Abstract
Objective
We explored the relationship between achievement of clinical disease control and improvements in and normative values for patient‐reported outcomes (PROs), including quality of life (QoL) measures, in patients with psoriatic arthritis (PsA).
Methods
This was a post hoc analysis of 104‐week data from the SELECT‐PsA 1 and 2 trials in adults with PsA and inadequate response to one or more conventional synthetic (SELECT‐PsA 1) or biologic (SELECT‐PsA 2) disease‐modifying antirheumatic drug. Patients were initially randomized to upadacitinib 15 mg once daily (QD) to placebo switched to upadacitinib 15 mg QD at week 24 or to adalimumab 40 mg every other week (SELECT‐PsA 1 only), and data were pooled across treatments and analyzed. We evaluated several clinical disease control measures (minimal disease activity [MDA]; very low disease activity [VLDA]; and low disease activity [LDA] and/or remission by Disease Activity in Psoriatic Arthritis [DAPSA], Psoriatic Arthritis Disease Activity Score [PASDAS], and Routine Assessment of Patient Index Data 3 [RAPID3]) and examined their associations with improvements and normative values for various PROs.
Results
A total of 1,069 and 317 patients were analyzed for SELECT‐PsA 1 and 2, respectively. In both studies, responders (patients who achieved MDA or VLDA, and DAPSA, PASDAS, and RAPID3 LDA or remission) at week 104 achieved more marked changes from baseline, and more responders achieved normative values in PROs compared with nonresponders (most nominal P < 0.0001). Furthermore, numerically larger proportions of responders achieved minimal clinically important differences across PROs compared with nonresponders in both studies. In addition, patients who achieved MDA or VLDA were more likely to achieve DAPSA, PASDAS, and RAPID3 LDA or remission (all nominal P < 0.0001) for upadacitinib 15 mg QD and when treatment arms were pooled.
Conclusion
Patients with PsA who achieve clinical disease control are more likely to achieve improvements and normative values in PROs and QoL measures, which reinforces disease control as a treatment target.
INTRODUCTION
Psoriatic arthritis (PsA) is a heterogeneous condition associated with a high disease burden and significant comorbidities. 1 The primary treatment goal in PsA is to maximize health‐related quality of life (QoL) by achieving the lowest possible level of disease activity, managing symptoms, and preventing irreversible joint damage and disability. 1 , 2 Disease activity and its control in PsA can be assessed using a variety of measures, including minimal disease activity (MDA) and very low disease activity (VLDA), 3 , 4 as well as other composite measures, such as Disease Activity in Psoriatic Arthritis (DAPSA), 5 Psoriatic Arthritis Disease Activity Score (PASDAS), 6 and Routine Assessment of Patient Index Data 3 (RAPID3). 7 , 8 , 9 Owing to the substantial impact of PsA manifestations, such as enthesitis, dactylitis, tender and swollen joints, and skin and nail psoriasis, on patients’ QoL, patient‐reported outcomes (PROs) are an important part of clinical assessment. 10 , 11
Upadacitinib is an oral, reversible Janus kinase (JAK) inhibitor with selectivity for JAK1 (over JAK2, JAK3, and tyrosine kinase 2). 12 The efficacy and safety of upadacitinib in patients with active PsA were investigated in the Phase 3 SELECT‐PsA clinical trials. 13 , 14 In patients with prior inadequate response or intolerance to one or more conventional synthetic disease‐modifying antirheumatic drug (csDMARD; SELECT‐PsA 1) or biologic disease‐modifying antirheumatic drug (bDMARD; SELECT‐PsA 2), upadacitinib 15 mg once daily (QD) was efficacious in improving the signs and symptoms of PsA, including achievement of MDA, compared with placebo. 13 , 14 , 15 , 16 , 17 , 18 Results from both trials also showed that upadacitinib provided rapid, sustained, and clinically meaningful improvements in a range of PROs. 19 , 20
To further investigate the association between disease activity control and improvement in PROs that are most meaningful to patients, this post hoc analysis of the SELECT‐PsA 1 and 2 clinical trials explored the relationship between achievement of clinical disease control and improvements in and normative values for various PROs, including QoL measures. We also assessed the associations between achievement of MDA and VLDA and responses by other composite disease activity measures.
METHODS
Study design and patient population
Details of the SELECT‐PsA 1 (NCT03104400) and SELECT‐PsA 2 (NCT03104374) Phase 3 trials have been published previously. 13 , 14 In brief, both studies were multicenter, double‐blind, placebo‐controlled trials with an initial duration of 24 weeks, followed by a further 32 weeks of blinded treatment and long‐term, open‐label extensions. This post hoc analysis of pooled treatment arms evaluated 104‐week data from the open‐label extension of both studies.
