Abstract
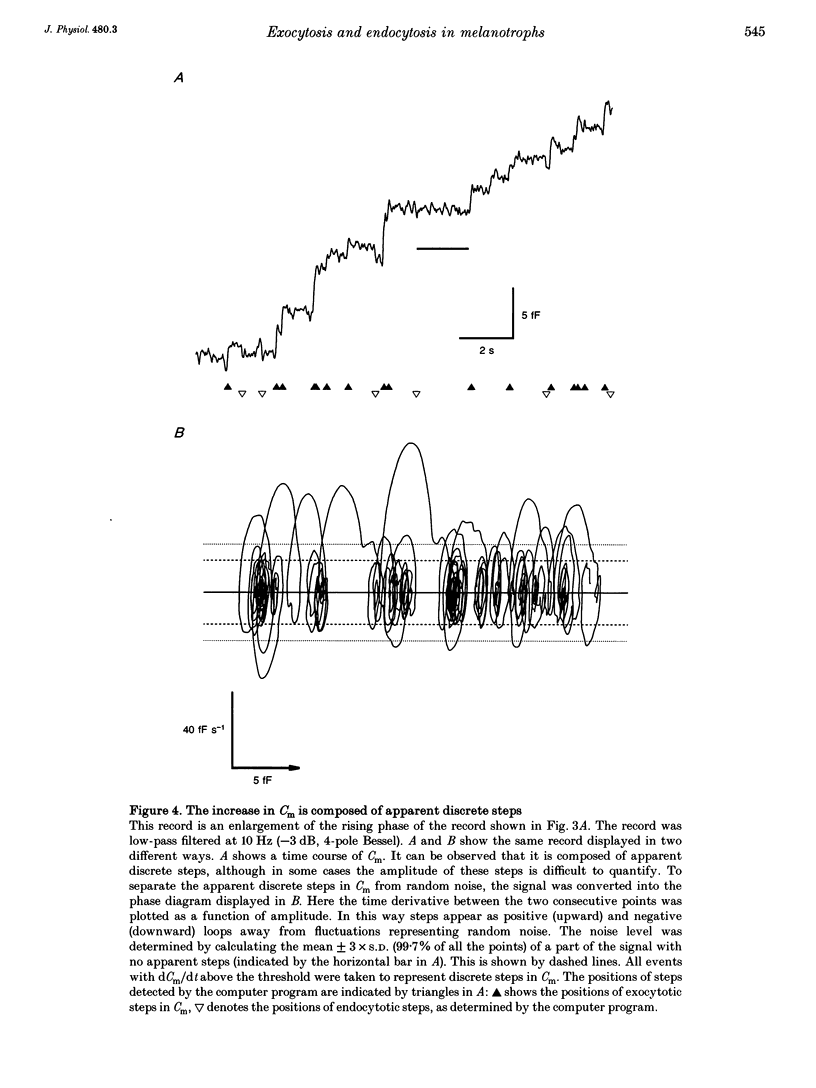
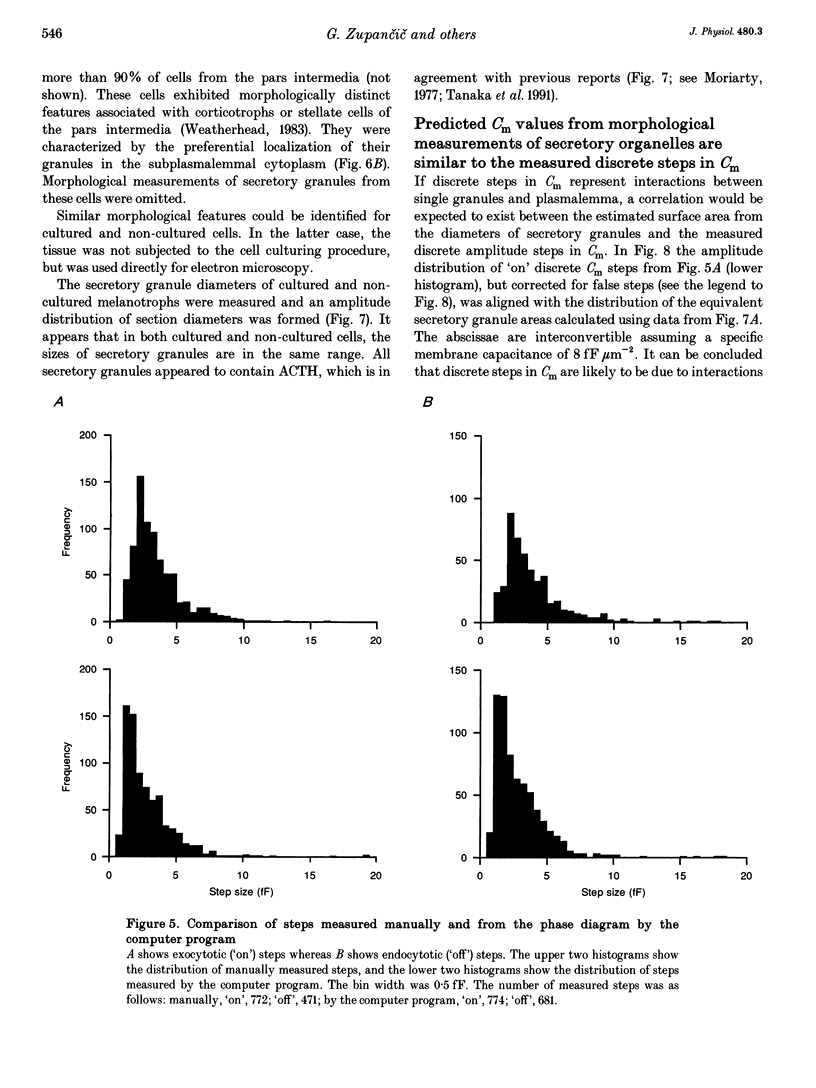
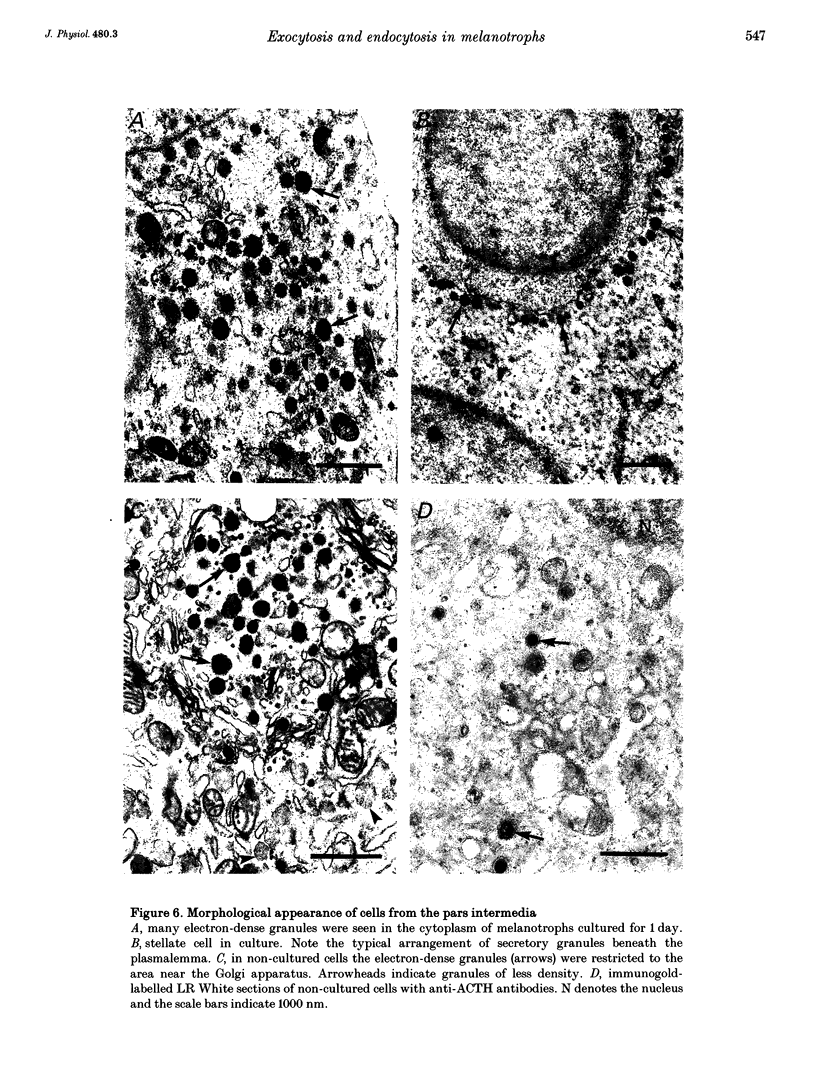
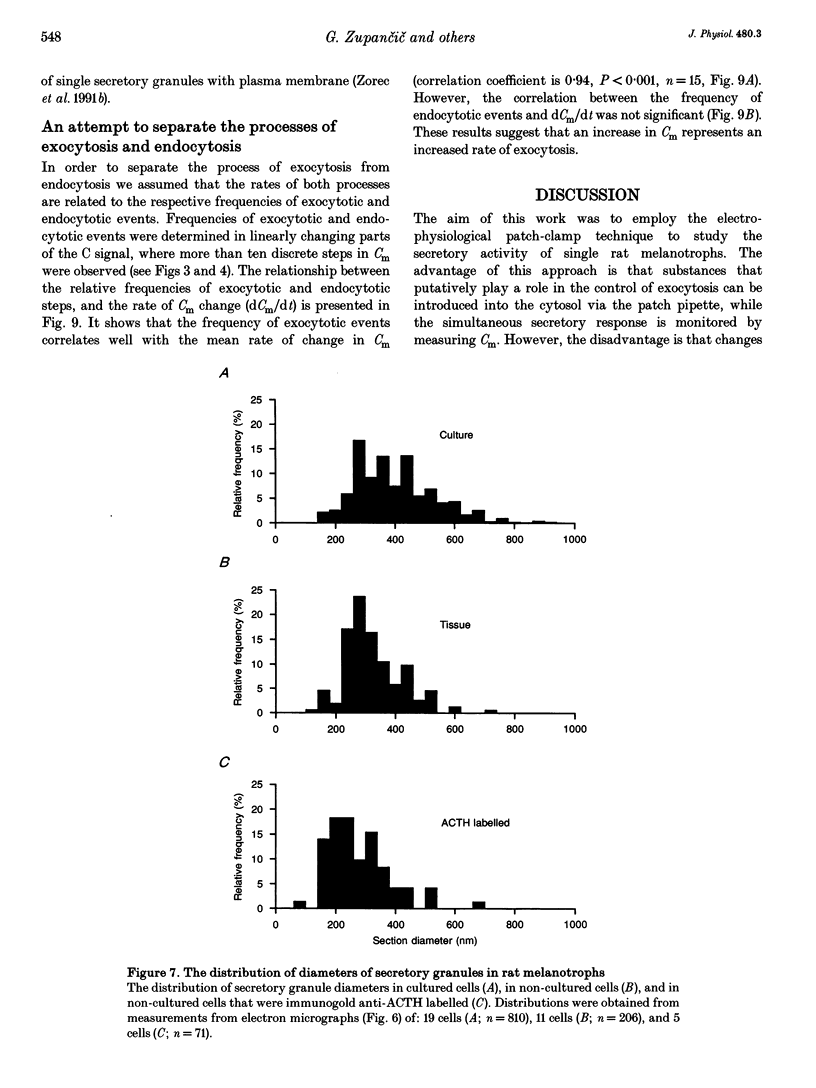
1. Using the patch-clamp technique, we have monitored the secretory activity of single rat melanotrophs. Changes in membrane capacitance (Cm) were measured to detect small discrete femtofarad steps. These are believed to be due to interactions between single secretory organelles (granules) and plasmalemma. 2. A new approach was introduced to measure the amplitude of discrete steps in Cm. Records of Cm were converted into time derivatives, where discrete steps appeared as transients. A transient due to a 2 fF discrete step in Cm was easily distinguished from random noise, since the probability of such a transient being due to random noise was less than 0.01. To distinguish apparent steps from noise the computer-based analysis employed