Abstract
Objectives
Current therapies show limited efficacy against peritoneal metastases (PM) from pancreatic cancer. Pressurized intra-peritoneal aerosol chemotherapy (PIPAC) has emerged as a novel intraperitoneal drug delivery method. Recently, a dose-escalation study identified the safe dose of Nabpaclitaxel for PIPAC administration, an ideal intraperitoneal chemotherapy agent against pancreatic cancer. Combining systemic NabPaclitaxel-Gemcitabine with NabPaclitaxel-PIPAC may enhance disease control in pancreatic cancer patients with PM.
Methods
The Nab-PIPAC trial is a single-center, prospective, open-label, phase II study (ClinicalTrials.gov identifier: NCT05371223). Its primary goal is to evaluate the antitumor activity of the combined treatment based on Disease Control Rate (DCR) using RECISTv.1.1 criteria. Secondary objectives include feasibility, safety, pathological response, progression-free and overall survival, nutritional status, quality of life, pharmacokinetics of NabPaclitaxel-PIPAC, and PM molecular evolution via translational research. The treatment protocol consists of three courses, each with two cycles of intravenous NabPaclitaxel-Gemcitabine and one cycle of NabPaclitaxel-PIPAC, with standard metastatic pancreatic cancer doses for the former and 112.5 mg/m2 for the latter. Sample size follows Simon’s two-stage design: 12 patients in stage one and 26 in stage two (80 % power, 0.1 alpha).
Results
Partial results will be available after first stage enrollment.
Conclusions
This trial aims to determine the antitumor efficacy and safety of combining NabPaclitaxel-PIPAC with systemic NabPaclitaxel-Gemcitabine in pancreatic cancer patients with PM.
Keywords: peritoneal metastasis, pancreatic cancer, PIPAC, bidirectional chemotherapy, combined chemotherapy, locoregional chemotherapy
Background
Pancreatic cancer is the fourth leading cause of cancer mortality in the West despite its relatively low incidence, with 43.500 estimated deaths in 2022 in the European Union [1].
Prognosis has been consistently poor over the last decades [2], with a 5-year survival rate of only 8 % following initial diagnosis [3]. Contrary to other malignant diseases, few advances have been made and the observed trends in mortality rates remained stable or slightly increased [4].
Surgery represents the only treatment option with curative potential but only 15 % of patients can undergo primary tumor resection [5]. Most of patients have already developed at time of diagnosis a locally advanced or metastatic disease and are treated with systemic chemotherapy or palliative care.
The peritoneum is the second most common site of metastasis after the liver. Indeed, peritoneal dissemination is a major concern in pancreatic cancer affecting at least 1 in every 8 patients at the time of diagnosis [6] and ultimately developing in up to 50 % of cases [7]. These patients are striving for new treatment options effectively addressing peritoneal disease as systemic chemotherapy hardly reaches peritoneal implants, resulting in very poor outcomes. Compared to systemic chemotherapy, intraperitoneal chemotherapy provides higher drug concentrations directly targeting tumor nodules with less systemic exposure and might be more advantageous in treating peritoneal disease. The results of recent Asian trials on the combination of systemic and port-based intraperitoneal chemotherapy are encouraging 8], [9], [10.
Recently, pressurized intraperitoneal aerosol chemotherapy (PIPAC) emerged as a novel intraperitoneal drug-delivery system of low-dose chemotherapy as a pressurized aerosol. It combines high intraperitoneal concentration, low systemic concentration and toxicity with the homogeneous intraperitoneal distribution and deeper tissue penetration of aerosol. Data from several non-comparative clinical studies with various intraperitoneal chemotherapy drugs suggest that PIPAC is a safe, feasible, and well-tolerated treatment showing good preliminary response rates on peritoneal metastases (PM) of various origins. Furthermore, due to its repeatable, minimally-invasive nature, PIPAC was successfully combined with current systemic chemotherapy regimens [11, 12].
So far, prospective series have reported promising feasibility, safety and antitumor activity data on PIPAC with cisplatin/doxorubicin or oxaliplatin for pancreatic cancer peritoneal metastases 13], [14], [15], [16], [17. However, no phase-II PIPAC trials addressed to pancreatic cancer peritoneal metastases have been conducted.
Based on preclinical and clinical data, Nabpaclitaxel is an ideal candidate for intraperitoneal chemotherapy and is highly active on pancreatic cancer cells 18], [19], [20.
