcoma | 96 | 81 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Malignant peripheral nerve sheath tumor (MPNST) | 15 | 13 | 92.3 | 0.0 | 0.0 | 7.7 | |
| Myofibrosarcoma | 26 | 25 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Angiosarcoma | 42 | 28 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Angiomyolipoma | 91 | 69 | 89.9 | 10.1 | 0.0 | 0.0 | |
| Dermatofibrosarcoma protuberans | 21 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Ganglioneuroma | 14 | 10 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Kaposi sarcoma | 8 | 2 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Neurofibroma | 117 | 82 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Sarcoma, not otherwise specified (NOS) | 74 | 62 | 96.8 | 0.0 | 3.2 | 0.0 | |
| Paraganglioma | 41 | 28 | 96.4 | 3.6 | 0.0 | 0.0 | |
| Ewing sarcoma | 23 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Rhabdomyosarcoma | 7 | 6 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Schwannoma | 122 | 92 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Synovial sarcoma | 12 | 9 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Osteosarcoma | 19 | 11 | 90.9 | 9.1 | 0.0 | 0.0 | |
| Chondrosarcoma | 15 | 8 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Rhabdoid tumor | 5 | 5 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Solitary fibrous tumor | 17 | 17 | 100.0 | 0.0 | 0.0 | 0.0 | |
FIGURE 3.
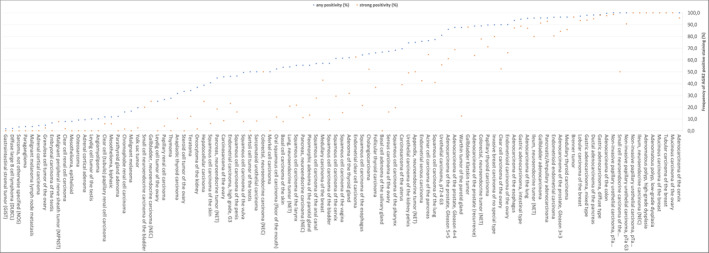
Ranking order of AGR2‐positive immunostaining in different human tumors. Orange dots show the percentage of strongly stained samples, whereas blue dots show the percentage of positive samples of any intensity.
TABLE 2.
AGR2 immunostaining and tumor phenotype.
| AGR2 immunostaining | |||||||
|---|---|---|---|---|---|---|---|
| n | Negative (%) | Weak (%) | Moderate (%) | Strong (%) | p | ||
| Colorectal cancers | All cancers | 2168 | 0.5 | 0.7 | 1.3 | 97.5 | |
| pT1 | 83 | 1.2 | 0 | 1.2 | 97.6 | 0.1215 | |
| pT2 | 421 | 0 | 0.2 | 0.7 | 99 | ||
| pT3 | 1200 | 0.5 | 0.6 | 1.4 | 97.5 | ||
| pT4 | 430 | 0.7 | 1.6 | 1.6 | 96 | ||
| pN0 | 1109 | 0.2 | 0.5 | 0.9 | 98.4 | 0.0275 | |
| pN+ | 1018 | 0.8 | 0.9 | 1.9 | 98.5 | ||
| V0 | 1535 | 0.3 | 0.5 | 1.4 | 97.9 | 0.0514 | |
| V+ | 558 | 1.1 | 1.3 | 1.3 | 96.4 | ||
| L0 | 696 | 0.1 | 0.7 | 0.9 | 98.3 | 0.1817 | |
| L1 | 1407 | 0.6 | 0.7 | 1.6 | 97.1 | ||
| Left colon | 1228 | 0.2 | 0.6 | 1.1 | 98 | 0.3608 | |
| Right colon | 465 | 0.9 | 0.4 | 1.5 | 97.2 | ||
| MSI | 88 | 0 | 0 | 1.1 | 98.9 | 0.7543 | |
| MSS | 1158 | 0.2 | 0.5 | 1.3 | 98 | ||
| RAS mutated | 356 | 0 | 0 | 1.4 | 98.6 | 0.0136 | |
| RAS wildtype | 451 | 0.7 | 1.3 | 1.1 | 96.9 | ||
| BRAF mutated | 22 | 0 | 0 | 0 | 100 | 0.6245 | |
| BRAF wildtype | 131 | 0 | 0.8 | 1.5 | 97.7 | ||
| Endometrioid endometrial carcinoma | pT1 | 104 | 1.9 | 2.9 | 12.5 | 82.7 | 0.297 |
| pT2 | 24 | 4.2 | 0 | 29.2 | 66.7 | ||
| pT3‐4 | 37 | 5.4 | 5.4 | 18.9 | 70.3 | ||
| pN0 | 50 | 4 | 0 | 20 | 76 | 0.0482 | |
| pN+ | 30 | 0 | 10 | 16.7 | 73.3 | ||
| Serous carcinoma of the ovary | pT1 | 30 | 33.3 | 20 | 20 | 26.7 | 0.2592 |
| pT2 | 42 | 42.9 | 23.8 | 21.4 | 11.9 | ||
| pT3 | 245 | 32.2 | 16.3 | 33.9 | 17.6 | ||