Eligible patients were adults (age ≥18 years) with active PsA based upon the Classification Criteria for Psoriatic Arthritis, 21 historic or active plaque psoriasis, and both swollen joint count in 66 joints (SJC66) and tender joint count in 68 joints (TJC68) ≥3 at baseline. Patients had inadequate response or intolerance to one or more csDMARD in SELECT‐PsA 1, or one or more bDMARD in SELECT‐PsA 2.
Patients in both studies were initially randomized to receive oral upadacitinib 15 mg QD, upadacitinib 30 mg QD, or placebo. 13 , 14 In SELECT‐PsA 1, an additional cohort was randomized to subcutaneous adalimumab 40 mg every other week (EOW). In both trials, patients on placebo were switched in a blinded manner (1:1) to either upadacitinib 15 mg or 30 mg QD at week 24. At week 16, patients who did not achieve ≥20% improvement in SJC66 and TJC68 were permitted to add or modify background medications, and from week 36, patients who had not achieved ≥20% improvement in SJC66 and TJC68 in two consecutive visits discontinued the study drug. 15 , 16
Both trials were conducted according to the International Conference on Harmonization guidelines and the principles of the Declaration of Helsinki of 1964 and its later amendments. All patients provided written informed consent. The trial protocols were approved by the relevant independent ethics committees and institutional review boards of all participating institutions. 13 , 14
Outcomes
Disease activity was evaluated by MDA and VLDA based on the MDA and VLDA criteria (fulfillment of ≥5 of 7 and 7 of 7 criteria, respectively; Supplementary Table 1). Disease activity was also measured by DAPSA, PASDAS, and RAPID3 and grouped into the following ordinal categories of low disease activity (LDA) and remission (REM): DAPSA LDA ≤14, DAPSA REM ≤4; PASDAS LDA ≤3.2, PASDAS REM ≤1.9; and RAPID3 LDA ≤6, RAPID3 REM ≤3. RAPID3 was included because, although it was developed as a patient‐reported disease activity measure, 22 it demonstrates good agreement with validated physician‐led composite scores, such as MDA, VLDA, and DAPSA, and provides comparable results in clinical trials and clinical practice. 23 Achievements of individual components of MDA were also compared between MDA responders and nonresponders at week 104. Change from baseline, achievement of minimal clinically important differences (MCIDs; patient‐derived scores that reflect changes in a clinical intervention that are meaningful for the patient and require a clinical change in a patient's health status 24 , 25 ), and normative values (observations that describe what is expected in a defined reference population, and at a specific point or period of time 25 , 26 ) for PROs were compared between responders and nonresponders of the disease activity measures listed above at week 104. The PROs and QoL measures included Health Assessment Questionnaire‐Disability Index (HAQ‐DI), 36‐item short‐form quality of life questionnaire (SF‐36) physical component summary (PCS) and mental component summary (MCS), 5‐Level EuroQol 5‐Dimension (EQ‐5D‐5L) index, patients’ global assessment of disease activity (PtGA), patients’ assessment of pain (PtPain), Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), morning stiffness (mean of BASDAI questions 5 and 6), and Work Productivity and Activity Impairment questionnaire (WPAI) overall work impairment. The definitions of MCIDs and normative values used in this study are listed in Table 1.
Table 1.
Definitions of MCID and normative values for patient‐reported outcomes
| PRO | MCID | Normative value |
|---|---|---|
| HAQ‐DI 19 , 22 , 27 , 28 | ≥0.35‐point decrease | ≤0.25 points |
| SF‐36 PCS 19 , 22 | ≥2.5‐point increase | ≥50 points |
| SF‐36 MCS 19 , 22 | ≥2.5‐point increase | ≥50 points |
| EQ‐5D‐5L 19 , 22 , 29 | ≥0.05‐point increase | ≥0.915 points |
| FACIT‐F 19 , 22 , 30 | ≥4‐point increase | ≥40.1 points |
| PtGA 19 , 22 , 31 | ≥1‐point decrease | ≤2 points |
| PtPain 19 , 22 | ≥1‐point decrease | ≤2 points |
| BASDAI 19 , 22 | ≥1.1‐point decrease | — |
| Morning stiffness a , 19 , 22 | ≥1‐point decrease | — |
| WPAI overall work impairment 32 | ≥15% improvement | — |
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; EQ‐5D‐5L, 5‐Level EuroQol 5‐Dimension index; FACIT‐F, Functional Assessment of Chronic Illness Therapy‐Fatigue; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; MCID, minimal clinically important difference; MCS, mental component summary; PCS, physical component summary; PRO, patient‐reported outcome; PtGA, patients’ global assessment of disease activity; PtPain, patients’ assessment of pain; SF‐36, 36‐item short‐form quality of life questionnaire; WPAI, Work Productivity and Activity Impairment questionnaire.