Recently, a phase I study (NCT03304210) explored its use with PIPAC resulting well tolerated, with a favorable PK profile and promising anticancer activity in patients with PM. This study identified the dose to safely start a phase-II trial [21]. A phase IB trial from Switzerland is ongoing to determine the MDT of IP Nab-paclitaxel administered by PIPAC in concomitant with IP Cisplatin and to access the safety and tolerability of this combined PIPAC treatment [22].
We designed the present study to test Nabpaclitaxel-PIPAC in combination with systemic Nabpaclitaxel-Gemcitabine for the treatment of pancreatic cancer PM.
Patients and methods
The Nab-PIPAC trial is a monocentric prospective, open-label, phase II study assessing the combined treatment of endovenous Nabpaclitaxel-Gemcitabine and intraperitoneal Nabpaclitaxel administered through PIPAC for pancreatic cancer peritoneal metastasis.
Primary objective
The main objective is to evaluate the antitumor activity of this combined chemotherapy. The primary endpoint is the disease control rate (DCR) defined as the combined incidence of complete response (CR), partial response (PR), and stable disease (SD) according to the RECIST v. 1.1 criteria.
Secondary objectives
The secondary objectives include the assessment of feasibility, safety, pathological tumor response, progression-free and overall survival, and quality of life of the combined treatment. In addition, the pharmacokinetics of Nab-PIPAC, the nutritional status assessment and a translational study on the mutational, transcriptomic, and immune cells profiles have been planned. Table 1 shows the endpoints considered for the evaluation of the secondary objectives.
Table 1:
Secondary endpoints.
| Objectives | Endpoints |
|---|---|
| Feasibility |
|
| Safety |
|
| Antitumor activity |
|
PIPAC, Pressurized Intraperitoneal Aerosol Chemotherapy; CTCAE, Common Terminology Criteria for Adverse Events; PRGS, Peritoneal Regression Grading Score; PCI, Peritoneal Cancer Index; QoL, quality of live; QLQ-30, Quality of Life Questionnaire; PFS, Progression-Free Survival; OS, Overall Survival.
Study assessment and time points
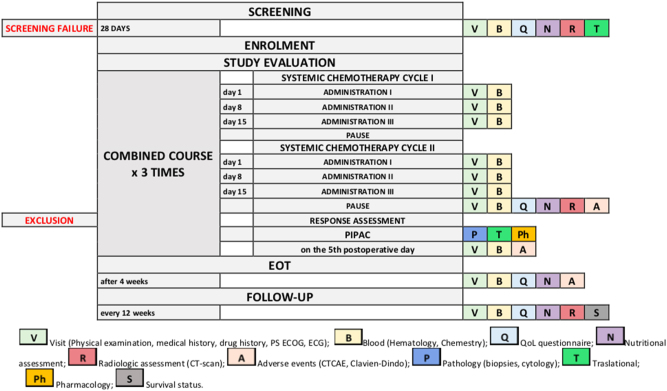
Each patient will be treated to complete three combined courses of endovenous Nabpaclitaxel-Gemcitabine chemotherapy and Nab-PIPAC. Patients will undergo disease response assessment at each combined course.
The study period will include the screening phase, the study treatment phase (until 30 days after the last dose of Nabpaclitaxel- Gemcitabine or last PIPAC) and the follow-up period. The treatment phase will start with the administration of systemic Nabpaclitaxel-Gemcitabine, for two cycles, according to the standard protocol. Nabpaclitaxel-PIPAC will be started 10–13 days after the last dose of the second cycle of systemic chemotherapy and repeated once every two cycles of systemic Nabpaclitaxel-Gemcitabine. Each patient will have three repetitive combined courses.
Figure 1 shows the timing of the assessments performed during the study period.
Figure 1:
Flow-chart of the study with time-points evaluations.
Radiological tumor response assessment according to RECIST is performed after every two cycles of systemic chemotherapy just before each PIPAC. Patients who progressed will not proceed to PIPAC and drop out the protocol. Study treatment is discontinued in case of physician-determined disease progression, unacceptable toxicity or physician’s or patient’s decision to discontinue participation. Study treatment ends after the third PIPAC, regardless of response to therapy.
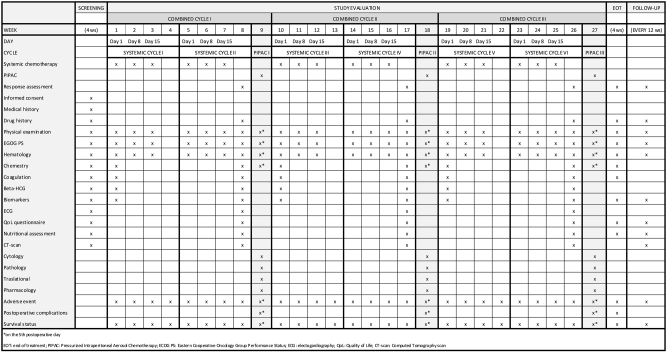
The schedule of enrolment, interventions and assessments is shown in Figure 2.
