Abstract
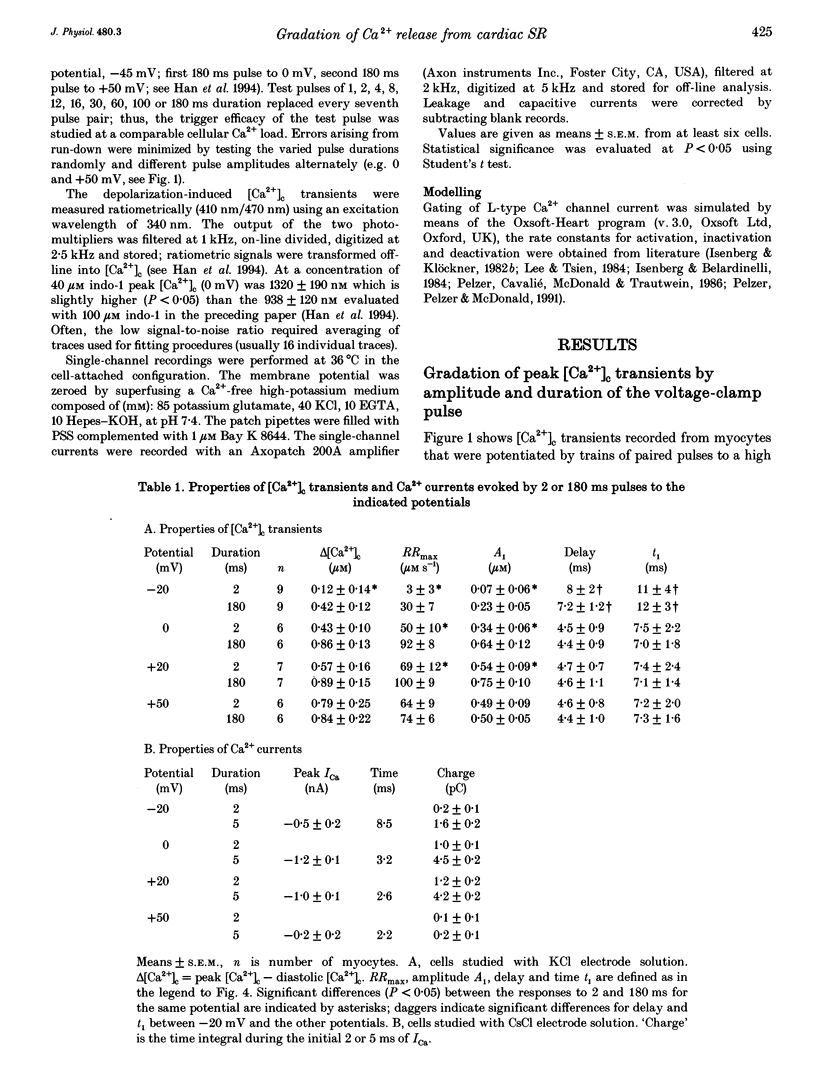
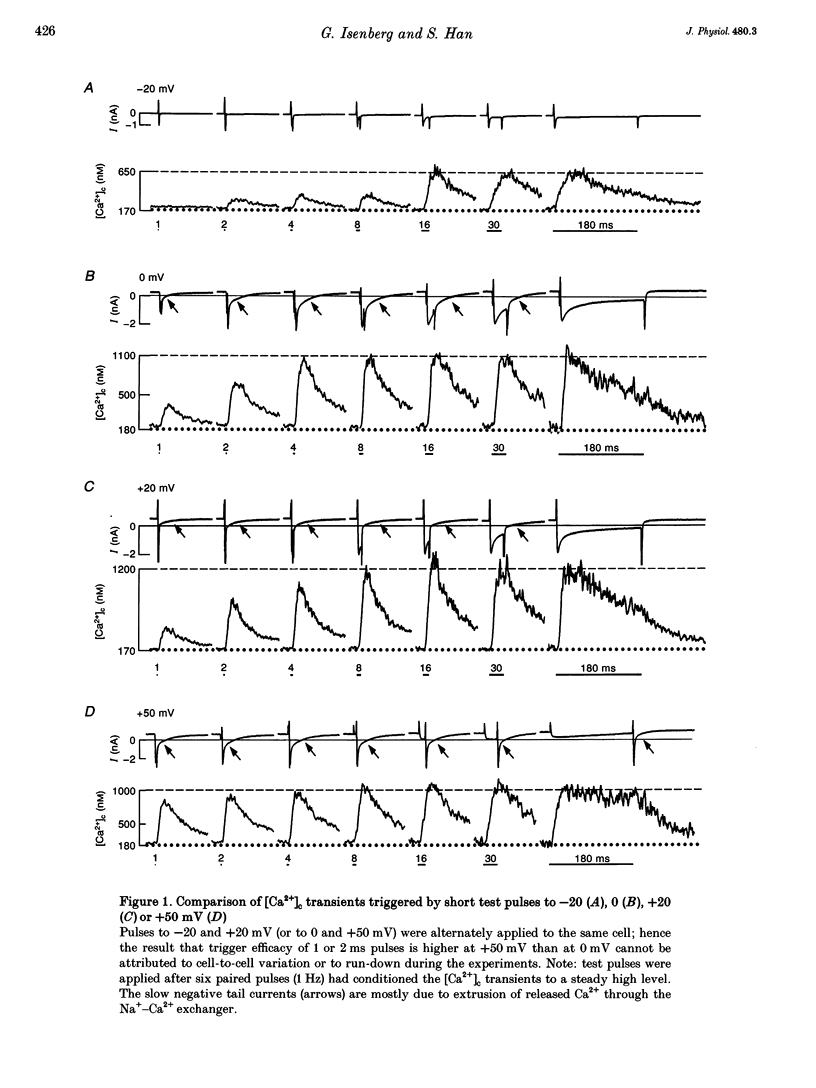
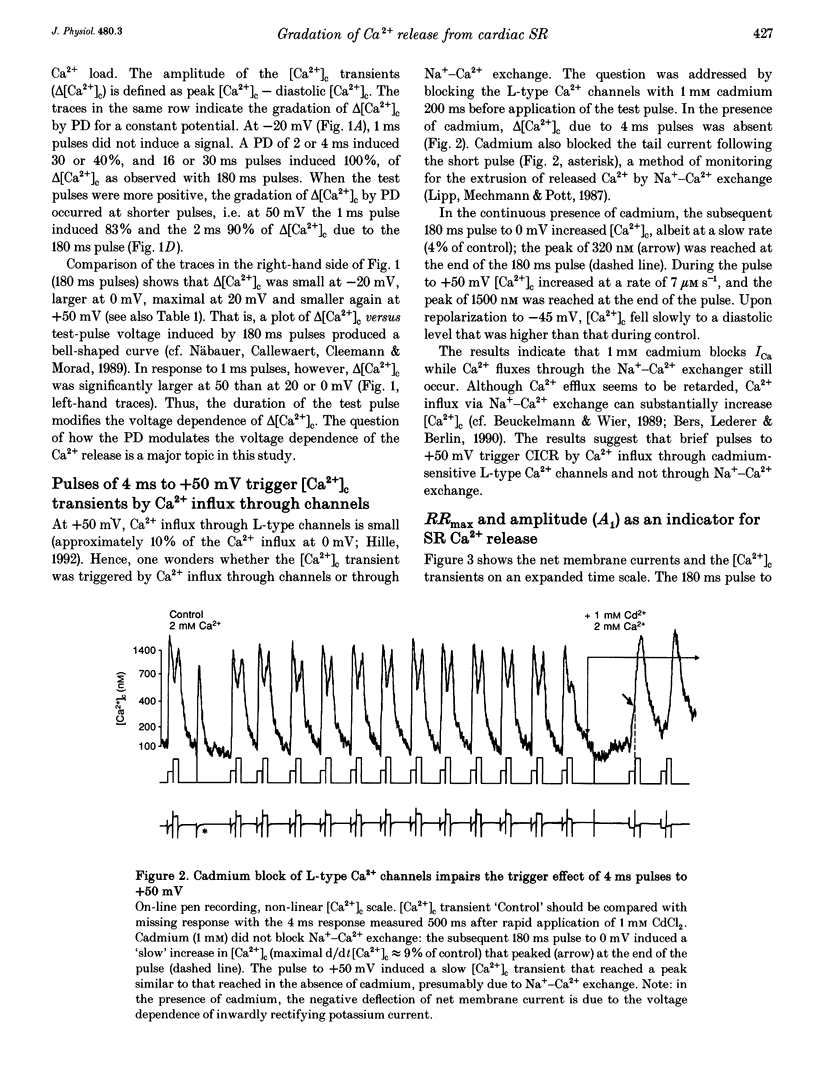
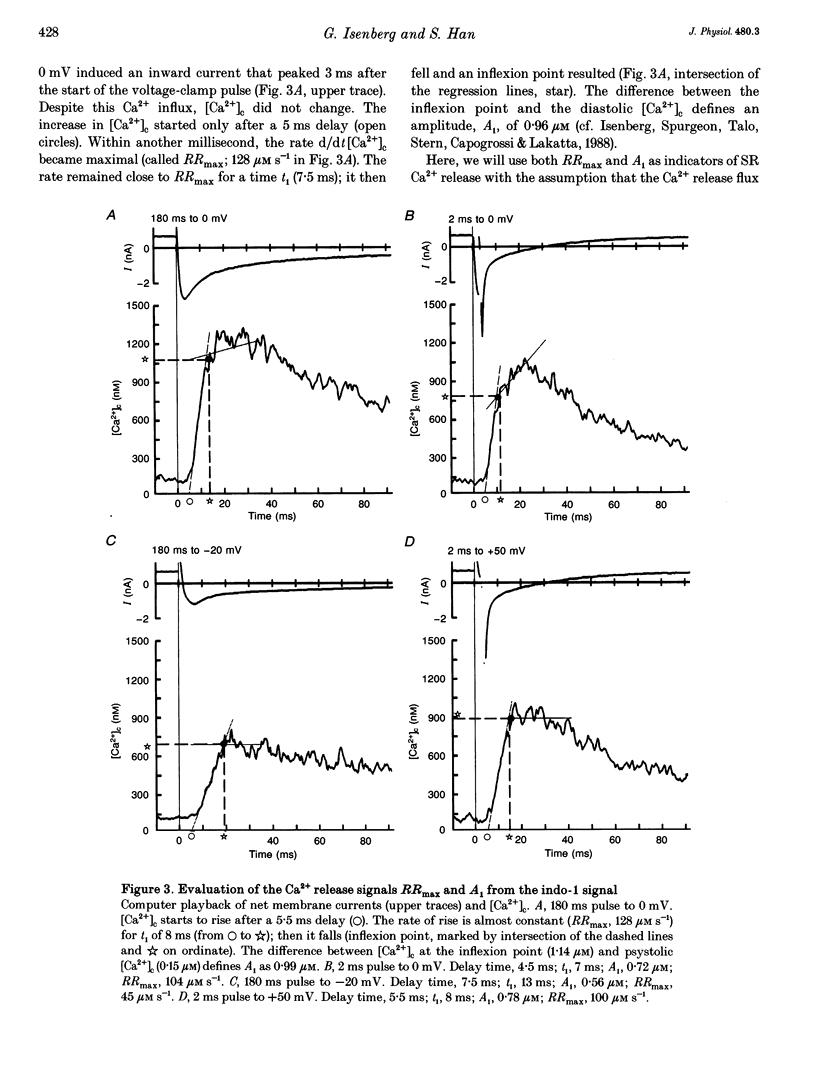
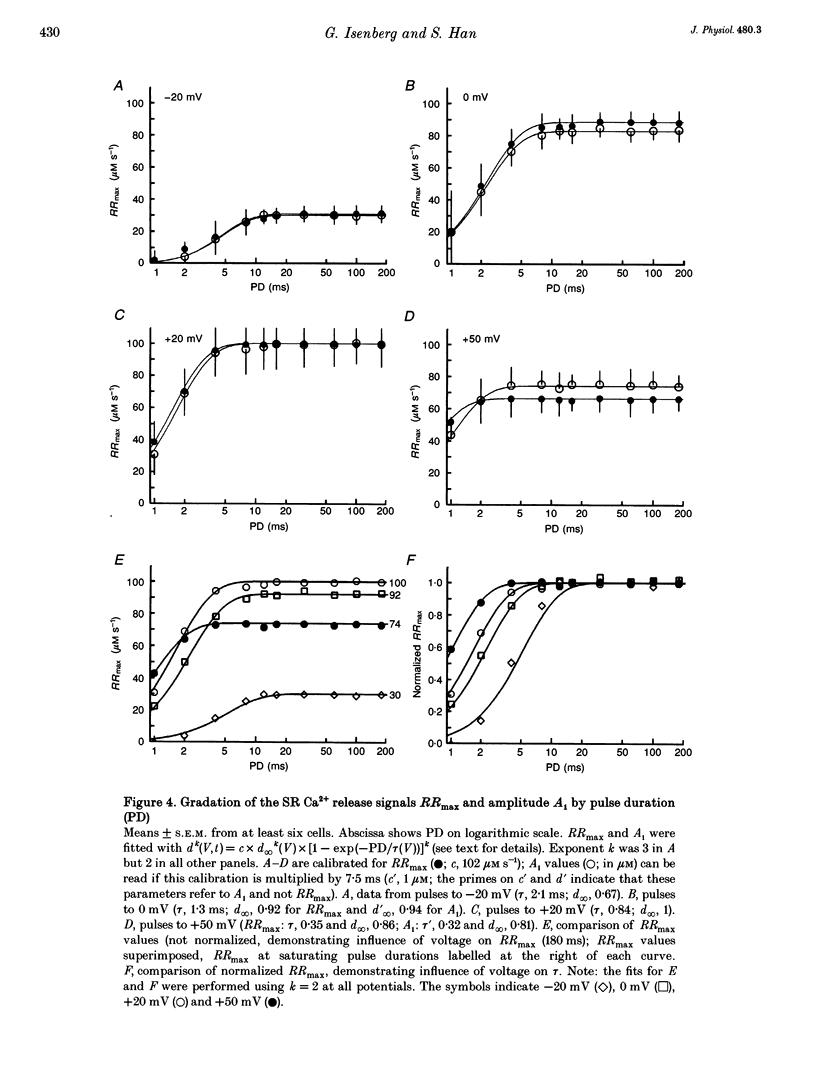
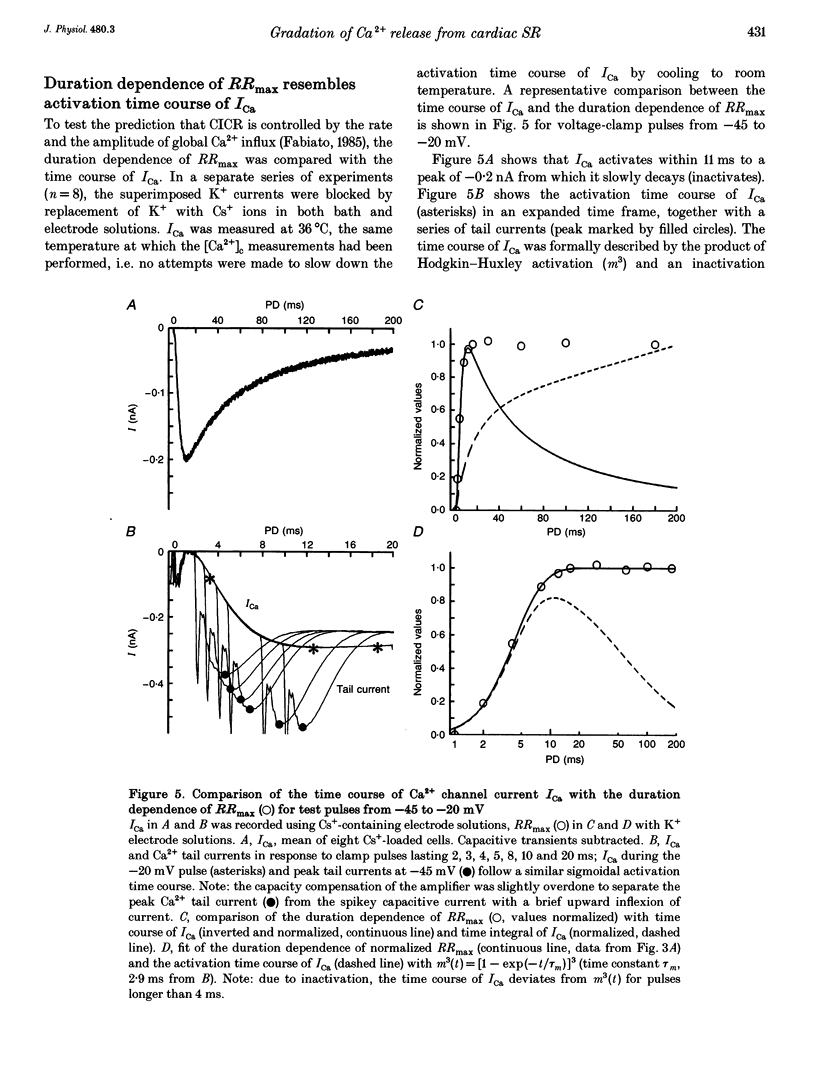
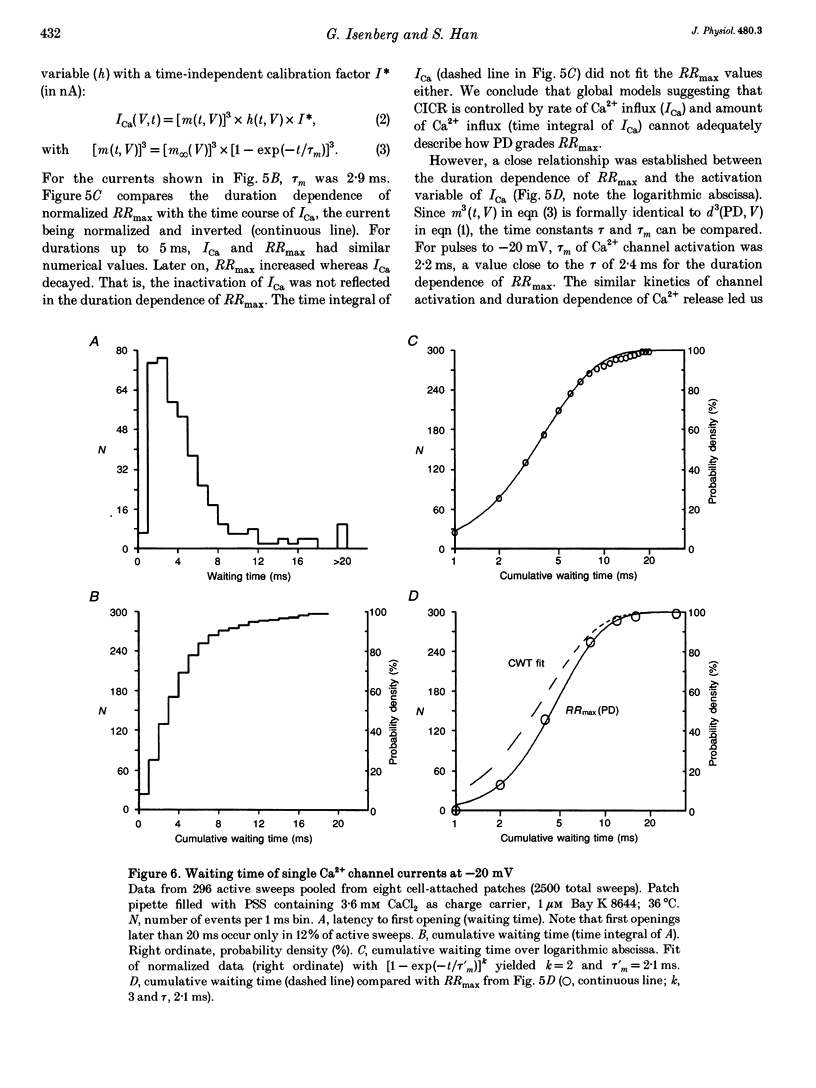
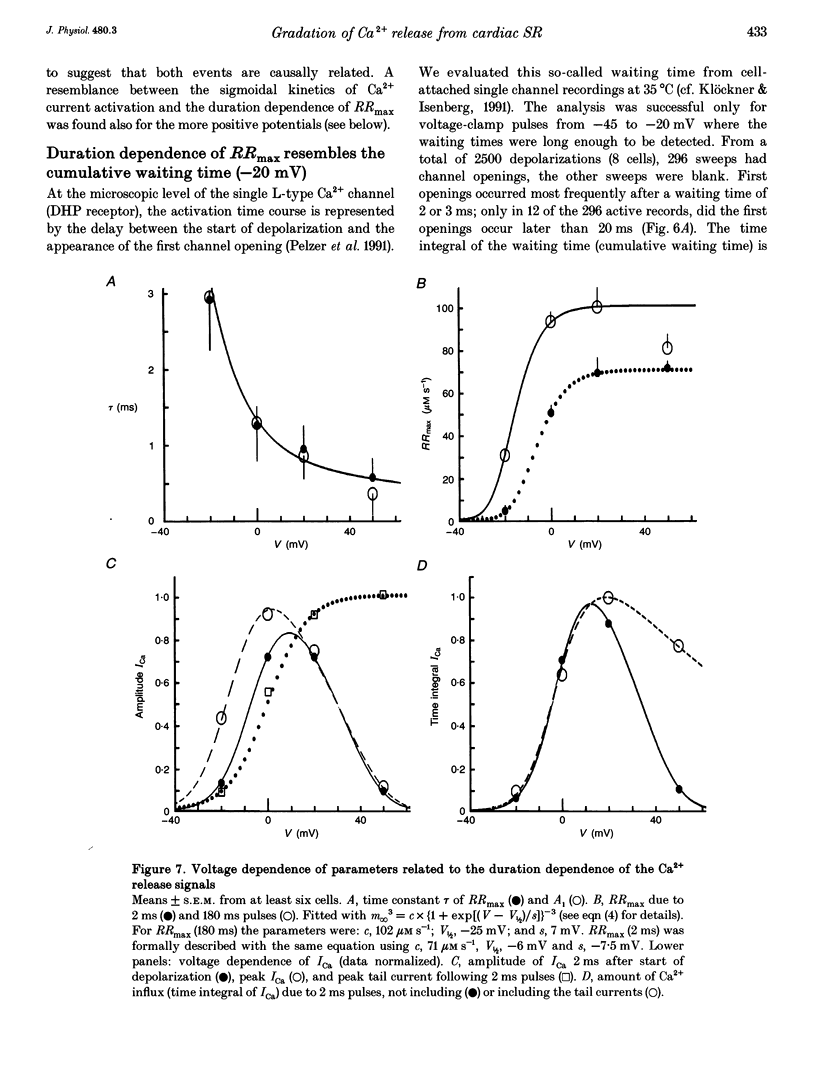
1. This study tests the hypothesis that whole-cell cardiac SR Ca2+ release is graded by recruitment of independent 'release units'. Structurally, an individual release unit may comprise ca four sarcolemmal L-type Ca2+ channels, adjacent ryanodine-sensitive sarcoplasmic reticulum (SR) Ca2+ release channels and the junctional gap between them. After depolarization, the first