Mean of BASDAI questions 5 and 6.
Associations of MDA and VLDA response with ordinal categories of DAPSA, PASDAS, and RAPID3 at week 104 were also analyzed for upadacitinib alone and the different pooled treatment combinations (upadacitinib 15 mg QD + placebo to upadacitinib 15 mg QD + adalimumab 40 mg EOW for SELECT‐PsA 1; and upadacitinib 15 mg QD + placebo to upadacitinib 15 mg QD for SELECT‐PsA 2).
Statistical analyses
Data were analyzed for SELECT‐PsA 1 and SELECT‐PsA 2 separately, using an as‐observed approach without imputation of data. The analyses included patients who were initially randomized to upadacitinib 15 mg QD, patients who were randomized to placebo and switched to upadacitinib 15 mg QD at week 24 (placebo to upadacitinib 15 mg QD group), and patients who were randomized to adalimumab 40 mg EOW (SELECT‐PsA 1 only). Patients in the upadacitinib 30 mg QD and placebo to upadacitinib 30 mg groups were excluded from the analyses.
Change from baseline analyses were performed for continuous endpoints in analysis of covariance (ANCOVA) models that included responder status at week 104, treatment, current DMARD use (yes or no), and baseline measure. The proportions of patients achieving MCIDs or normative values were assessed with the Mantel‐Haenszel test, adjusting for treatment and current DMARD use (yes or no). Associations of MDA and VLDA with ordinal categories of DAPSA, PASDAS, and RAPID3 were evaluated using the Mantel‐Haenszel chi‐square test with modified ridit scores. Nominal P values were generated for each analysis.
RESULTS
Achievement of clinical disease control response at week 104
In total, 1,069 patients were included in the analysis of SELECT‐PsA 1, and 317 patients were included in the analysis of SELECT‐PsA 2. In both trials, disease control at week 104 was achieved by similar proportions of patients across the upadacitinib 15 mg QD, placebo to upadacitinib 15 mg QD, and adalimumab 40 mg EOW (SELECT‐PsA 1 only) treatment arms. In SELECT‐PsA 1, MDA was achieved by 50 – 55% of patients, whereas VLDA was achieved by 22 – 23% (Figure 1A). Achievement of LDA and REM by DAPSA, PASDAS, and RAPID3 across treatment arms was as follows: DAPSA LDA, 62% to 67% and DAPSA REM, 17 – 23%; PASDAS LDA, 58 – 65% and PASDAS REM, 24 – 27%; RAPID3 LDA, 45 – 51% and RAPID3 REM, 27 – 29% (Figure 1B–D). Treatment responses were similar, although slightly numerically lower, in SELECT‐PsA 2: MDA and VLDA at week 104 across treatment arms were achieved by 34 – 41% and 9 – 14% of patients, respectively (Figure 1A). Achievement of LDA and REM was as follows: DAPSA LDA, 50 – 57% and DAPSA REM, 9 – 17%; PASDAS LDA, 49 – 52% and PASDAS REM, 17 – 19%; RAPID3 LDA, 40 – 44% and RAPID3 REM, 20 – 23% (Figure 1B–D).
Figure 1.

Proportion of patients achieving (A) MDA and VLDA, (B) DAPSA LDA and REM, (C) PASDAS LDA and REM, and (D) RAPID3 LDA and REM by treatment arm in SELECT‐PsA 1 and SELECT‐PsA 2 at week 104 (AO data). MDA response was defined as achievement of ≥5 of the 7 MDA components. VLDA response was defined as achievement of all seven MDA components. DAPSA LDA and REM were defined as ≤14 and ≤4, respectively. PASDAS LDA and REM were defined as ≤3.2 and ≤1.9, respectively. RAPID3 LDA and REM were defined as ≤6 and ≤3, respectively. ADA, adalimumab; AO, as observed; DAPSA, Disease Activity in Psoriatic Arthritis; EOW, every other week; LDA, low disease activity; MDA, minimal disease activity; PASDAS, Psoriatic Arthritis Disease Activity Score; PBO, placebo; PsA, psoriatic arthritis; QD, once daily; REM, remission; UPA, upadacitinib; VLDA, very low disease activity.
Achievement of individual MDA components at week 104 was lower among MDA nonresponders compared with MDA responders, with PtPain ≤1.5 being the MDA component achieved by the lowest proportions of patients across both trials (Figure 2).
Figure 2.