| pN0 | 80 | 28.8 | 22.5 | 26.3 | 22.5 | 0.1028 | |
| pN1 | 158 | 39.9 | 17.1 | 30.4 | 12.7 | ||
| Endometrioid carcinoma of the ovary | pT1 | 26 | 0 | 11.5 | 19.2 | 69.2 | 0.2769 |
| pT2 | 5 | 0 | 20 | 0 | 80 | ||
| pT3 | 6 | 16.7 | 16.7 | 0 | 66.7 | ||
| pN0 | 24 | 0 | 12.5 | 4.2 | 83.3 | 0.0673 | |
| pN1 | 8 | 12.5 | 0 | 25 | 62.5 | ||
| Clear cell renal cell carcinoma | all cancers | 1001 | 96.9 | 0.7 | 0.7 | 1.7 | |
| ISUP | |||||||
| 1 | 227 | 99.6 | 0 | 0 | 0.4 | 0.0013 | |
| 2 | 356 | 97.8 | 0.3 | 0.3 | 1.7 | ||
| 3 | 219 | 95.9 | 0.5 | 0.9 | 2.7 | ||
| 4 | 70 | 87.1 | 5.7 | 1.4 | 5.7 | ||
| Fuhrman | |||||||
| 1 | 55 | 98.2 | 0 | 0 | 1.8 | < 0.0001 | |
| 2 | 596 | 98.8 | 0.2 | 0.2 | 0.8 | ||
| 3 | 249 | 96.4 | 0.4 | 1.6 | 1.6 | ||
| 4 | 84 | 83.3 | 6 | 2.4 | 8.3 | ||
| Thoenes | |||||||
| 1 | 302 | 100 | 0 | 0 | 0 | < 0.0001 | |
| 2 | 414 | 97.1 | 0.2 | 0.7 | 1.9 | ||
| 3 | 92 | 85.9 | 5.4 | 2.2 | 6.5 | ||
| UICC | |||||||
| 1 | 259 | 97.7 | 0.8 | 0 | 1.5 | 0.2976 | |
| 2 | 31 | 96.8 | 0 | 3.2 | 0 | ||
| 3 | 83 | 97.6 | 1.2 | 0 | 1.2 | ||
| 4 | 66 | 92.4 | 3 | 1.5 | 3 | ||
| pT1 | 569 | 98.2 | 0.4 | 0.4 | 1.1 | 0.0035 | |
| pT2 | 118 | 99.2 | 0 | 0.8 | 0 | ||
| pT3‐4 | 303 | 93.4 | 1.7 | 1.3 | 3.6 | ||
| pN0 | 149 | 96 | 2 | 0 | 2 | 0.5371 | |
| pN+ | 25 | 96 | 0 | 0 | 4 | ||
| pM0 | 88 | 98.9 | 0 | 0 | 1.1 | 0.0542 | |
| pM+ | 86 | 91.9 | 3.5 | 2.3 | 2.3 | ||
| Papillary renal cell carcinoma | All cancers | 240 | 72.1 | 17.9 | 6.3 | 3.8 | |
| ISUP | |||||||
| 1 | 29 | 89.7 | 6.9 | 3.4 | 0 | 0.194 | |
| 2 | 112 | 64.3 | 21.4 | 8.9 | 5.4 | ||
| 3 | 64 | 71.9 | 21.9 | 3.1 | 3.1 | ||
| 4 | 5 | 80 | 20 | 0 | 0 | ||
| Fuhrman | |||||||
| 1 | 2 | 100 | 0 | 0 | 0 | 0.5608 | |
| 2 | 151 | 70.9 | 17.9 | 7.9 | 3.3 | ||
| 3 | 65 | 73.8 | 21.5 | 1.5 | 3.1 | ||
| 4 | 9 | 77.8 | 11.1 | 11.1 | 0 | ||
| Thoenes | |||||||
| 1 | 45 | 77.8 | 15.6 | 6.7 | 0 | 0.2377 | |
| 2 | 128 | 66.4 | 23.4 | 6.3 | 3.9 | ||
| 3 | 15 | 86.7 | 6.7 | 6.7 | 0 | ||
| UICC | |||||||
| 1 | 76 | 72.4 | 15.8 | 6.6 | 5.3 | 0.7206 | |
| 2 | 11 | 63.6 | 27.3 | 9.1 | 0 | ||
| 3 | 4 | 50 | 25 | 25 | 0 | ||
| 4 | 9 | 55.6 | 11.1 | 22.2 | 11.1 | ||
| pT1 | 166 | 72.3 | 17.5 | 6.6 | 3.6 | 0.9371 | |
| pT2 | 41 | 70.7 | 22 | 4.9 | 2.4 | ||
| pT3‐4 | 27 | 70.4 | 18.5 | 3.7 | 7.4 | ||
| pN0 | 18 | 50 | 27.8 | 22.2 | 0 | 0.1321 | |
| pN+ | 13 | 61.5 | 7.7 | 15.4 | 15.4 | ||
| pM0 | 22 | 40.9 | 27.3 | 18.2 | 13.6 | 0.3536 | |
| pM+ | 11 | 72.7 | 9.1 | 9.1 | 9.1 | ||
| Ductal adenocarcinoma of the pancreas | All cancers | 327 | 2.1 | 0.6 | 3.1 | 94.2 | |
| pT1 | 7 | 0 | 0 | 0 | 100 | 0.8717 | |
| pT2 | 44 | 2.3 | 2.3 | 2.3 | 93.2 | ||
| pT3 | 259 | 1.9 | 0.4 | 3.5 | 94.2 | ||
| pT4 | 17 | 5.9 | 0 | 0 | 94.1 | ||
| G1 | 9 | 0 | 0 | 0 | 100 | 0.073 | |
| G2 | 230 | 2.6 | 0.9 | 1.7 | 94.8 | ||
| G3 | 70 | 0 | 0 | 8.6 | 91.4 | ||
| pN0 | 65 | 0 | 1.5 | 3.1 | 95.4 | 0.3114 | |
| pN+ | 261 | 2.3 | 0.4 | 3.1 | 94.3 | ||
| Papillary thyroid carcinoma |
pT1 |
152 | 9.2 | 10.5 | 9.2 | 71.1 | 0.086 |
| pT2 | 79 | 15.2 | 1.3 | 11.4 | 72.2 | ||
| pT3‐4 | 97 | 8.2 | 9.3 | 12.4 | 70.1 | ||
| pN0 | 91 | 11 | 9.9 | 11 | 68.1 | 0.1302 | |