opening of a single L-type Ca2+ channel of the unit provides sufficient Ca2+ influx to increase local [Ca2+] beyond the threshold activating Ca(2+)-induced Ca2+ release (CICR), which amplifies local [Ca2+] until all release channels of the unit are active. This all-or-none activation does not spread to other release units. Gradation of whole-cell Ca2+ release is predicted to correlate with the cumulative probability density distribution of first latency of L-type Ca2+ channels or the activation time course of the calcium current, ICa. 2. Guinea-pig ventricular myocytes were potentiated by paired voltage-clamp pulses (1 Hz, 2 mM [Ca2+]o, 40 microM K5-indo-1, 36 degrees C). When the cellular Ca2+ load was at a steady high level, cytosolic calcium concentration ([Ca2+]c) transients were measured in response to test pulses of varied pulse duration (PD, 1-180 ms) and amplitude (-20, 0, 20 and 50 mV). The maximal rate of rise (RRmax) of the [Ca2+]c transient was used as an indicator for SR Ca2+ release. 3. Fast [Ca2+]c transients due to 4 ms pulses to 0 or 50 mV were blocked by 1 mM cadmium suggesting that these Ca2+ release signals are triggered by Ca2+ influx through L-type Ca2+ channels and not by Ca2+ influx through Na(+)-Ca2+ exchange. 4. RRmax increased with longer PD along the sigmoidal curve [1-exp(-PD/tau)]kappa(exponent k: 2 < k < 3). The time constant, tau, resembled the activation time