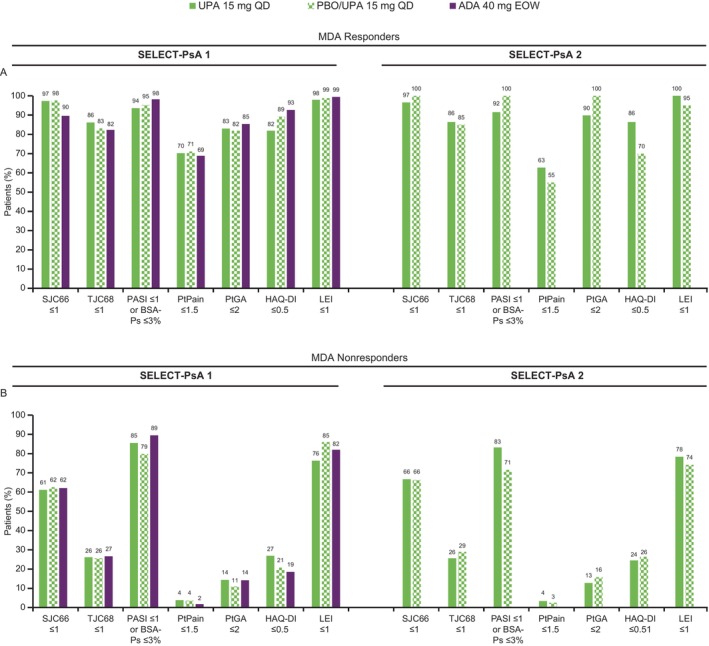
Proportion of patients achieving MDA components among (A) MDA responders and (B) MDA nonresponders by treatment arm in SELECT‐PsA 1 and SELECT‐PsA 2 at week 104 (AO data). MDA response was defined as achievement of ≥5 of the 7 MDA components. ADA, adalimumab; AO, as observed; BSA‐PS, psoriasis body surface area; EOW, every other week; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; LEI, Leeds Enthesitis Index; MDA, minimal disease activity; PASI, Psoriasis Area and Severity Index; PtGA, patients’ global assessment of disease activity; PBO, placebo; PsA, psoriatic arthritis; PtPain, patients’ assessment of pain; QD, once daily; SJC66, swollen joint count in 66 joints; TJC68, tender joint count in 68 joints; UPA, upadacitinib.
Changes in PROs in disease activity measure responders and nonresponders
Changes in a wide range of PROs were assessed by disease activity measure responder status and are presented as changes from baseline (MDA and LDA, Figure 3; VLDA and REM, Supplementary Figure 1), achievement of MCID (MDA and LDA, Figure 4; VLDA and REM, Supplementary Figure 2), and achievement of normative values (MDA and LDA, Supplementary Table 2; VLDA and REM, Supplementary Table 3). Patients in both studies who achieved clinical disease control (defined as MDA or LDA by DAPSA, PASDAS, or RAPID3) at week 104 achieved markedly larger changes from baseline in all investigated PROs compared with nonresponders (Figure 3A–D). A higher proportion of responders also achieved MCID for most PROs versus nonresponders, except for SF‐36 MCS, although numerically more responders achieved SF‐36 MCS MCID compared with nonresponders (Figure 4A–D). Similarly, a larger proportion of responders achieved normative values compared with nonresponders in both studies (Supplementary Table 2).
Figure 3.

Change from baseline in PROs by (A) MDA, (B) DAPSA LDA, (C) PASDAS LDA, and (D) RAPID3 LDA responder status in SELECT‐PsA 1 and SELECT‐PsA 2 at week 104 (AO data). MDA response was defined as achievement of ≥5 of the 7 MDA components. DAPSA LDA was defined as ≤14. PASDAS LDA was defined as ≤3.2. RAPID3 LDA was defined as ≤6. Results are based on an analysis of covariance model with categorical effects for responder status at week 104, treatment, and current disease‐modifying antirheumatic drug use. Baseline measure is included as a covariate. AO, as observed; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CI, confidence interval; DAPSA, Disease Activity in Psoriatic Arthritis; EQ‐5D‐5L, 5‐Level EuroQol 5‐Dimension index; FACIT‐F, Functional Assessment of Chronic Illness Therapy‐Fatigue; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; LDA, low disease activity; LS, least squares; MCS, mental component summary; MDA, minimal disease activity; OWI, overall work impairment; NR, nonresponders; PASDAS, Psoriatic Arthritis Disease Activity Score; PCS, physical component summary; PRO, patient‐reported outcome; PsA, psoriatic arthritis; PtGA, patients’ global assessment of disease activity; PtPain, patients’ assessment of pain; RAPID3, Routine Assessment of Patient Index Data 3; R, responders; SF‐36, 36‐item short‐form quality of life questionnaire; WPAI, Work Productivity and Activity Impairment questionnaire. Nominal P values are provided. *P ≤ 0.05. **P ≤ 0.01. ***P ≤ 0.001. ****P ≤ 0.0001.