| pN+ | 121 | 3.3 | 7.4 | 12.4 | 76.9 | ||
| Follicular thyroid carcinoma | pT1 | 15 | 33.3 | 13.3 | 13.3 | 40 | 0.9763 |
| pT2 | 51 | 33.3 | 13.7 | 13.7 | 39.2 | ||
| pT3‐4 | 38 | 34.2 | 13.2 | 21.1 | 31.6 | ||
| pN0 | 30 | 46.7 | 3.3 | 10 | 40 | 0.3741 | |
| pN+ | 2 | 50 | 0 | 50 | 0 | ||
| Invasive breast carcinoma of no special type | pT1 | 115 | 7.8 | 0.9 | 6.1 | 85.2 | 0.6069 |
| pT2 | 147 | 9.5 | 4.8 | 4.8 | 81 | ||
| pT3‐4 | 27 | 7.4 | 3.7 | 3.7 | 85.2 | ||
| G1 | 8 | 0 | 0 | 0 | 100 | 0.0049 | |
| G2 | 162 | 4.3 | 1.9 | 4.3 | 89.5 | ||
| G3 | 122 | 15.6 | 4.9 | 7.4 | 72.1 | ||
| pN0 | 147 | 7.5 | 2 | 6.1 | 84.4 | 0.7739 | |
| pN+ | 117 | 7.7 | 4.3 | 6 | 8.2 | ||
| Urothelial carcinoma | All cancers | 637 | 11.3 | 7.5 | 9.9 | 71.3 | |
| pTa G2 low | 81 | 0 | 0 | 0 | 100 | 0.0003 | |
| pTa high | 76 | 0 | 0 | 1.3 | 98.7 | ||
| pTa G3 | 86 | 0 | 4.7 | 5.8 | 89.5 | ||
| pT2 | 101 | 16.8 | 7.9 | 9.9 | 65.3 | 0.2196 | |
| pT3 | 190 | 17.4 | 12.1 | 17.4 | 53.2 | ||
| pT4 | 93 | 22.6 | 14 | 15.1 | 48.4 | ||
| G2 | 18 | 0 | 5.6 | 5.6 | 88.9 | 0.0066 | |
| G3 | 366 | 19.4 | 11.7 | 15.3 | 53.6 | ||
| pN0 | 212 | 18.9 | 11.8 | 13.7 | 55.7 | 0.8212 | |
| pN+ | 146 | 17.1 | 12.3 | 17.1 | 53.4 | ||
4. Discussion
The successful analysis of 12,434 tumors from 134 different tumor categories provides a comprehensive overview on AGR2 expression in cancer. The data show that the AGR2 findings in tumors largely mirror the observations in normal tissues. A significant AGR2 expression was found in large fractions of cases in virtually all epithelial tumor entities but only rarely in nonepithelial neoplasms. That adenocarcinomas are more commonly positive than squamous cell carcinomas reflects the limited AGR2 expression in normal squamous epithelium. That only few renal cell carcinomas were AGR2 positive also parallels our normal tissue findings as only few tubuli and collecting ducts had shown AGR2 staining in the normal kidney. For the few tumor entities that were previously analyzed for AGR2 expression, our positivity rates were generally in the upper range of earlier data (Figure 4). This demonstrates a comparatively high sensitivity of our assay. Accordingly, we found higher positivity rates for squamous cell carcinomas of the lung (77.3%) than the 49% of Fritzsche et al. [28] and the 45% of Pizzi et al. [24], for squamous cell carcinomas of the esophagus (64.3%) than the 28.4% of Takabatake et al. [29] and the 37% of DiMaio et al. [30] for clear cell carcinomas of the ovary (90%) than the 45% of Armes et al. [25] and the 11.1% of Darb‐Esfahani et al. [31] and for serous high‐grade carcinomas of the ovary (67.2%) than the 32.3% of Darb‐Esfahani et al. [31], the 50% of Park et al. [13], and the 19% of Armes et al. [25]. We also found an AGR2 positivity in 38.6% of our 277 hepatocellular carcinomas, a cancer type where Lepreux et al. [32] had previously not found AGR2 staining in a cohort of 12 cancers. Possible reasons that might have caused these discrepancies include differences in the staining protocols and antibodies used as well as different definitions of thresholds to determine positivity.
FIGURE 4.