Figure 2:
Study procedures chart.
Eligibility criteria
Patients with pancreatic cancer peritoneal metastasis will be evaluated for eligibility to the study by the multidisciplinary tumor board (MTB) based on the screening assessment and the following criteria. Inclusion and exclusion criteria are reported in Table 2.
Table 2:
Inclusion and exclusion criteria for Nab-PIPAC trial.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Pancreatic cancer with peritoneal metastases determined based on abdominal CT or MR and/or diagnostic laparotomy or laparoscopy | -urgical or medical contraindications to laparoscopy |
| Histological or cytological proof of pancreatic cancer | Advanced metastatic systemic disease with clinical deterioration |
| ECOG performance status 0 or 1 | Patients with extra-abdominal tumor spread |
| Life expectancy of at least 3 months | Patients with a germline or somatic pathogenic variant involving an HRR-related gene |
| Absolute neutrophil count ≥1,500 cell/mm3 | Symptoms of gastrointestinal occlusion and total parenteral nutritional support |
| Platelets ≥100,000 cell/mm3 | -atients defined as “refractory” to previous systemic treatment with Nabpaclitaxel and Gemcitabine administered for locally advanced pancreatic cancer |
| Hemoglobin ≥9 g/dL | -nown hypersensitivity reaction to drugs chemically related to Nabpaclitaxel, Gemcitabine and their excipients |
| Adequate renal function | History of severe and unexpected reactions to Nabpaclitaxel or Gemcitabine derivates |
| Resolution of all toxic effects of prior therapies or surgical procedures to grade ≤1 (except alopecia and peripheral neuropathy) | |
| In absence of liver metastases, ALT and AST ≤2.5 × ULN. With liver metastases, ALT and AST <5 × ULN, total bilirubin ≤ULN, or total bilirubin 1.5 × ULN with direct bilirubin ≤ULN of the laboratory in subjects with documented Gilbert’s syndrome | |
| Age≥18 years | |
| Informed consent |
CT, Computed Tomography; MR, Magnetic Resonance; ECOG, Eastern Cooperative Oncology Group; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal; HRR, Homologous Recombination Repair; PM, peritoneal metastases.
Study treatments
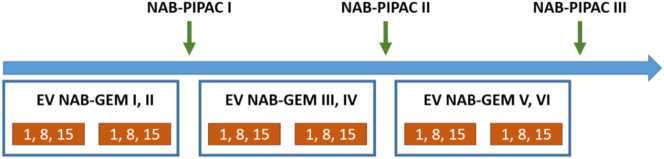
Each patient is scheduled for three combined courses of endovenous chemotherapy Nabpaclitaxel-Gemcitabine 125/1,000 mg/m2 and Nabpaclitaxel-PIPAC 112.5 mg/m2 (Figure 3). Each combined course lasts nine weeks and is constituted by two 28-day cycles of systemic chemotherapy (three administrations per cycle: days 1, 8 and 15) and one cycle of PIPAC administered within 10–13 days from the last administration of the second systemic cycle.
Figure 3:
Timeline of combined systemic Nabpaclitaxel-Gemcitabine and Nab-PIPAC.
The PIPAC procedure will be carried out according to the standard technique previously reported [23] and briefly described. After each PIPAC, systemic chemotherapy was resumed in 7–10 days. Hence, each patient will receive a total of VI cycles of systemic chemotherapy and III PIPAC administrations. The study treatment ends after the third PIPAC in all patients.
Statistical analysis
Concerning the analysis of the primary endpoint, the DCR, all time-points responses observed while on study treatment and during the EOT visit will be included in the derivation. The ratio of the rate and its 95 % CI will be presented.
Safety, feasibility, and QoL endpoints will be reported by descriptive statistics. PFS and OS will be presented by median times and associated 95 % CI as well as by survival curves using the Kaplan-Meier method. Continuous data will be summarized using the number of available data, mean, standard deviation. Categorical data will be summarized using the number and percentage of patients.
Sample size
A total of 38 patients affected by pancreatic carcinoma with peritoneal metastases undergoing combined systemic and intraperitoneal chemotherapy will be enrolled.