Figure 4.

Achievement of MCIDa in PROs by (A) MDA, (B) DAPSA LDA, (C) PASDAS LDA, and (D) RAPID3 LDA responder status in SELECT‐PsA 1 and SELECT‐PsA 2 at week 104 (AO data). MDA response was defined as achievement of ≥5 of the seven MDA components. DAPSA LDA was defined as ≤14. PASDAS LDA was defined as ≤3.2. RAPID3 LDA was defined as ≤6. AO, as observed; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; DAPSA, Disease Activity in Psoriatic Arthritis; EQ‐5D‐5L, 5‐Level EuroQol 5‐Dimension index; FACIT‐F, Functional Assessment of Chronic Illness Therapy‐Fatigue; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; LDA, low disease activity; MCID, minimal clinically important difference; MCS, mental component summary; MDA, minimal disease activity; NR, nonresponders; OWI, overall work impairment; PASDAS, Psoriatic Arthritis Disease Activity Score; PCS, physical component summary; PRO, patient‐reported outcome; PsA, psoriatic arthritis; PtGA, patients’ global assessment of disease activity; PtPain, patients’ assessment of pain; R, responders; RAPID3, Routine Assessment of Patient Index Data 3; R, responders; SF‐36, 36‐item short‐form quality of life questionnaire; WPAI, Work Productivity and Activity Impairment questionnaire. aHAQ‐DI, ≥0.35‐point decrease; SF‐36 PCS, ≥2.5‐point increase; SF‐36 MCS, ≥2.5‐point increase; EQ‐5D‐5L, ≥0.05‐point increase; FACIT‐F, ≥4‐point increase; PtGA, ≥1‐point decrease; PtPain, ≥1‐point decrease; BASDAI, ≥1.1‐point decrease; morning stiffness, ≥1‐point decrease; WPAI OWI, ≥15% improvement. Nominal P values are provided. *P ≤ 0.05. **P ≤ 0.01. ***P ≤ 0.001. ****P ≤ 0.0001.
Among patients who achieved VLDA or REM by DAPSA, PASDAS, or RAPID3, differences in changes from baseline in PROs and proportions of patients achieving normative values were broadly similar between the two studies (Supplementary Figure 1 and Supplementary Table 3). Although a numerically higher proportion of responders achieved MCID improvements in all PROs compared with nonresponders, several did not reach significance (nominal P > 0.05), particularly in SELECT‐PsA 2 (Supplementary Figure 2).
Association of MDA and VLDA with other composite disease activity measures
Associations were observed between MDA and VLDA and other composite measures of PsA disease activity (DAPSA, PASDAS, and RAPID3) in both studies for the upadacitinib 15 mg QD arm, and the pooled upadacitinib 15 mg QD and placebo to upadacitinib (and adalimumab 40 mg EOW for SELECT‐PsA 1 only) arms (Figure 5), with MDA and VLDA responders being more likely to achieve DAPSA, PASDAS, and RAPID3 LDA and REM (all nominal P < 0.0001).
Figure 5.

Association between achievement of MDA or VLDA and composite disease activity measures for upadacitinib 15 mg QD in (A) SELECT‐PsA 1 and (B) SELECT‐PsA 2, and for the pooled upadacitinib treatment arms in (C) SELECT‐PsA 1 and (D) SELECT‐PsA 2 at week 104 (AO data). Nonresponse was defined as achievement of ≤4 of 7 MDA components; MDA (not VLDA) response was defined as achievement of 5 or 6 of the 7 MDA components; VLDA response was defined as achievement of all 7 MDA components. AO, as observed; DAPSA, Disease Activity in Psoriatic Arthritis; EOW, every other week; MDA, minimal disease activity; NR, nonresponders; PASDAS, Psoriatic Arthritis Disease Activity Score; PsA, psoriatic arthritis; QD, once daily; RAPID3, Routine Assessment of Patient Index Data 3; UPA, upadacitinib; VLDA, very low disease activity. aUPA 15 mg QD + placebo to UPA 15 mg QD + adalimumab 40 mg EOW. bUPA 15 mg QD + placebo to UPA 15 mg QD. Percentages may not total 100% due to rounding. Nominal P values are provided. *P ≤ 0.0001.
