Abstract
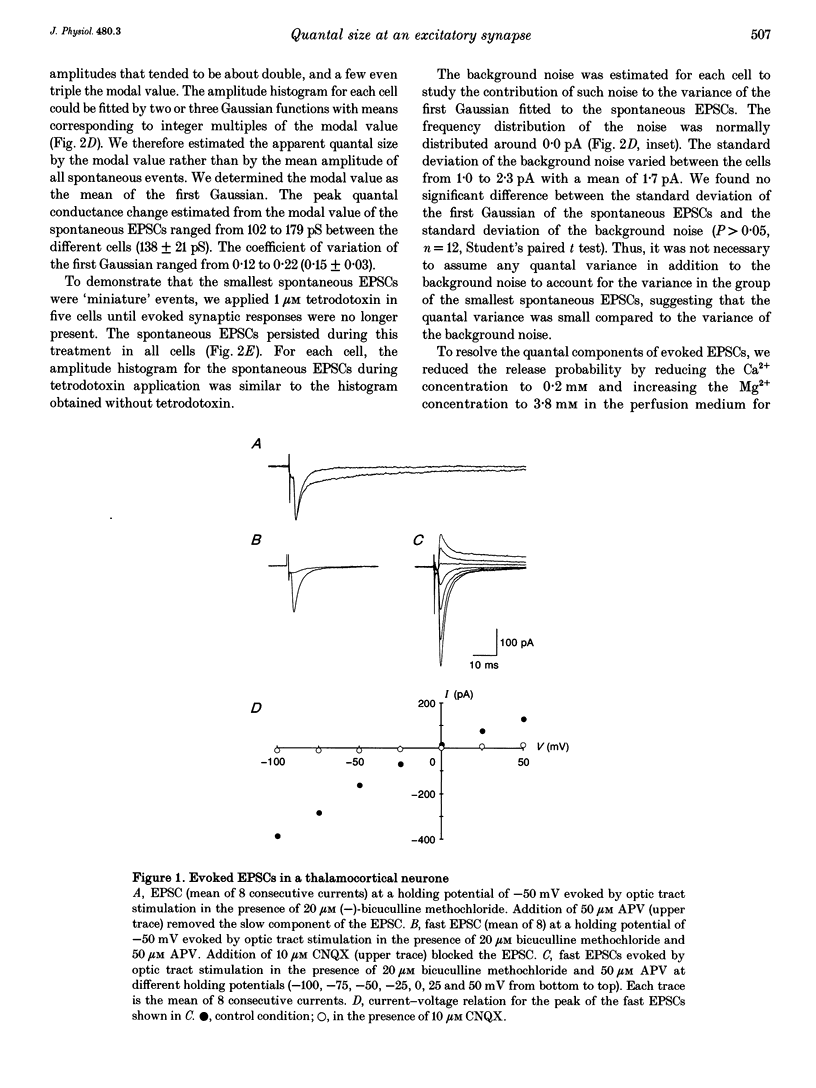
1. To determine the quantal size at retinogeniculate synapses, spontaneous and evoked excitatory postsynaptic currents (EPSCs) were recorded in twelve neurones of the dorsal lateral geniculate nucleus in guinea-pig thalamic slices using the whole-cell patch-clamp technique. We limited our study to the fast non-N-methyl-D-aspartate (NMDA) component of the EPSCs by adding the NMDA receptor antagonist DL-2-amino-5-phosphonovaleric acid to the perfusion medium. 2. Spontaneous EPSCs occurred at a frequency between 0.5 and 6.6 Hz (mean 2.5 Hz). The modal value of the peak conductance change of spontaneous excitatory events varied between cells from 102 to 179 pS. 3. EPSCs were evoked by electrical stimulation in the optic tract. The peak conductance change of EPSCs evoked by stimulation of a putative single input fibre ranged from 0.6 to 3.4 nS (mean 1.7 nS). 4. To resolve the quantal components of evoked EPSCs the external Ca2+ concentration was reduced and the external Mg2+ concentration increased for four cells. In this condition failures occurred and the amplitude histograms were multimodal with approximately equidistant peaks. 5. These multimodal histograms could be fitted by a sum of Gaussian functions with mean values corresponding to integer multiples of the modal value of the spontaneous EPSCs for the same cell. Thus, the quantal size of evoked EPSCs was the same as the modal value of spontaneous EPSCs. The mean of the apparent quantal conductance change was 138 pS. The estimated number of quanta released by stimulating a putative single input fibre in the control condition ranged from 4 to 27 (mean 13).