Simon’s two-stage design was used to calculate the sample size for this trial [24]. With a Power of 80 %, P0=40 %, and P1=60 %, 12 patients will be enrolled in the first stage; with 6 or more patients experiencing CR/PR/SD, at this stage, another 26 patients will be enrolled in the second stage. The study will be considered positive with an alpha error=0.1, whether 19 or more patients will experience CR/PR/SD. The planned duration of the study is 36 months.
Data collection and management
Data will be collected using an electronic case report form (CRF).
The sponsor maintains confidentiality standards by assigning a patient identification number.
On all trial-specific documents, other than the signed consent, the participant will be referred to by the trial participant number/code.
Monitoring
Data monitoring
According to the international conference on harmonisation good clinical practice (ICH GCP), the monitoring team must check the CRF entries against the source documents, except for the pre-identified source data directly recorded in the CRF. The informed consent form will include a statement by which the patient allows the Sponsor’s duly authorized personnel, the Ethics Committee (IRB/IEC), and the regulatory authorities to have direct access to original medical records, which support the data on the CRFs (e.g., patient’s medical file, appointment books, original laboratory records, etc.). These personnel, bound by professional secrecy, must maintain the confidentiality of all personal identity or personal medical information (according to confidentiality and personal data protection rules).
Safety reporting
The collection, assessment and presentation of safety reports will be carried out in accordance with the detailed guidance on the collection, verification and presentation of adverse event/reaction reports arising from clinical trials on medicinal products for human use (‘CT-3’).
Patients will be carefully monitored for any AE occurring during the trial conduct. Such monitoring also includes clinical laboratory tests. AEs will be assessed in terms of their seriousness, severity, and causal relation to the study treatment. Safety reporting to study investigators, ECs, competent authorities will then follow in accordance with the results of such assessment.
Ethics approval, consent to participate, and dissemination
The trial will be conducted in accordance with the ethical principles set out in the Declaration of Helsinki and are consistent with ICH/Good Clinical Practice and regulatory requirements for participant data protection.
Before participating in the investigation, participants will receive comprehensive information concerning the trial through both verbal communication and a written consent form. Participants will be duly informed of their prerogative to discontinue their involvement in the trial at any given juncture.
The study received the approval of the Italian drug agency (AIFA) (Approval Code: 2021-002539-51 SC 22540); Date of approval: 18/10/2021.
Institutional Review Board approval of the Ethical Committee of the Fondazione Policlinico Universitario Agostino Gemelli was obtained. Institutional Review Board number: ID CE 4368; Date of approval: 07/01/2022.
During the clinical trial, any amendment or modification to the clinical trial protocol should be submitted to the IRB/IEC before implementation, unless the change is necessary to eliminate an immediate hazard to the patients, in which case the IRB/IEC should be informed as soon as possible.
Property of data is of Fondazione Policlinico Universitario Agostino Gemelli, IRCCS. The main results of the clinical trial will be published in a peer-reviewed scientific journal. The final publication will be written by one of the Investigators on the basis of the final analysis performed by the Fondazione Policlinico Universitario Agostino Gemelli, IRCCS. All publications, abstract or presentations including data related to the present trial will be submitted for review to the PI prior to submission. Publication will be done in case of positive study results as well as negative results.
Discussion
The theory of limited diffusion of antiblastic drugs from systemic circulation into peritoneal cancer implants, due to the plasma-peritoneal barrier [25], supports combining intravenous and intraperitoneal chemotherapy. Intravenous chemotherapy targets systemic metastasis and accumulates antiblastic agents in the subperitoneal space, while intra-abdominal administration allows penetration into peritoneal nodules from the peritoneal side. Moreover, systemic drug uptake from the peritoneal cavity targets the liver due to the first-hepatic passage.
This approach seems rational, especially for pancreatic cancer, which often spreads to the liver and peritoneum. However, its efficacy lacks solid scientific evidence. This research aims to provide reliable data to design future controlled trials to determine if this combination is clinically advantageous for pancreatic cancer patients.
Two recent Asian trials combined systemic Gemcitabine-Nabpaclitaxel with intraperitoneal solvent-based paclitaxel, yielding encouraging results [9, 26]. Takahara et al. conducted a phase I study to determine the recommended dose of systemic Nabpaclitaxel-Gemcitabine and intraperitoneal solvent-based Paclitaxel given through an implanted peritoneal-access port on days 1, 8, and 15 every 28 days. The doses identified were 30 mg/m2 of intraperitoneal Paclitaxel, 1,000 mg/m2 of systemic Gemcitabine, and 125 mg/m2 of systemic Nabpaclitaxel. The study concluded that there was no systemic toxicity from adding intraperitoneal Paclitaxel, with no adverse events or deaths related to its administration. Major issues with intraperitoneal chemotherapy were related to port management, as 33 % of patients developed port-related complications. The response rate and disease control rates were 25 and 75 %, respectively, based on RECIST criteria.