DISCUSSION
This post hoc analysis of the SELECT‐PsA 1 and 2 studies explored the relationship between achievement of clinical disease control and improvements and normative values in PROs, as well as the association between MDA and VLDA and the achievement of DAPSA, PASDAS, and RAPID3 responses. Overall, patients who achieved disease control (defined as MDA or VLDA and LDA or REM by DAPSA, PASDAS, or RAPID3 ) were more likely to achieve MCID or normative values across a wide range of PROs, including QoL measures, compared with those who did not. Greater improvements from baseline in PROs were also observed in responders versus nonresponders at week 104. The results observed were generally similar for SELECT‐PsA 1 and 2 (ie, regardless of inadequate response or intolerance to csDMARDs or bDMARDs), which is of interest considering the more treatment‐refractory population in SELECT‐PsA 2. The patterns observed are consistent with an earlier analysis evaluating the association between disease activity and QoL using 24‐ and 56‐week pooled data from SELECT‐PsA 1 and 2. 33
Similar to the results observed here, associations between disease activity measures and PROs have been reported in a pooled analysis of two ixekizumab studies in patients with PsA who were bDMARD‐naive or had inadequate response or intolerance to prior bDMARD treatment. 34 In that study, patients who achieved MDA had significantly greater improvements versus nonresponders in a range of PROs, including SF‐36 PCS and EQ‐5D‐5L, and were significantly more likely to achieve MCIDs. Similarly, an analysis at two years from an ongoing five‐year, randomized study of secukinumab in adult patients with active PsA (FUTURE 2) demonstrated that improvements in PROs, including health‐related QoL, physical and social function, fatigue, and work productivity, were significantly better for patients with DAPSA LDA or REM versus DAPSA moderate‐to‐high disease activity, and for MDA responders versus nonresponders. 35 In a post hoc analysis of two tofacitinib studies in patients with PsA, approximately linear relationships were identified between disease activity (MDA as a continuous outcome [ScoreMDA] and PASDAS) and PROs, including EQ‐5D‐3L. 36 Similarly, cross‐sectional and longitudinal analyses from noninterventional studies of patients with PsA showed that DAPSA and clinical DAPSA (DAPSA without C‐reactive protein) are linked to patient perceptions of remission, flares, EQ‐5D utilities, and QoL. 37 , 38 , 39
In the present study, MDA and VLDA achievement was associated with a higher probability of lower composite DAPSA, PASDAS, and RAPID3 scores. Earlier results also showed a high degree of overlap between patients with LDA across composite indices, including MDA, DAPSA, and PASDAS at week 56 in SELECT‐PsA 1. 40
The results of this study highlight the association of disease activity measures with improvements in PROs, including QoL measures. MDA and DAPSA LDA are valid, comprehensive measures of disease activity in PsA, with patients achieving MDA or DAPSA LDA also appearing to consistently achieve important improvements in PROs. This is consistent with a previous analysis of MDA in patients with PsA based on 10 randomized controlled trials and two long‐term observational studies, which demonstrated that MDA is a clinically meaningful measure that can detect between‐group and intraindividual changes in clinical disease activity. 41
Limitations of this study include the post hoc nature of the analysis, which was not powered specifically to conduct responder analyses, although the data are derived from two robust randomized controlled trials. Other limitations include the limited generalizability of the results (although patients with inadequate response or intolerance to csDMARDs and bDMARDs were analyzed) and missing data due to attrition and loss to follow‐up. In addition, some of the PROs used are components of the disease measures evaluated and may therefore be expected to correlate with these measures; however, several PROs are used that are not components of disease measures. Although data on additional PROs that are independent of the composite disease activity measures used in this study (such as the Health‐Related QoL [HRQoL] measures SF‐36 total score and EQ‐5D) were available from the source studies, the correlations of these measures with disease activity measurements were not evaluated in this post hoc analysis. Future analyses of any potential correlations with such independent measures of HRQoL would be of interest to validate and expand on the associations observed here. The small size of some patient subgroups, particularly in the VLDA analyses, may also affect the robustness of the results. Finally, the attainment of MCIDs and normative values must be approached with caution, as the calculation of MCIDs is not standardized and may be subject to methodologic or interpretation problems, whereas normative values may vary among different countries and cultures, and the validity of their interpretation depends on the reference population.
In conclusion, patients with PsA who achieve responses in measures of disease control, including MDA, VLDA, and LDA and REM by DAPSA, PASDAS, and RAPID3, are more likely to achieve benefits in PROs, including QoL measures, demonstrated as changes from baseline, clinically meaningful improvements, and achievement of normative values. These results further suggest that clinical disease activity control measures are key to the treatment of PsA and are closely tied to the achievement of outcomes that are important to patients.
AUTHOR CONTRIBUTIONS
All authors contributed to at least one of the following manuscript preparation roles: conceptualization AND/OR methodology, software, investigation, formal analysis, data curation, visualization, and validation AND drafting or reviewing/editing the final draft. As corresponding author, Dr Kavanaugh confirms that all authors have provided the final approval of the version to be published, and takes responsibility for the affirmations regarding article submission (eg, not under consideration by another journal), the integrity of the data presented, and the statements regarding compliance with institutional review board/Helsinki Declaration requirements.
