Abstract
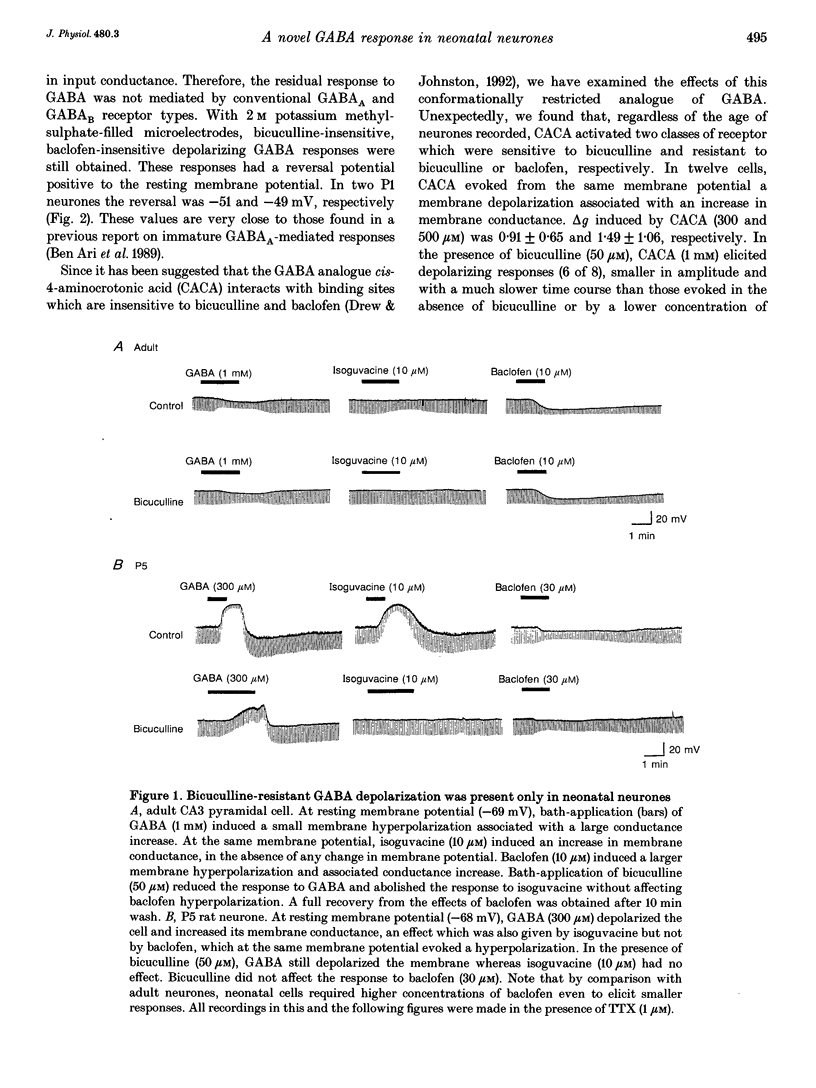
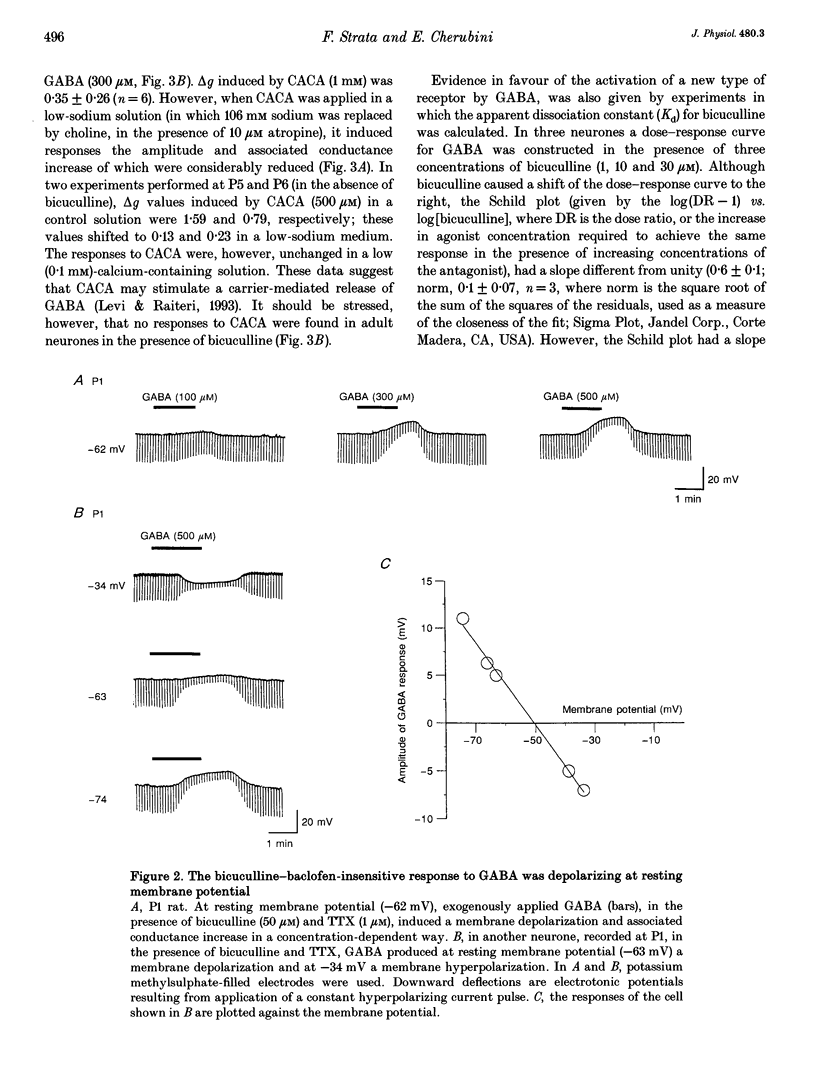
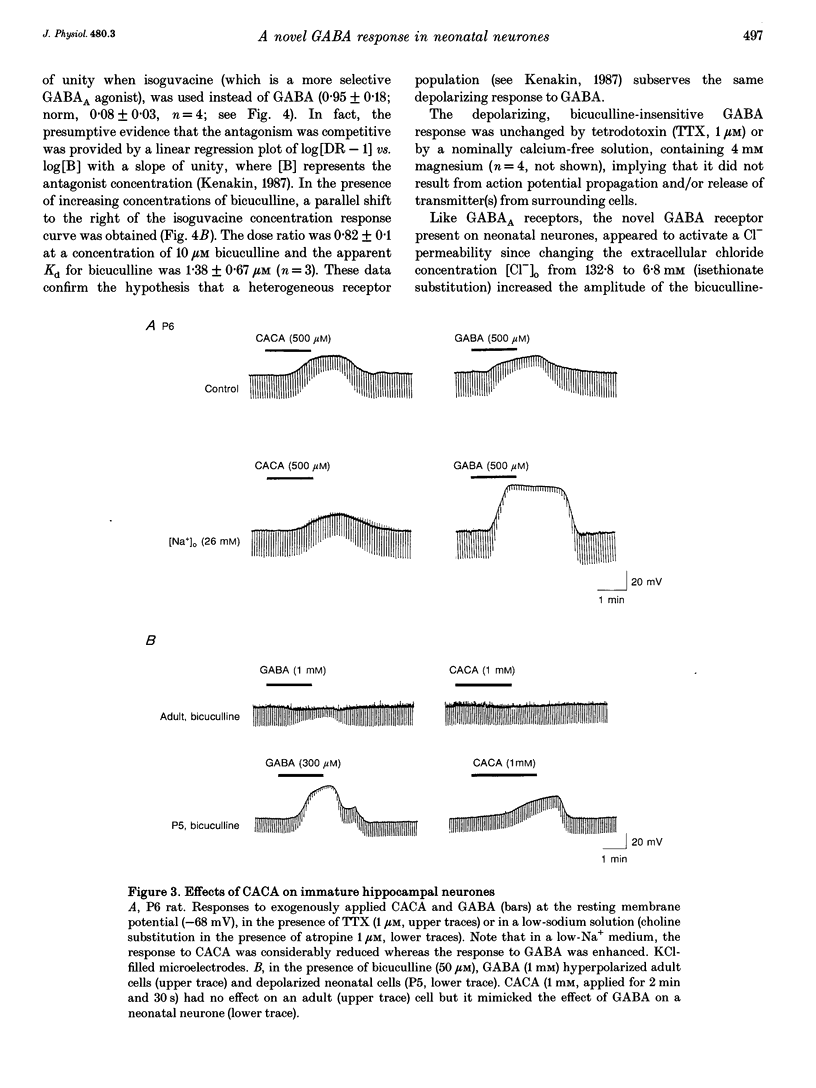
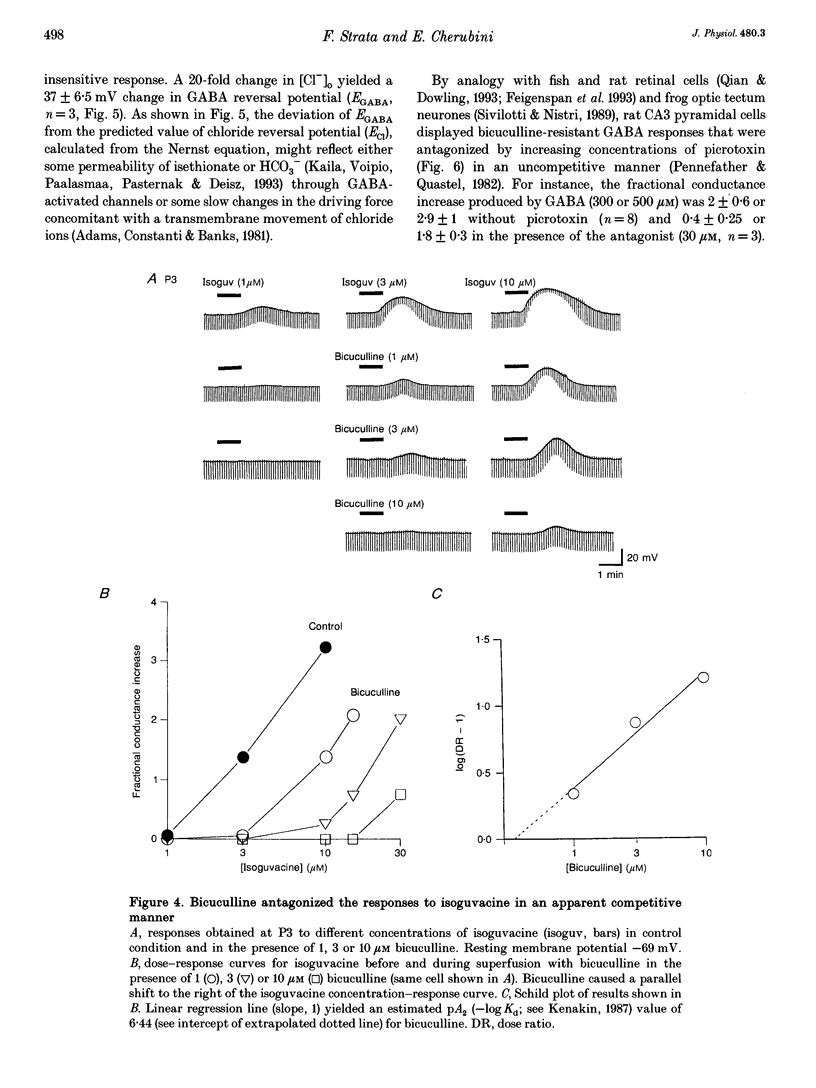
1. Intracellular recordings were used to study the effects of gamma-aminobutyric acid (GABA) on rat CA3 hippocampal neurones during the first two weeks of postnatal life. 2. In the presence of tetrodotoxin (TTX, 1 microM), from postnatal day 0 (P0) to P12 both associated with an increase in input conductance whereas baclofen (30-100 microM) produced a membrane hyperpolarization. 3. Bicuculline (50 microM) reduced the effects of GABA and abolished the response to isoguvacine without affecting the response to baclofen. 4. This novel bicuculline-insensitive GABA response was chloride dependent and was blocked by picrotoxin (10-100 microM) in an uncompetitive way. In bicuculline and picrotoxin, a GABAB-mediated hyperpolarization appeared. 5. Towards the end of the second postnatal week, bicuculline blocked the GABA-induced depolarization and revealed a small hyperpolarizing response which was blocked by the GABAB antagonist CGP 35348 (0.5-1 mM). 6. It is suggested that, during development, the GABA response was mediated through the conventional GABAA and GABAB receptors as well as a new bicuculline-baclofen-insensitive type of receptor.