Yamada et al. conducted a phase I/II study with the same drugs but encountered dose-limiting toxicities in three out of four patients at level 1 dose, leading to lower recommended doses compared to the previous study. They established the recommended phase II dose (RP2D) at 800-75 and 20 mg/m2 for intravenous Gemcitabine-Nabpaclitaxel and intraperitoneal Paclitaxel, respectively. In the subsequent phase II study on 46 patients, the response and disease control rates were 49 and 95 %, respectively.
In this trial, we combined systemic Nabpaclitaxel-Gemcitabine with intraperitoneal Nabpaclitaxel administered through PIPAC. PIPAC is a safe, repeatable, minimally invasive procedure that can be combined with various systemic chemotherapy regimens 27], [28], [29. Compared to port-based intraperitoneal chemotherapy, PIPAC offers deeper tissue penetration, better drug distribution within the peritoneal cavity, and no port-related complications such as infections, ascites effusion, bowel obstruction, or peritoneal adhesions. Furthermore, it allows repetitive evaluation of the abdominal cavity and PM tissue sampling.
Five published studies on PIPAC for pancreatic cancer PM documented its safety and ability to induce pathological tumor regression on pancreatic peritoneal metastases. Four used PIPAC with cisplatin-doxorubicin as monotherapy in a salvage setting 13], [14], [15], [16, and one combined PIPAC-cisplatin-doxorubicin or PIPAC-oxaliplatin with various systemic regimens [17].
The choice of Nabpaclitaxel for PIPAC was based on its documented activity against pancreatic cancer cells and its suitability for intraperitoneal administration [30]. Cristea et al. demonstrated in a phase I study that IP administration of Nabpaclitaxel has a favorable toxicity profile, significant pharmacologic advantages, and promising clinical activity [31]. From a pharmacokinetic perspective, besides its favorable molecular size, albumin presence enhances tumor drug penetration. Both stromal fibroblast and pancreatic tumor epithelial cells exhibit high levels of SPARC, promoting Paclitaxel delivery inside pancreatic cancer cells [32]. Additionally, the high pressure of aerosolized drugs in the PIPAC procedure can overcome the high interstitial pressure in tumor tissue, critical for pancreatic cancer pharmacokinetics.
A dose-escalation study explored the safety of IP Nabpaclitaxel and determined its RP2D for PIPAC at 140 mg/m2 [21]. The most frequent treatment-related toxicities were liver toxicity (75 %) and anemia (70 %). Forty percent of patients at the highest dose had surgical wound infection or dehiscence. Hematological toxicity was moderate, with one patient developing grade 3 neutropenia. No grade 4 or 5 morbidity was reported. The authors concluded that PIPAC with Nabpaclitaxel was well-tolerated. Considering no DLT was observed, the MTD and RP2D were defined as 140 mg/m2, with an advised RP2D of 112.5 mg/m2 for patients with hepatobiliary impairment. Thirteen patients combined PIPAC with systemic chemotherapy, though none received taxane-based systemic regimens. PIPAC was administered every four weeks.
PIPAC has been successfully combined with several intravenous regimens [33]. A recent review concluded that combining systemic chemotherapy with PIPAC is feasible [11]. The regimen under investigation comprises six systemic cycles (according to the standard regimen [34]), each consisting of three administrations of Gemcitabine-Nabpaclitaxel on days 1, 8, and 15 plus three Nabpaclitaxel-PIPACs, one every two systemic cycles.
To reduce toxicity risk, we set the PIPAC Nabpaclitaxel dose at 112.5 mg/m2, lower than the 140 mg/m2 recommended by the Ghent group. PIPAC cycles are spaced every eight weeks, with PIPAC application 13–15 days after the last systemic administration and chemotherapy resumption 7–10 days after PIPAC. A dose level reduction of systemic chemotherapy according to EMA is also planned.
We expect the combined systemic/intraperitoneal treatment to be feasible, safe, and well-tolerated. The primary endpoint, disease control rate (DCR), will assess antitumor activity. Despite the challenges of assessing PM through RECIST criteria, DCR, comprising complete responses, partial responses, and stable diseases, should reflect the combined treatment’s activity. Histologic tumor regression and visual macroscopic disease evaluation by laparoscopy are secondary endpoints.