ROLE OF THE STUDY SPONSOR
AbbVie funded this trial and participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Supporting information
Disclosure form
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
AbbVie and the authors thank the participants, study sites, and investigators who participated in these clinical trials. Medical writing support was provided by Dan Booth, PhD, on behalf of 2 the Nth (Cheshire, UK), and was funded by AbbVie.
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis datasets), as well as other information (eg, protocols, clinical study reports, or analysis plans), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan, and execution of a Data Sharing Agreement. Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing.
Additional supplementary information cited in this article can be found online in the Supporting Information section (https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/acr2.11714).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11714.
REFERENCES
- 1. Coates LC, Soriano ER, Corp N, et al; GRAPPA Treatment Recommendations domain subcomittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022;18(8):465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79(6):700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69(1):48–53. [DOI] [PubMed] [Google Scholar]
- 4. Coates LC, Helliwell PS. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol 2016;43(2):371–375. [DOI] [PubMed] [Google Scholar]
- 5. Schoels M, Aletaha D, Funovits J, et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69(8):1441–1447. [DOI] [PubMed] [Google Scholar]
- 6. Helliwell PS, FitzGerald O, Fransen J, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72(6):986–991. [DOI] [PubMed] [Google Scholar]
- 7. Coates LC, Tillett W, Shaddick G, et al. Value of the routine assessment of Patient Index Data 3 in patients with psoriatic arthritis: results from a tight‐control clinical trial and an observational cohort. Arthritis Care Res (Hoboken) 2018;70(8):1198–1205. [DOI] [PubMed] [Google Scholar]
- 8. Leung YY, Tillett W, de Wit M, et al. Initiating evaluation of composite outcome measures for psoriatic arthritis: 2022 updates from the GRAPPA‐OMERACT working group. J Rheumatol 2023;50(suppl 2):53–57. [DOI] [PubMed] [Google Scholar]
- 9. Aouad K, Moysidou G, Rakotozafiarison A, et al. Outcome measures used in psoriatic arthritis registries and cohorts: A systematic literature review of 27 registries or 16,183 patients. Semin Arthritis Rheum 2021;51(4):888–894. [DOI] [PubMed] [Google Scholar]
- 10. Wervers K, Luime JJ, Tchetverikov I, et al. Influence of disease manifestations on health‐related quality of life in early psoriatic arthritis. J Rheumatol 2018;45(11):1526–1531. [DOI] [PubMed] [Google Scholar]
- 11. Salaffi F, Carotti M, Gasparini S, et al. The health‐related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes 2009;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT‐494). BMC Rheumatol 2018;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med 2021;384(13):1227–1239. [DOI] [PubMed] [Google Scholar]
- 14. Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT‐PsA 2. Ann Rheum Dis 2021;80(3):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McInnes IB, Kato K, Magrey M, et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non‐biological therapy: 56‐week data from the phase 3 SELECT‐PsA 1 study. RMD Open 2021;7(3):e001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mease PJ, Lertratanakul A, Papp KA, et al. Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 56‐week data from the randomized controlled Phase 3 SELECT‐PsA 2 study. Rheumatol Ther 2021;8(2):903–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mease P, Setty A, Papp K, et al. Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 3‐year results from the open‐label extension of the randomised controlled phase 3 SELECT‐PsA 2 study. Clin Exp Rheumatol 2023;41(11):2286–2297. [DOI] [PubMed] [Google Scholar]
- 18. McInnes IB, Kato K, Magrey M, et al. Efficacy and safety of upadacitinib in patients with psoriatic arthritis: 2‐year results from the phase 3 SELECT‐PsA 1 study. Rheumatol Ther 2023;10(1):275–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strand V, Mease PJ, Soriano ER, et al. Improvement in patient‐reported outcomes in patients with psoriatic arthritis treated with upadacitinib versus placebo or adalimumab: results from SELECT‐PsA 1. Rheumatol Ther 2021;8(4):1789–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strand V, Van den Bosch F, Ranza R, et al. Patient‐reported outcomes in psoriatic arthritis patients with an inadequate response to biologic disease‐modifying antirheumatic drugs: SELECT‐PsA 2. Rheumatol Ther 2021;8(4):1827–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tillett W, Costa L, Jadon D, et al. The ClASsification for Psoriatic ARthritis (CASPAR) criteria–a retrospective feasibility, sensitivity, and specificity study. J Rheumatol 2012;39(1):154–156. [DOI] [PubMed] [Google Scholar]