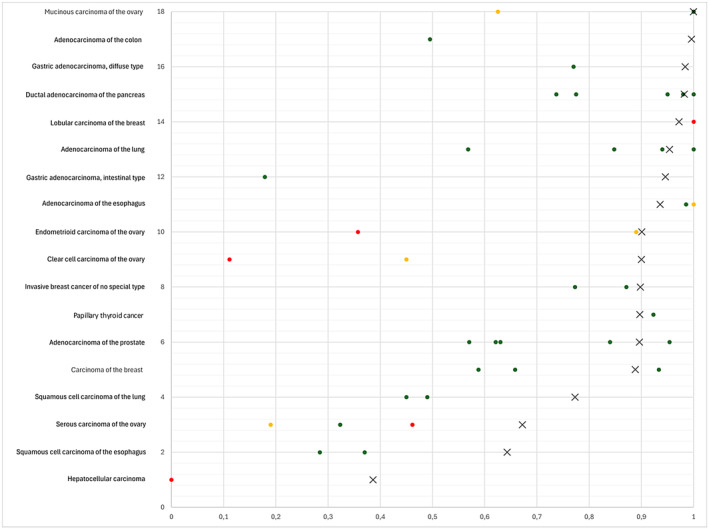
AGR2 protein expression in cancer (own findings vs. literature data). Graphical representation of AGR2 data from this study (X) compared to the previous literature. The colors of the dots represent the number of tumors analyzed in these studies: Red: n ≤ 20, yellow: n = 21 to 100, green: n > 100. For raw data and references, see Table S1.
The comprehensive analysis of a broad range of cancer types and their corresponding normal tissues is an important prerequisite to assess the potential diagnostic utility of an IHC assay. The most striking diagnostic aspect for AGR2 was its significant upregulation in a large number of thyroidal neoplasms. While AGR2 expression was weak or absent in our normal thyroid, a moderate to strong AGR2 staining was observed in 46% of adenomas, 52.8% of follicular carcinomas, 81.8% of papillary carcinomas, and 31.6% of anaplastic carcinomas of the thyroid. AGR2 IHC may therefore be helpful for the distinction of neoplastic from nonneoplastic thyroidal tissues, which is especially challenging in cytology where sensitivity ranges from 45% to 95.2% [33, 34, 35, 36, 37, 38] depending on the subtype. Other potential diagnostic applications of AGR2 IHC may include the distinction of renal cell carcinomas (usually negative) from urothelial carcinomas (usually positive) from the kidney and mesotheliomas (usually negative) from adenocarcinomas (usually positive) of the lung. However, all these potential diagnostic applications need to be further evaluated.
The availability of large subsets of tumors from various important cancer entities enabled us to also address the clinical/prognostic relevance of AGR2 expression in diverse cancers. These data demonstrate that the prognostic role of AGR2 is cancer‐type dependent. In most cancers derived from tissues with high AGR2 expression in normal cells, such as the colorectum, the breast, the endometrium, and the urothelium, a reduced expression paralleled morphological parameters of tumor aggressiveness such as high histologic grade and advanced stage. These findings are in line with earlier data. Associations between reduced AGR2 expression and unfavorable tumor features have been described for colorectal cancer [39], ductal adenocarcinoma of the pancreas [40], prostate cancer [41], ovarian cancer [16, 25], and adenocarcinoma of the lung [42] although other groups could not confirm these findings for ovarian [31] and lung cancer [28] and others even found a link between low AGR expression and favorable tumor features for lung [10], ovarian, [31] and prostate cancer [23]. The reasons for the role of AGR2 loss in tumor progression are not clear. Functional studies have suggested a higher resistance to apoptosis [43] and a reduction of cellular adhesion [40, 43]. Alternatively, it is possible that reduced AGR2 expression in tumors derived from AGR2‐expressing normal cells just reflects tumor cell dedifferentiation which usually parallels cancer progression.
Elevated AGR2 expression was associated with unfavorable tumor features in only one of our cancer entities. The strong link of high AGR2 with high grade and advanced stage in clear cell renal cell carcinoma (ccRCC) is in line with RNA data from the Cancer Genome Atlas (TCGA) which also suggested a poor prognosis in case of high AGR2 expression levels [44]. Although the number of AGR2‐positive cases was low (7.8%) in this cancer entity, AGR2 expression analysis might become clinically useful in this cancer type because patients with high‐risk renal cell carcinomas are increasingly subjected to adjuvant chemotherapy, and risk assessment by histology alone is not sufficient for a safe individual risk assessment. Elevated levels of AGR2 expression have earlier also been linked to unfavorable tumor features in breast cancer [12], prostate cancer [23], head and neck squamous cell carcinoma [45], and squamous cell carcinoma of the esophagus [29].
Potential cancer promoting effects of AGR2 upregulation include a stimulating role on cell proliferation [29, 46, 47], migration [47, 48], epithelial–mesenchymal transition [48], invasion [49, 50], and chemoresistance [47]. Arumugam et al. [47] reported that AGR2 stimulated the proliferation, migration, invasion, and chemoresistance of pancreatic ductal adenocarcinoma cells by interacting with the metastasis‐associated protein C4.4A that is co‐expressed on the cell surface. Jia et al. [51] found that AGR2 directly interacts with VEGFA and enhances VEGFR/VEGFR2 signaling in prostate cancer cells, leading to the acquisition of a mesenchymal phenotype in vivo and in vitro, which is associated with increased invasiveness and metastatic growths. Fessart et al. [46] described a mechanism by which secreted AGR2 acts like a growth factor and stimulates cell proliferation in lung cancer cells by repressing cyclin‐dependent kinase inhibitor 1A (CDKN1A). Zhang et al. [48] reported that intracellular but not extracellular AGR2 promotes the expression of the SNAIL and SLUG transcription factors through direct transcriptional activation and histone 3 acetylation. This drives epithelial–mesenchymal transition in colorectal cancer cells [48], an important cellular process leading to increased migratory capability and invasive potential [52]. Others have found that extracellular AGR2 may promote cell migration and metastasis in CRC through noncanonical Wnt signaling [53]. Takabatake et al. [29] described a direct interaction of AGR2 with the p53 tumor suppressor that results in attenuation of p53 activity and increased cell proliferation in esophageal squamous cell carcinoma cells. Again, it cannot be excluded that AGR2 neo‐expression in tumors derived from non‐AGR2‐expressing normal cells is caused by random alterations occurring because of tumor cell dedifferentiation.