The main weakness of this trial is the lack of a preliminary phase I study assessing the drug combination. However, dose-escalation trials have documented the feasibility and safety of combining systemic Nabpaclitaxel-Gemcitabine with intraperitoneal solvent-based Paclitaxel [9, 26]. The well-established Nabpaclitaxel-Gemcitabine regimen for metastatic pancreatic cancer includes defined dose reduction steps [34]. Celeen et al. documented that PIPAC Nabpaclitaxel results in a median PIPAC total PTX plasma Cmax and AUC 0–24 h 2.5 times lower compared to catheter-based Nabpaclitaxel IP delivery, suggesting better peritoneal tissue drug uptake with less systemic exposure [21]. This pharmacokinetic data supports the combined intraperitoneal/intravenous treatment’s availability.
After 10–13 days from the last intravenous Nabpaclitaxel administration, Paclitaxel should be cleared, avoiding overdose following PIPAC administration. Safety will be closely monitored with dose adjustments for subsequent PIPAC cycles if toxicity occurs. Simon’s two-stage design allows stopping the study for futility if seven or more patients out of twelve demonstrate disease progression in the first stage.
Despite the pancreas being a retroperitoneal organ, pancreatic cancer frequently spreads to the peritoneum, requiring adequate treatment. This research addresses these needs. If results support adding Nabpaclitaxel-PIPAC to standard systemic treatment for metastatic pancreatic cancer, further controlled trials can be planned.
Acknowledgments
The authors warmly thanks Ministero della Salute, Ricerca Corrente 2024.
Footnotes
Research ethics: The study received the approval of the Italian drug agency (AIFA) (Approval Code: 2021-002539-51 SC 22540); Date of approval: 18/10/2021.Institutional Review Board approval of the Ethical Committee of the Fondazione Policlinico Agostino Gemelli was obtained. Institutional Review Board number: ID CE 4368; Date of approval: 07/01/2022.
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
Author contributions: AD, FF, CC, CB, and GT, have made substantial contributions to the conception and design of this study, have been involved in drafting the manuscript or revising it critically for important intellectual content, and have given final approval of the version to be published. AS, LS, SA, and FP have made contributions to the design of this study and have made substantial contributions to the organization of this trial. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Use of Large Language Models, AI and Machine Learning Tools: None declared.
Conflict of interest: The authors state no conflict of interest.
Research funding: Not applicable.
Data availability: Not applicable.
References
- 1.Dalmartello M, La Vecchia C, Bertuccio P, Boffetta P, Levi F, Negri E, et al. European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann Oncol. 2022;33:330–9. doi: 10.1016/j.annonc.2021.12.007. Epub 2022 Jan 26. PMID: 35090748. [DOI] [PubMed] [Google Scholar]
- 2.De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. Epub 2013 Dec 5. PMID: 24314615. [DOI] [PubMed] [Google Scholar]
- 3.Minicozzi P, Cassetti T, Vener C, Sant M. Analysis of incidence, mortality and survival for pancreatic and biliary tract cancers across Europe, with assessment of influence of revised European age standardisation on estimates. Cancer Epidemiol. 2018;55:52–60. doi: 10.1016/j.canep.2018.04.011. Epub 2018 May 25. PMID: 29777994. [DOI] [PubMed] [Google Scholar]
- 4.Carioli G, Malvezzi M, Bertuccio P, Boffetta P, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann Oncol. 2021;32:478–87. doi: 10.1016/j.annonc.2021.01.006. Epub 2021 Feb 21. PMID: 33626377. [DOI] [PubMed] [Google Scholar]
- 5.Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128:1606–25. doi: 10.1053/j.gastro.2005.04.001. PMID: 15887154. [DOI] [PubMed] [Google Scholar]
- 6.Thomassen I, Lemmens VE, Nienhuijs SW, Luyer MD, Klaver YL, de Hingh IH. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: a population-based study. Pancreas. 2013;42:72–5. doi: 10.1097/MPA.0b013e31825abf8c. PMID: 22850624. [DOI] [PubMed] [Google Scholar]