Full text
PDF

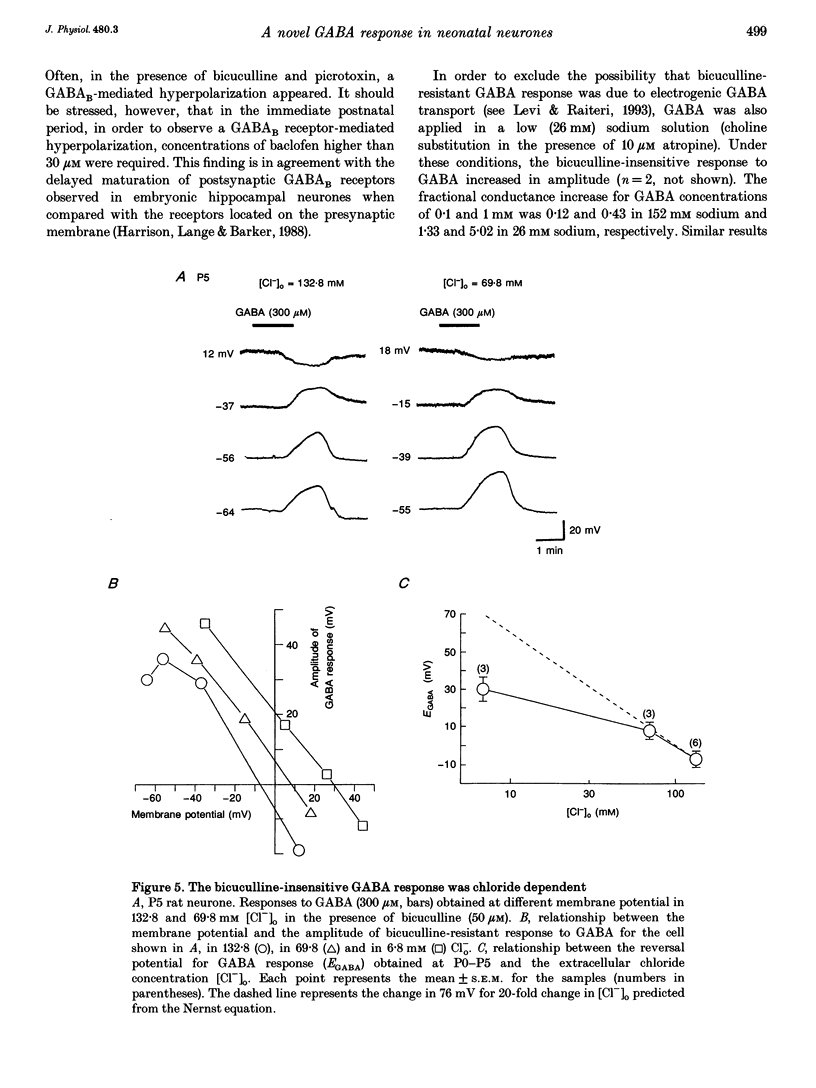
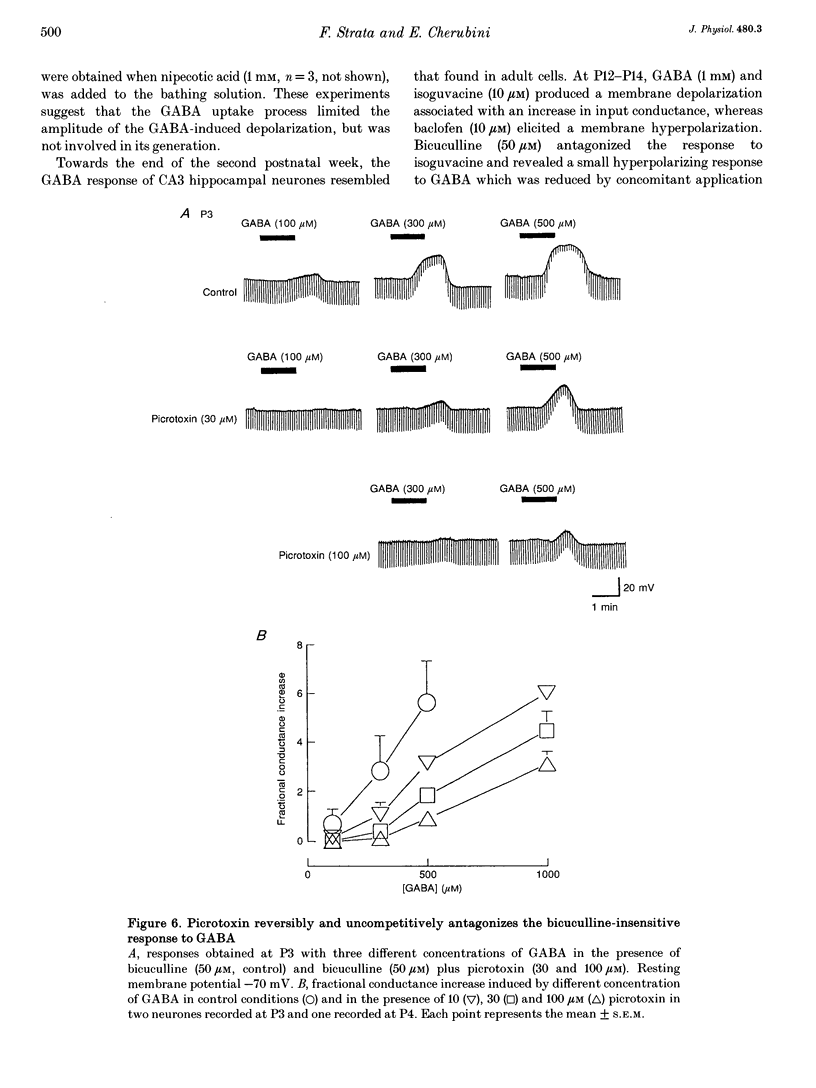


Selected References
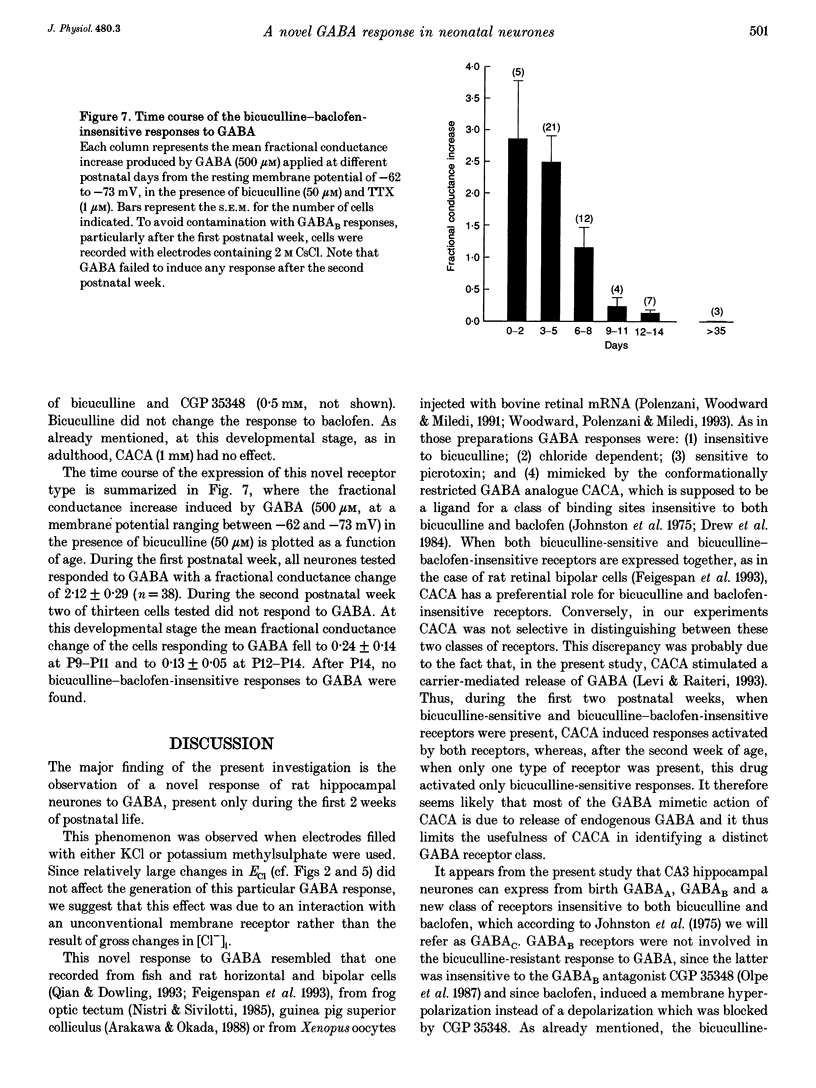
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Banks F. W. Voltage clamp analysis of inhibitory synaptic action in crayfish stretch receptor neurons. Fed Proc. 1981 Sep;40(11):2637–2641. [PubMed] [Google Scholar]
- Arakawa T., Okada Y. Excitatory and inhibitory action of GABA on synaptic transmission in slices of guinea pig superior colliculus. Eur J Pharmacol. 1988 Dec 13;158(3):217–224. doi: 10.1016/0014-2999(88)90070-2. [DOI] [PubMed] [Google Scholar]
- Becker C. M., Betz H., Schröder H. Expression of inhibitory glycine receptors in postnatal rat cerebral cortex. Brain Res. 1993 Mar 26;606(2):220–226. doi: 10.1016/0006-8993(93)90988-y. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989 Sep;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J. L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991 Dec;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cutting G. R., Lu L., O'Hara B. F., Kasch L. M., Montrose-Rafizadeh C., Donovan D. M., Shimada S., Antonarakis S. E., Guggino W. B., Uhl G. R. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew C. A., Johnston G. A. Bicuculline- and baclofen-insensitive gamma-aminobutyric acid binding to rat cerebellar membranes. J Neurochem. 1992 Mar;58(3):1087–1092. doi: 10.1111/j.1471-4159.1992.tb09366.x. [DOI] [PubMed] [Google Scholar]