Additional interest in AGR2 is based on its potential as a therapeutic target. Wu et al. [54] showed inhibition of breast cancer growth in vitro using the first developed monoclonal antibody directed against AGR2, termed 184A. Subsequent studies showed the potential therapeutic benefit of a humanized version of 184A in ovarian cancer xenografts [55] and improved survival in lung cancer in mice [56]. Further therapeutic strategies are targeting the influence of AGR2 on chemotherapy resistance. Studies have shown that AGR2 is involved in cellular survival and the development of tamoxifen resistance in breast cancer [57]. Antibodies directed against AGR2 were able to reduce tumor growth in endocrine therapy‐resistant breast cancer [58].
Considering the large scale of our study, our assay was extensively validated according to the recommendations of the international working group of antibody validation (IWGAV) [59] by comparing our IHC findings in normal tissues with data obtained by another independent anti‐AGR2 antibody and RNA data derived from three different publicly accessible databases [60, 61, 62, 63]. To ensure an as broad as possible range of proteins to be tested for a possible cross‐reactivity, 76 different normal tissue categories were included in this analysis. These diverse tissues are likely to contain a large fraction of the proteins expressed in cells of adult humans all of which are screened for potential cross‐reactivities of antibodies. Our normal tissue analysis revealed AGR2 immunostaining in all organs for which RNA expression had been described (salivary glands, stomach, duodenum small intestine, appendix, colorectum, gallbladder, urinary bladder, lung, pituitary gland, epididymis, prostate, seminal vesicle, breast, cervix, and fallopian tube). While significant RNA expression had not been reported for other tissues with IHC‐positive cell types, such as endometrium, intercalated ducts and acinar cells of the pancreas, a subset of tubuli/collecting ducts of the kidney, squamous epithelial cells of the tonsil, hair follicles, and of corpuscles of Hassall's of the thymus, these staining obtained by our assay were confirmed by an independent second AGR2 antibody. It is likely that these cell types are made up of too small fractions of their entire organs to be detected in RNA analysis of disintegrated tissues. Overall, these validation data document a high level of specificity for our AGR2 IHC assay.
In summary, our data provide a comprehensive overview of AGR2 expression in different tumor entities, identify AGR2 IHC as a potential diagnostic aid for the identification of thyroidal neoplasms, and demonstrate a potential prognostic role of AGR2 in various cancer types.
Author Contributions
Nina Schraps: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), visualization (equal), writing – original draft (equal). Jacob Constantin Port: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), visualization (equal), writing – original draft (equal). Anne Menz: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Florian Viehweger: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Seyma Büyücek: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), visualization (equal), writing – review and editing (supporting). David Dum: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Ria Schlichter: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Andrea Hinsch: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Christoph Fraune: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Christian Bernreuther: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Martina Kluth: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Claudia Hube‐Magg: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Katharina Möller: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Viktor Reiswich: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Andreas M. Luebke: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Patrick Lebok: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Sören Weidemann: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Guido Sauter: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), supervision (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Maximilian Lennartz: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Frank Jacobsen: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Till S. Clauditz: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Andreas H. Marx: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Ronald Simon: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), supervision (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Stefan Steurer: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Baris Mercanoglu: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Nathaniel Melling: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Thilo Hackert: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (equal). Eike Burandt: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Natalia Gorbokon: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Sarah Minner: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Till Krech: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), writing – review and editing (supporting). Florian Lutz: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), supervision (equal), writing – original draft (equal), writing – review and editing (equal).
Ethics Statement
The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF‐049/09), allowing the use of leftover diagnostic tissues and corresponding clinical data for scientific research without the patients written consent. All work has been carried out in compliance with the Helsinki Declaration.
Conflicts of Interest
The AGR2 antibody clone HMV‐325 was provided by ardoci GmbH (owned by a family member of G.S.).
Supporting information
Data S1.
Data S2.
Acknowledgments
We are grateful to Laura Behm, Melanie Steurer, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance. Open Access funding enabled and organized by Projekt DEAL.
Funding: The authors received no specific funding for this work.