- 7.Del Castillo CF, Warshaw L. Peritoneal metastases in pancreatic carcinoma. Hepato-Gastroenterology. 1993;40:430–2. PMID: 8270231. [PubMed] [Google Scholar]
- 8.Satoi S, Fujii T, Yanagimoto H, Motoi F, Kurata M, Takahara N, et al. Multicenter phase II study of intravenous and intraperitoneal paclitaxel with S-1 for pancreatic ductal adenocarcinoma patients with peritoneal metastasis. Ann Surg. 2017;265:397–401. doi: 10.1097/SLA.0000000000001705. PMID: 28059968. [DOI] [PubMed] [Google Scholar]
- 9.Yamada S, Fujii T, Yamamoto T, Takami H, Yoshioka I, Yamaki S, et al. Phase I/II study of adding intraperitoneal paclitaxel in patients with pancreatic cancer and peritoneal metastasis. Br J Surg. 2020;107:1811–17. doi: 10.1002/bjs.11792. Epub 2020 Jul 7. PMID: 32638367; PMCID: PMC7689756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018;36:1922–9. doi: 10.1200/JCO.2018.77.8613. Epub 2018 May 10. PMID: 29746229. [DOI] [PubMed] [Google Scholar]
- 11.Ploug M, Graversen M, Pfeiffer P, Mortensen MB. Bidirectional treatment of peritoneal metastasis with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) and systemic chemotherapy: a systematic review. BMC Cancer. 2020;20:105. doi: 10.1186/s12885-020-6572-6. PMID: 32041558; PMCID: PMC7011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Giorgio A, Macrì A, Ferracci F, Robella M, Visaloco M, De Manzoni G, et al. 10 Years of pressurized intraperitoneal aerosol chemotherapy (PIPAC): a systematic review and meta-analysis. Cancers. 2023;15:1125. doi: 10.3390/cancers15041125. PMID: 36831468; PMCID: PMC9954579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khosrawipour T, Khosrawipour V, Giger-Pabst U. Pressurized Intra Peritoneal Aerosol Chemotherapy in patients suffering from peritoneal carcinomatosis of pancreatic adenocarcinoma. PLoS One. 2017;12:e0186709. doi: 10.1371/journal.pone.0186709. PMID: 29049340; PMCID: PMC5648228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graversen M, Detlefsen S, Bjerregaard JK, Pfeiffer P, Mortensen MB. Peritoneal metastasis from pancreatic cancer treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) Clin Exp Metastasis. 2017;34:309–14. doi: 10.1007/s10585-017-9849-7. Epub 2017 May 17. PMID: 28516306. [DOI] [PubMed] [Google Scholar]
- 15.Horvath P, Beckert S, Struller F, Königsrainer A, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastases of pancreas and biliary tract cancer. Clin Exp Metastasis. 2018;35:635–40. doi: 10.1007/s10585-018-9925-7. Epub 2018 Jul 30. PMID: 30062506. [DOI] [PubMed] [Google Scholar]
- 16.Falkenstein TA, Götze TO, Ouaissi M, Tempfer CB, Giger-Pabst U, Demtröder C. First clinical data of pressurized intraperitoneal aerosol chemotherapy (PIPAC) as salvage therapy for peritoneal metastatic biliary tract cancer. Anticancer Res. 2018;38:373–8. doi: 10.21873/anticanres.12232. PMID: 29277797. [DOI] [PubMed] [Google Scholar]
- 17.Di Giorgio A, Sgarbura O, Rotolo S, Schena CA, Bagalà C, Inzani F, et al. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin or oxaliplatin for peritoneal metastasis from pancreatic adenocarcinoma and cholangiocarcinoma. Ther Adv Med Oncol. 2020;12:1758835920940887. doi: 10.1177/1758835920940887. PMID: 32782488; PMCID: PMC7383654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita J, Fushida S, Tsukada T, Oyama K, Watanabe T, Shoji M, et al. Comparative study of the antitumor activity of Nab-paclitaxel and intraperitoneal solvent-based paclitaxel regarding peritoneal metastasis in gastric cancer. Oncol Rep. 2014;32:89–96. doi: 10.3892/or.2014.3210. Epub 2014 May 23. PMID: 24859429. [DOI] [PubMed] [Google Scholar]
- 19.Coccolini F, Acocella F, Morosi L, Brizzola S, Ghiringhelli M, Ceresoli M, et al. High penetration of paclitaxel in abdominal wall of rabbits after hyperthermic intraperitoneal administration of nab-paclitaxel compared to standard paclitaxel formulation. Pharm Res. 2017;34:1180–6. doi: 10.1007/s11095-017-2132-4. Epub 2017 Feb 28. PMID: 28247168. [DOI] [PubMed] [Google Scholar]