constant of whole-cell ICa (Cs(+)-dialysed cells). A PD longer than a limiting duration did not modify RRmax. That is, inactivation of ICa was not reflected in the duration dependence. 5. Single L-type Ca2+ channels (cell-attached patches, 36 degrees C, -20 mV, 3.6 mM CaCl2 and 1 microM Bay K 8644 in patch pipette) opened with a waiting time the cumulative probability distribution of which resembled the duration dependence of RRmax, suggesting that the first opening of L-type Ca2+ channels determines whether the corresponding release unit contributes to the [Ca2+]c transient activated during a short voltage-clamp pulse. 6. The time constant, tau, of the duration dependence was shorter at positive than at negative potentials.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bers D. M., Lederer W. J., Berlin J. R. Intracellular Ca transients in rat cardiac myocytes: role of Na-Ca exchange in excitation-contraction coupling. Am J Physiol. 1990 May;258(5 Pt 1):C944–C954. doi: 10.1152/ajpcell.1990.258.5.C944. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Stiffel V. M. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am J Physiol. 1993 Jun;264(6 Pt 1):C1587–C1593. doi: 10.1152/ajpcell.1993.264.6.C1587. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in guinea-pig cardiac cells: exchange current and changes in intracellular Ca2+. J Physiol. 1989 Jul;414:499–520. doi: 10.1113/jphysiol.1989.sp017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Schiefer A., Isenberg G. Ca2+ load of guinea-pig ventricular myocytes determines efficacy of brief Ca2+ currents as trigger for Ca2+ release. J Physiol. 1994 Nov 1;480(Pt 3):411–421. doi: 10.1113/jphysiol.1994.sp020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Belardinelli L. Ionic basis for the antagonism between adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res. 1984 Sep;55(3):309–325. doi: 10.1161/01.res.55.3.309. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Mechmann S., Pott L. Effects of calcium release from sarcoplasmic reticulum on membrane currents in guinea pig atrial cardioballs. Pflugers Arch. 1987 Sep;410(1-2):121–131. doi: 10.1007/BF00581904. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- O'Neill S. C., Mill J. G., Eisner D. A. Local activation of contraction in isolated rat ventricular myocytes. Am J Physiol. 1990 Jun;258(6 Pt 1):C1165–C1168. doi: 10.1152/ajpcell.1990.258.6.C1165. [DOI] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Sipido K. R., Wier W. G. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. J Physiol. 1991 Apr;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D., Lakatta E. G. Excitation-contraction coupling in the heart: the state of the question. FASEB J. 1992 Sep;6(12):3092–3100. doi: 10.1096/fasebj.6.12.1325933. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]