- Drew C. A., Johnston G. A., Weatherby R. P. Bicuculline-insensitive GABA receptors: studies on the binding of (-)-baclofen to rat cerebellar membranes. Neurosci Lett. 1984 Dec 21;52(3):317–321. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- Feigenspan A., Wässle H., Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993 Jan 14;361(6408):159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Lange G. D., Barker J. L. (-)-Baclofen activates presynaptic GABAB receptors on GABAergic inhibitory neurons from embryonic rat hippocampus. Neurosci Lett. 1988 Feb 15;85(1):105–109. doi: 10.1016/0304-3940(88)90437-5. [DOI] [PubMed] [Google Scholar]
- Ito S., Cherubini E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. J Physiol. 1991;440:67–83. doi: 10.1113/jphysiol.1991.sp018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A., Curtis D. R., Beart P. M., Game C. J., McCulloch R. M., Twitchin B. Cis- and trans-4-aminocrotonic acid as GABA analogues of restricted conformation. J Neurochem. 1975 Jan;24(1):157–160. doi: 10.1111/j.1471-4159.1975.tb07642.x. [DOI] [PubMed] [Google Scholar]
- Kaila K., Voipio J., Paalasmaa P., Pasternack M., Deisz R. A. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurones. J Physiol. 1993 May;464:273–289. doi: 10.1113/jphysiol.1993.sp019634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater S. B., Mattson M. P., Cohan C., Connor J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988 Jul;11(7):315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Wisden W., Seeburg P. H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992 Nov;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Raiteri M. Carrier-mediated release of neurotransmitters. Trends Neurosci. 1993 Oct;16(10):415–419. doi: 10.1016/0166-2236(93)90010-j. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri A., Sivilotti L. An unusual effect of gamma-aminobutyric acid on synaptic transmission of frog tectal neurones in vitro. Br J Pharmacol. 1985 Aug;85(4):917–921. doi: 10.1111/j.1476-5381.1985.tb11092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe H. R., Karlsson G., Pozza M. F., Brugger F., Steinmann M., Van Riezen H., Fagg G., Hall R. G., Froestl W., Bittiger H. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990 Oct 2;187(1):27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Modification of dose-response curves by effector blockade and uncompetitive antagonism. Mol Pharmacol. 1982 Sep;22(2):369–380. [PubMed] [Google Scholar]
- Polenzani L., Woodward R. M., Miledi R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Dowling J. E. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993 Jan 14;361(6408):162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Kunkel D. D. Electrophysiology and morphology of the developing hippocampus of fetal rabbits. J Neurosci. 