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

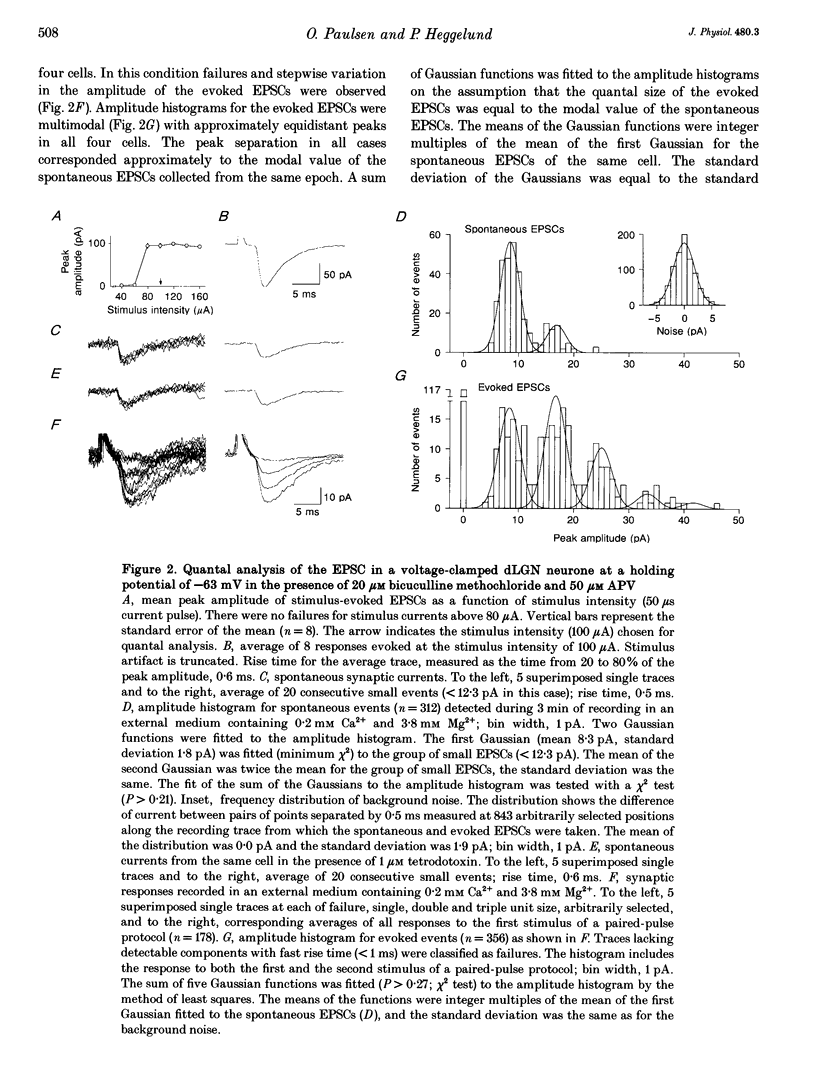
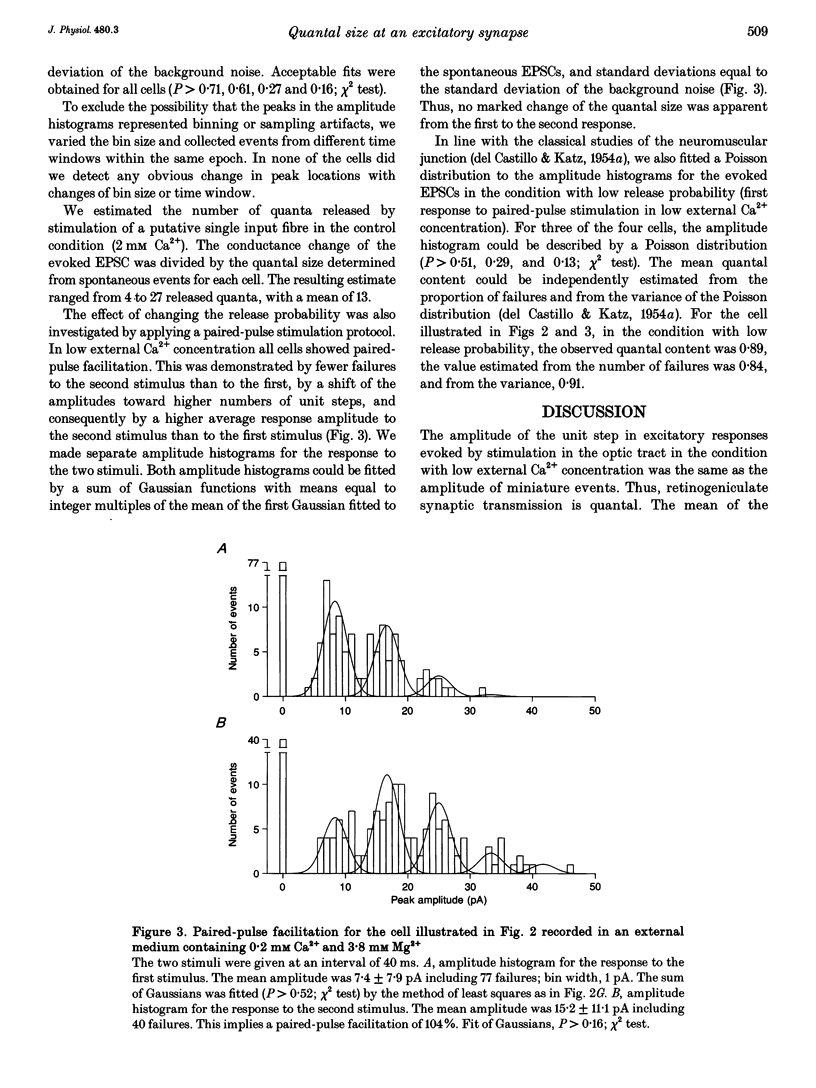


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomfield S. A., Hamos J. E., Sherman S. M. Passive cable properties and morphological correlates of neurones in the lateral geniculate nucleus of the cat. J Physiol. 1987 Feb;383:653–692. doi: 10.1113/jphysiol.1987.sp016435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C. Spontaneous multiquantal release at synapses in guinea-pig hypogastric ganglia: evidence that release can occur in bursts. J Physiol. 1978 Sep;282:375–398. doi: 10.1113/jphysiol.1978.sp012470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Leresche N., Parnavelas J. G. Membrane properties of morphologically identified X and Y cells in the lateral geniculate nucleus of the cat in vitro. J Physiol. 1987 Sep;390:243–256. doi: 10.1113/jphysiol.1987.sp016697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamos J. E., Van Horn S. C., Raczkowski D., Sherman S. M. Synaptic circuits involving an individual retinogeniculate axon in the cat. J Comp Neurol. 1987 May 8;259(2):165–192. doi: 10.1002/cne.902590202. [DOI] [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Faber D. S. Quantal analysis and synaptic efficacy in the CNS. Trends Neurosci. 1991 Oct;14(10):439–445. doi: 10.1016/0166-2236(91)90042-s. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Nicoll R. A. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992 May 21;357(6375):240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde D. N. Two classes of single-input X-cells in cat lateral geniculate nucleus. I. Receptive-field properties and classification of cells. J Neurophysiol. 1987 Feb;57(2):357–380. doi: 10.1152/jn.1987.57.2.357. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature. 1988 Jul 21;334(6179):246–248. doi: 10.1038/334246a0. [DOI] [PubMed] [Google Scholar]
- Raastad Morten, Storm Johan F., Andersen Per. Putative Single Quantum and Single Fibre Excitatory Postsynaptic Currents Show Similar Amplitude Range and Variability in Rat Hippocampal Slices. Eur J Neurosci. 1992 Oct;4(1):113–117. doi: 10.1111/j.1460-9568.1992.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Robson J. A. Qualitative and quantitative analyses of the patterns of retinal input to neurons in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1993 Aug 8;334(2):324–336. doi: 10.1002/cne.903340212. [DOI] [PubMed] [Google Scholar]
- Ropert N., Miles R., Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. J Physiol. 1990 Sep;428:707–722. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P., Edwards F. A., Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol. 1992 Apr;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]