Nina Schraps and Jacob Constantin Port contributed equally to this work.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
References
- 1. Jach D., Cheng Y., Prica F., Dumartin L., and Crnogorac‐Jurcevic T., “From Development to Cancer ‐ An Ever‐Increasing Role of AGR2,” American Journal of Cancer Research 11 (2021): 5249–5262. [PMC free article] [PubMed] [Google Scholar]
- 2. Aberger F., Weidinger G., Grunz H., and Richter K., “Anterior Specification of Embryonic Ectoderm: The Role of the Xenopus Cement Gland‐Specific Gene XAG‐2,” Mechanisms of Development 72 (1998): 115–130. [DOI] [PubMed] [Google Scholar]
- 3. Zhu Q., Mangukiya H. B., Mashausi D. S., et al., “Anterior Gradient 2 Is Induced in Cutaneous Wound and Promotes Wound Healing Through Its Adhesion Domain,” FEBS Journal 284 (2017): 2856–2869. [DOI] [PubMed] [Google Scholar]
- 4. Park S.‐W., Zhen G., Verhaeghe C., et al., “The Protein Disulfide Isomerase AGR2 Is Essential for Production of Intestinal Mucus,” Proceedings of the National Academy of Sciences of the United States of America 106 (2009): 6950–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tereshina M. B., Ivanova A. S., Eroshkin F. M., et al., “Agr2‐Interacting Prod1‐Like Protein Tfp4 From Xenopus laevis Is Necessary for Early Forebrain and Eye Development as Well as for the Tadpole Appendage Regeneration,” Genes N. Y. N 2000 57 (2019): e23293. [DOI] [PubMed] [Google Scholar]
- 6. Ivanova A. S., Tereshina M. B., Ermakova G. V., Belousov V. V., and Zaraisky A. G., “Agr Genes, Missing in Amniotes, Are Involved in the Body Appendages Regeneration in Frog Tadpoles,” Scientific Reports 3 (2013): 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al‐Shaibi A. A., “Human AGR2 Deficiency Causes Mucus Barrier Dysfunction and Infantile Inflammatory Bowel Disease,” Cellular and Molecular Gastroenterology and Hepatology 12 (2021): 1809–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao F., Edwards R., Dizon D., et al., “Disruption of Paneth and Goblet Cell Homeostasis and Increased Endoplasmic Reticulum Stress in Agr2−/− Mice,” Developmental Biology 338 (2010): 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumartin L., Whiteman H. J., Weeks M. E., et al., “AGR2 Is a Novel Surface Antigen That Promotes the Dissemination of Pancreatic Cancer Cells Through Regulation of Cathepsins B and D ,” Cancer Research 71 (2011): 7091–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ci H. and Wu L., “Expression of KAI1 and AGR2 in Lung Adenocarcinoma and Their Clinicopathological Significance,” Medicine (Baltimore) 101 (2022): e32498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang G., Wang X., Li C., et al., “Integrated Stress Response Couples Mitochondrial Protein Translation With Oxidative Stress Control,” Circulation 144 (2021): 1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barraclough D. L., Platt‐Higgins A., de Silva Rudland S., et al., “The Metastasis‐Associated Anterior Gradient 2 Protein Is Correlated With Poor Survival of Breast Cancer Patients,” American Journal of Pathology 175 (2009): 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park K., Chung Y. J., So H., et al., “AGR2, a Mucinous Ovarian Cancer Marker, Promotes Cell Proliferation and Migration,” Experimental & Molecular Medicine 43 (2011): 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hrstka R., Bouchalova P., Michalova E., et al., “AGR2 Oncoprotein Inhibits p38 MAPK and p53 Activation Through a DUSP10‐Mediated Regulatory Pathway,” Molecular Oncology 10 (2016): 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Z., Hao Y., and Lowe A. W., “The Adenocarcinoma‐Associated Antigen, AGR2, Promotes Tumor Growth, Cell Migration, and Cellular Transformation,” Cancer Research 68 (2008): 492–497. [DOI] [PubMed] [Google Scholar]
- 16. Alves M. R., e Melo N. C., Barros‐Filho M. C., et al., “Downregulation of agr 2, p21, and Cyclin D and Alterations in p53 Function Were Associated With Tumor Progression and Chemotherapy Resistance in Epithelial Ovarian Carcinoma,” Cancer Medicine 7 (2018): 3188–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fritzsche F. R., Dahl E., Pahl S., et al., “Prognostic Relevance of AGR2 Expression in Breast Cancer,” Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 12 (2006): 1728–1734. [DOI] [PubMed] [Google Scholar]
- 18. Guo J., Gong G., and Zhang B., “Identification and Prognostic Value of Anterior Gradient Protein 2 Expression in Breast Cancer Based on Tissue Microarray,” Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine 39 (2017): 1010428317713392. [DOI] [PubMed] [Google Scholar]
- 19. Zhou M., Gan X. L., Ren Y. X., et al., “AGR2 and FOXA1 as Prognostic Markers in ER‐Positive Breast Cancer,” BMC Cancer 23 (2023): 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bu H., Schweiger M. R., Manke T., et al., “Anterior Gradient 2 and 3– Two Prototype Androgen‐Responsive Genes Transcriptionally Upregulated by Androgens and by Oestrogens in Prostate Cancer Cells,” FEBS Journal 280 (2013): 1249–1266. [DOI] [PubMed] [Google Scholar]
- 21. Bu H., Bormann S., Schäfer G., et al., “The Anterior Gradient 2 (AGR2) Gene Is Overexpressed in Prostate Cancer and May Be Useful as a Urine Sediment Marker for Prostate Cancer Detection,” Prostate 71 (2011): 575–587. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J., Jin Y., Xu S., et al., “AGR2 Is Associated With Gastric Cancer Progression and Poor Survival,” Oncology Letters 11 (2016): 2075–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y., Forootan S. S., Liu D., et al., “Increased Expression of Anterior Gradient‐2 Is Significantly Associated With Poor Survival of Prostate Cancer Patients,” Prostate Cancer and Prostatic Diseases 10 (2007): 293–300. [DOI] [PubMed] [Google Scholar]