- 20.Reni M, Zanon S, Peretti U, Chiaravalli M, Barone D, Pircher C, et al. Nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in metastatic pancreatic adenocarcinoma (PACT-19): a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:691–7. doi: 10.1016/S2468-1253(18)30196-1. Epub 2018 Jul 7. PMID: 30220407. [DOI] [PubMed] [Google Scholar]
- 21.Ceelen W, Sandra L, de Sande LV, Graversen M, Mortensen MB, Vermeulen A, et al. Phase I study of intraperitoneal aerosolized nanoparticle albumin based paclitaxel (NAB-PTX) for unresectable peritoneal metastases. EBioMedicine. 2022;82:104151. doi: 10.1016/j.ebiom.2022.104151. Epub 2022 Jul 15. PMID: 35843174; PMCID: PMC9297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang N, Diciola A, Labidi-Galy I, Ris F, Di Marco M, Mach N, et al. Nab-PIPAC: a phase IB study protocol of intraperitoneal cisplatin and nab-paclitaxel administered by pressurised intraperitoneal aerosol chemotherapy (PIPAC) in the treatment of advanced malignancies confined to the peritoneal cavity. BMJ Open. 2023;13:e067691. doi: 10.1136/bmjopen-2022-067691. PMID: 36604127; PMCID: PMC9827272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sgarbura O, Eveno C, Alyami M, Bakrin N, Guiral DC, Ceelen W, et al. Consensus statement for treatment protocols in pressurized intraperitoneal aerosol chemotherapy (PIPAC) Pleura Peritoneum. 2022;7:1–7. doi: 10.1515/pp-2022-0102. PMID: 35602919; PMCID: PMC9069497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon R. Optimal two-stage designs for phase II clinical trials. Contr Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. PMID: 2702835. [DOI] [PubMed] [Google Scholar]
- 25.Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53–63. doi: 10.1007/978-1-4613-1247-5_4. PMID: 8849943. [DOI] [PubMed] [Google Scholar]
- 26.Takahara N, Nakai Y, Ishigami H, Saito K, Sato T, Hakuta R, et al. A phase I study of intraperitoneal paclitaxel combined with gemcitabine plus nab-paclitaxel for pancreatic cancer with peritoneal metastasis. Invest N Drugs. 2021;39:175–81. doi: 10.1007/s10637-020-00982-7. Epub 2020 Aug 8. PMID: 32772340. [DOI] [PubMed] [Google Scholar]
- 27.Alyami M, Hübner M, Grass F, Bakrin N, Villeneuve L, Laplace N, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20:e368–77. doi: 10.1016/S1470-2045(19)30318-3. PMID: 31267971. [DOI] [PubMed] [Google Scholar]
- 28.Winkler CS, Sandhu J, Pettke E, Merchea A, Fong Y, Kumara HMCS, et al. Pressurized intraperitoneal aerosol chemotherapy, a palliative treatment approach for patients with peritoneal carcinomatosis: description of method and systematic review of literature. Dis Colon Rectum. 2020;63:242–55. doi: 10.1097/DCR.0000000000001565. PMID: 31914116. [DOI] [PubMed] [Google Scholar]
- 29.Hübner M, Grass F, Teixeira-Farinha H, Pache B, Mathevet P, Demartines N. Pressurized IntraPeritoneal aerosol chemotherapy - practical aspects. Eur J Surg Oncol. 2017;43:1102–9. doi: 10.1016/j.ejso.2017.03.019. Epub 2017 Apr 8. PMID: 28431896. [DOI] [PubMed] [Google Scholar]
- 30.Van de Sande L, Cosyns S, Willaert W, Ceelen W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: a review. Drug Deliv. 2020;27:40–53. doi: 10.1080/10717544.2019.1704945. PMID: 31858848; PMCID: PMC6968566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cristea MC, Frankel P, Synold T, Rivkin S, Lim D, Chung V, et al. A phase I trial of intraperitoneal nab-paclitaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Cancer Chemother Pharmacol. 2019;83:589–98. doi: 10.1007/s00280-019-03767-9. Epub 2019 Jan 8. PMID: 30623229; PMCID: PMC8919712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–25. doi: 10.1200/JCO.2006.07.8824. PMID: 17235047. [DOI] [PubMed] [Google Scholar]
- 33.Di Giorgio A, Schena CA, El Halabieh MA, Abatini C, Vita E, Strippoli A, et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): a bidirectional approach for gastric cancer peritoneal metastasis. Surg Oncol. 2020;34:270–5. doi: 10.1016/j.suronc.2020.05.006. Epub 2020 Jun 3. PMID: 32891341. [DOI] [PubMed] [Google Scholar]
- 34.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. Epub 2013 Oct 16. PMID: 24131140; PMCID: PMC4631139. [DOI] [PMC free article] [PubMed] [Google Scholar]