1982 Apr;2(4):448–462. doi: 10.1523/JNEUROSCI.02-04-00448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönrock B., Bormann J. Functional heterogeneity of hippocampal GABAA receptors. Eur J Neurosci. 1993 Aug 1;5(8):1042–1049. doi: 10.1111/j.1460-9568.1993.tb00957.x. [DOI] [PubMed] [Google Scholar]
- Segal M. GABA induces a unique rise of [Ca]i in cultured rat hippocampal neurons. Hippocampus. 1993 Apr;3(2):229–238. doi: 10.1002/hipo.450030214. [DOI] [PubMed] [Google Scholar]
- Shimada S., Cutting G., Uhl G. R. gamma-Aminobutyric acid A or C receptor? gamma-Aminobutyric acid rho 1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid responses in Xenopus oocytes. Mol Pharmacol. 1992 Apr;41(4):683–687. [PubMed] [Google Scholar]
- Sivilotti L., Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Sivilotti L., Nistri A. Pharmacology of a novel effect of gamma-aminobutyric acid on the frog optic tectum in vitro. Eur J Pharmacol. 1989 May 19;164(2):205–212. doi: 10.1016/0014-2999(89)90460-3. [DOI] [PubMed] [Google Scholar]
- Woodward R. M., Polenzani L., Miledi R. Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acidA and gamma-aminobutyric acidB receptor agonists and antagonists. Mol Pharmacol. 1993 Apr;43(4):609–625. [PubMed] [Google Scholar]
- Zhang L., Spigelman I., Carlen P. L. Development of GABA-mediated, chloride-dependent inhibition in CA1 pyramidal neurones of immature rat hippocampal slices. J Physiol. 1991 Dec;444:25–49. doi: 10.1113/jphysiol.1991.sp018864. [DOI] [PMC free article] [PubMed] [Google Scholar]