- 24. Pizzi M., Fassan M., Balistreri M., Galligioni A., Rea F., and Rugge M., “Anterior Gradient 2 Overexpression in Lung Adenocarcinoma,” Applied Immunohistochemistry & Molecular Morphology: AIMM 20 (2012): 31–36. [DOI] [PubMed] [Google Scholar]
- 25. Armes J. E., Davies C. M., Wallace S., Taheri T., Perrin L. C., and Autelitano D. J., “AGR2 Expression in Ovarian Tumours: A Potential Biomarker for Endometrioid and Mucinous Differentiation,” Pathology (Philadelphia, Pa.) 45 (2013): 49–54. [DOI] [PubMed] [Google Scholar]
- 26. Dancau A.‐M., Simon R., Mirlacher M., and Sauter G., “Tissue Microarrays,” Methods in Molecular Biology (Clifton, N.J.) 1381 (2016): 53–65. [DOI] [PubMed] [Google Scholar]
- 27. Kononen J., Bubendorf L., Kallionimeni A., et al., “Tissue Microarrays for High‐Throughput Molecular Profiling of Tumor Specimens,” Nature Medicine 4 (1998): 844–847. [DOI] [PubMed] [Google Scholar]
- 28. Fritzsche F. R., Dahl E., Dankof A., et al., “Expression of AGR2 in Non Small Cell Lung Cancer,” Histology and Histopathology 22 (2007): 703–708. [DOI] [PubMed] [Google Scholar]
- 29. Takabatake K., Konishi H., Arita T., et al., “Anterior Gradient 2 Regulates Cancer Progression in TP53‐Wild‐Type Esophageal Squamous Cell Carcinoma,” Oncology Reports 46 (2021): 260. [DOI] [PubMed] [Google Scholar]
- 30. DiMaio M. A., Kwok S., Montgomery K. D., Lowe A. W., and Pai R. K., “Immunohistochemical Panel for Distinguishing Esophageal Adenocarcinoma From Squamous Cell Carcinoma: A Combination of p63, Cytokeratin 5/6, MUC5AC, and Anterior Gradient Homolog 2 Allows Optimal Subtyping,” Human Pathology 43 (2012): 1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Darb‐Esfahani S., Fritzsche F., Kristiansen G., et al., “Anterior Gradient Protein 2 (AGR2) is an Independent Prognostic Factor in Ovarian High‐Grade Serous Carcinoma,” Virchows Archiv: An International Journal of Pathology 461 (2012): 109–116. [DOI] [PubMed] [Google Scholar]
- 32. Lepreux S., Bioulac‐Sage P., and Chevet E., “Differential Expression of the Anterior Gradient Protein‐2 Is a Conserved Feature During Morphogenesis and Carcinogenesis of the Biliary Tree,” Liver International: Official Journal of the International Association for the Study of the Liver 31 (2011): 322–328. [DOI] [PubMed] [Google Scholar]
- 33. Trimboli P., Treglia G., Guidobaldi L., et al., “Detection Rate of FNA Cytology in Medullary Thyroid Carcinoma: A Meta‐Analysis,” Clinical Endocrinology 82 (2015): 280–285. [DOI] [PubMed] [Google Scholar]
- 34. Hajmanoochehri F. and Rabiee E., “FNAC Accuracy in Diagnosis of Thyroid Neoplasms Considering all Diagnostic Categories of the Bethesda Reporting System: A Single‐Institute Experience,” Journal of Cytology 32 (2015): 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sinna E. A. and Ezzat N., “Diagnostic Accuracy of Fine Needle Aspiration Cytology in Thyroid Lesions,” Journal of the Egyptian National Cancer Institute 24 (2012): 63–70. [DOI] [PubMed] [Google Scholar]
- 36. Musani M. A., Khan F. A., Malik S., and Khambaty Y., “Fine Needle Aspiration Cytology: Sensitivity and Specificity in Thyroid Lesions,” Journal of Ayub Medical College, Abbottabad: JAMC 23 (2011): 34–36. [PubMed] [Google Scholar]
- 37. Elisei R., Bottici V., Luchetti F., et al., “Impact of Routine Measurement of Serum Calcitonin on the Diagnosis and Outcome of Medullary Thyroid Cancer: Experience in 10,864 Patients With Nodular Thyroid Disorders,” Journal of Clinical Endocrinology and Metabolism 89 (2004): 163–168. [DOI] [PubMed] [Google Scholar]
- 38. Cáp J., Ryska A., Rehorková P., Hovorková E., Kerekes Z., and Pohnetalová D., “Sensitivity and Specificity of the Fine Needle Aspiration Biopsy of the Thyroid: Clinical Point of View,” Clinical Endocrinology 51 (1999): 509–515. [DOI] [PubMed] [Google Scholar]
- 39. Riener M.‐O., Thiesler T., Hellerbrand C., et al., “Loss of Anterior Gradient‐2 Expression Is an Independent Prognostic Factor in Colorectal Carcinomas,” European Journal of Cancer (Oxford, England: 1990) 1990, no. 50 (2014): 1722–1730. [DOI] [PubMed] [Google Scholar]
- 40. Mizuuchi Y., Aishima S., Ohuchida K., et al., “Anterior Gradient 2 Downregulation in a Subset of Pancreatic Ductal Adenocarcinoma Is a Prognostic Factor Indicative of Epithelial‐Mesenchymal Transition,” Laboratory Investigation; A Journal of Technical Methods and Pathology 95 (2015): 193–206. [DOI] [PubMed] [Google Scholar]
- 41. Ho M. E., Quek S. I., True L. D., et al., “Prostate Cancer Cell Phenotypes Based on AGR2 and CD10 Expression,” Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc 26 (2013): 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung K., Nishiyama N., Wanibuchi H., et al., “AGR2 as a Potential Biomarker of Human Lung Adenocarcinoma,” Osaka City Medical Journal 58 (2012): 13–24. [PubMed] [Google Scholar]
- 43. Chanda D., Lee J. H., Sawant A., et al., “Anterior Gradient Protein‐2 Is a Regulator of Cellular Adhesion in Prostate Cancer,” PLoS One 9 (2014): e89940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. TCGA , “Research Network,” https://www.cancer.gov/tcga, 2024.