- 22. Orbai AM, Ogdie A. Patient‐reported outcomes in psoriatic arthritis. Rheum Dis Clin North Am 2016;42(2):265–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ward L, Oliffe M, Kane B, et al. Correlation of patient‐reported routine assessment of patient index data with clinical measures of disease activity in psoriatic arthritis. Int J Rheum Dis 2022;25(5):584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cook CE. Clinimetrics corner: the minimal clinically important change score (MCID): a necessary pretense. J Man Manip Ther 2008;16(4):E82–E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strand V, Boers M, Idzerda L, et al. It's good to feel better but it's better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol 2011;38(8):1720–1727. [DOI] [PubMed] [Google Scholar]
- 26. O'Connor PJ. Normative data: their definition, interpretation, and importance for primary care physicians. Fam Med 1990;22(4):307–311. [PubMed] [Google Scholar]
- 27. Mease PJ, Woolley JM, Bitman B, et al. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient‐rated importance and satisfaction. J Rheumatol 2011;38(11):2461–2465. [DOI] [PubMed] [Google Scholar]
- 28. Krishnan E, Sokka T, Häkkinen A, et al. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum 2004;50(3):953–960. [DOI] [PubMed] [Google Scholar]
- 29. Hinz A, Kohlmann T, Stöbel‐Richter Y, et al. The quality of life questionnaire EQ‐5D‐5L: psychometric properties and normative values for the general German population. Qual Life Res 2014;23(2):443–447. [DOI] [PubMed] [Google Scholar]
- 30. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson JK, Zimmerman L, Caplan L, et al. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28‐Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score‐II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index‐5 (RADAI‐5), Chronic Arthritis Systemic Index (CASI), Patient‐Based Disease Activity Score With ESR (PDAS1) and Patient‐Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI‐RA). Arthritis Care Res (Hoboken) 2011;63(suppl 11):S14–S36. [DOI] [PubMed] [Google Scholar]
- 32. Tillett W, Lin CY, Zbrozek A, et al. A threshold of meaning for work disability improvement in psoriatic arthritis measured by the Work Productivity and Activity Impairment questionnaire. Rheumatol Ther 2019;6(3):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kavanaugh A, Mease PJ, Douglas K, et al. AB0547 Association between achievement of low disease activity or remission with improvement in quality of life in upadacitinib‐treated patients in the phase 3 SELECT‐PsA 1 and 2 studies. Ann Rheum Dis 2021;80(suppl 1):1306–1307.33762264 [Google Scholar]
- 34. Coates LC, Orbai AM, Morita A, et al. Achieving minimal disease activity in psoriatic arthritis predicts meaningful improvements in patients' health‐related quality of life and productivity. BMC Rheumatol 2018;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coates LC, Nash P, Kvien TK, et al. Comparison of remission and low disease activity states with DAPSA, MDA and VLDA in a clinical trial setting in psoriatic arthritis patients: 2‐year results from the FUTURE 2 study. Semin Arthritis Rheum 2020;50(4):709–718. [DOI] [PubMed] [Google Scholar]
- 36. Coates LC, Bushmakin AG, FitzGerald O, et al. Relationships between psoriatic arthritis composite measures of disease activity with patient‐reported outcomes in phase 3 studies of tofacitinib. Arthritis Res Ther 2021;23(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mlcoch T, Tuzil J, Sedova L, et al. Mapping quality of Life (EQ‐5D) from DAPSA, clinical DAPSA and HAQ in psoriatic arthritis. Patient 2018;11(3):329–340. [DOI] [PubMed] [Google Scholar]
- 38. Gorlier C, Orbai AM, Puyraimond‐Zemmour D, et al. Comparing patient‐perceived and physician‐perceived remission and low disease activity in psoriatic arthritis: an analysis of 410 patients from 14 countries. Ann Rheum Dis 2019;78(2):201–208. [DOI] [PubMed] [Google Scholar]
- 39. Sousa M, Lubrano E, Smolen JS, et al. Patient‐defined flares and disease activity worsening in 222 patients with psoriatic arthritis from 14 countries. Joint Bone Spine 2023;90(3):105511. [DOI] [PubMed] [Google Scholar]
- 40. Smolen JS, Lubrano E, Kishimoto M, et al. POS1025 Comparison of composite indices for disease activity in patients with psoriatic arthritis treated with upadacitinib: a post‐hoc analysis from SELECT‐PsA 1. Ann Rheum Dis 2022;81(suppl 1):824. [Google Scholar]
- 41. Coates LC, Strand V, Wilson H, et al. Measurement properties of the minimal disease activity criteria for psoriatic arthritis. RMD Open 2019;5(2):e001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Appendix S1: Supplementary Information