- 45. Sun B., Cheng Z., and Sun J., “Associations of MACC1, AGR2, and KAI1 Expression With the Metastasis and Prognosis in Head and Neck Squamous Cell Carcinoma,” International Journal of Clinical and Experimental Pathology 11 (2018): 822–830. [PMC free article] [PubMed] [Google Scholar]
- 46. Fessart D., de Barbeyrac C., Boutin I., et al., “Extracellular AGR2 Triggers Lung Tumour Cell Proliferation Through Repression of p21CIP1 ,” Biochimica et Biophysica Acta, Molecular Cell Research 1868 (2021): 118920. [DOI] [PubMed] [Google Scholar]
- 47. Arumugam T., Deng D., Bover L., Wang H., Logsdon C. D., and Ramachandran V., “New Blocking Antibodies Against Novel AGR2‐C4.4A Pathway Reduce Growth and Metastasis of Pancreatic Tumors and Increase Survival in Mice,” Molecular Cancer Therapeutics 14 (2015): 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang H., Chi J., Hu J., et al., “Intracellular AGR2 Transduces PGE2 Stimuli to Promote Epithelial‐Mesenchymal Transition and Metastasis of Colorectal Cancer,” Cancer Letters 518 (2021): 180–195. [DOI] [PubMed] [Google Scholar]
- 49. Luu T.‐T., Bach D. H., Kim D., Hu R., Park H. J., and Lee S. K., “Overexpression of AGR2 Is Associated With Drug Resistance in Mutant Non‐small Cell Lung Cancers,” Anticancer Research 40 (2020): 1855–1866. [DOI] [PubMed] [Google Scholar]
- 50. Di Maro G., Salerno P., Unger K., et al., “Anterior Gradient Protein 2 Promotes Survival, Migration and Invasion of Papillary Thyroid Carcinoma Cells,” Molecular Cancer 13 (2014): 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jia M., Guo Y., Zhu D., et al., “Pro‐Metastatic Activity of AGR2 Interrupts Angiogenesis Target Bevacizumab Efficiency via Direct Interaction With VEGFA and Activation of NF‐κB Pathway,” Biochimica et Biophysica Acta ‐ Molecular Basis of Disease 1864 (2018): 1622–1633. [DOI] [PubMed] [Google Scholar]
- 52. Kalluri R. and Weinberg R. A., “The Basics of Epithelial‐Mesenchymal Transition,” Journal of Clinical Investigation 119 (2009): 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tian S., Hu J., Tao K., et al., “Secreted AGR2 Promotes Invasion of Colorectal Cancer Cells via Wnt11‐Mediated Non‐Canonical Wnt Signaling,” Experimental Cell Research 364 (2018): 198–207. [DOI] [PubMed] [Google Scholar]
- 54. Wu Z.‐H., Zhu Q., Gao G.‐W., Zhou C.‐C., and Li D.‐W., “Preparation, Characterization and Potential Application of Monoclonal Antibody 18A4 Against AGR2,” Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin Journal of Cellular and Molecular Immunology 26 (2010): 49–51. [PubMed] [Google Scholar]
- 55. Guo H., Chen H., Zhu Q., et al., “A Humanized Monoclonal Antibody Targeting Secreted Anterior Gradient 2 Effectively Inhibits the Xenograft Tumor Growth,” Biochemical and Biophysical Research Communications 475 (2016): 57–63. [DOI] [PubMed] [Google Scholar]
- 56. Negi H., Merugu S. B., Mangukiya H. B., et al., “Anterior Gradient‐2 Monoclonal Antibody Inhibits Lung Cancer Growth and Metastasis by Upregulating p53 Pathway and Without Exerting any Toxicological Effects: A Preclinical Study,” Cancer Letters 449 (2019): 125–134. [DOI] [PubMed] [Google Scholar]
- 57. Hrstka R., Nenutil R., Fourtouna A., et al., “The Pro‐Metastatic Protein Anterior Gradient‐2 Predicts Poor Prognosis in Tamoxifen‐Treated Breast Cancers,” Oncogene 29 (2010): 4838–4847. [DOI] [PubMed] [Google Scholar]
- 58. Cocce K. J., Jasper J. S., Desautels T. K., et al., “The Lineage Determining Factor GRHL2 Collaborates With FOXA1 to Establish a Targetable Pathway in Endocrine Therapy‐Resistant Breast Cancer,” Cell Reports 29 (2019): 889–903.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uhlen M., Bandrowski A., Carr S., et al., “A Proposal for Validation of Antibodies,” Nature Methods 13 (2016): 823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thul P. J., Åkesson L., Wiking M., et al., “A Subcellular Map of the Human Proteome,” Science 356 (2017): eaal3321. [DOI] [PubMed] [Google Scholar]
- 61. Lizio M., Harshbarger J., Shimoji H., et al., “Gateways to the FANTOM5 Promoter Level Mammalian Expression Atlas,” Genome Biology 16 (2015): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. GTEx Consortium , “The Genotype‐Tissue Expression (GTEx) Project,” Nature Genetics 45 (2013): 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lizio M., Abugessaisa I., Noguchi S., et al., “Update of the FANTOM Web Resource: Expansion to Provide Additional Transcriptome Atlases,” Nucleic Acids Research 47 (2019): D752–D758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data S2.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