a threshold of 3 times the standard deviation of the noise time derivative (dCm/dt). A phase diagram was created by plotting dCm/dt versus Cm, from which the magnitude and direction of transients were determined. Transients due to 2 fF steps (equivalent to a signal-to-noise ratio of 1) were detected with a reliability of 100%, whereas steps of 1 fF were detected with a reliability of more than 60%. The amplitude of false steps detected by the program was less than 1 fF, and the frequency of false detections of 0.075 S-1 was equal for exocytotic and endocytotic events. 3. Electron microscopy was used to measure secretory organelle size and an immunogold technique was used to label the electron micrographs with an anti-adrenocorticotrophin (ACTH) antibody. Secretory organelles in cultured and non-cultured cells were of similar diameter. All sizes of secretory granules appear to contain ACTH, since secretory organelles of similar diameter stained positively with the anti-ACTH antibodies. 4. Small discrete steps in Cm, recorded with the whole-cell configuration and loosely buffered cytosolic calcium, were similar to the estimated Cm of secretory organelles from morphological data. Thus, measured discrete steps in Cm reflect interactions between single organelle size and plasma membrane. Exocytotic and endocytotic steps were found to be of similar size. 5. To separate exocytosis from endocytosis in Cm records, we assumed that the rates of exocytosis and endocytosis were related to the respective frequencies of discrete steps in Cm. A relationship between the frequency of exocytotic, but not endocytotic events, and the rate of change in Cm was observed. Thus, under our experimental conditions, an increase in Cm could be explained by an increased rate of exocytosis in rat melanotrophs.
Full text
PDF


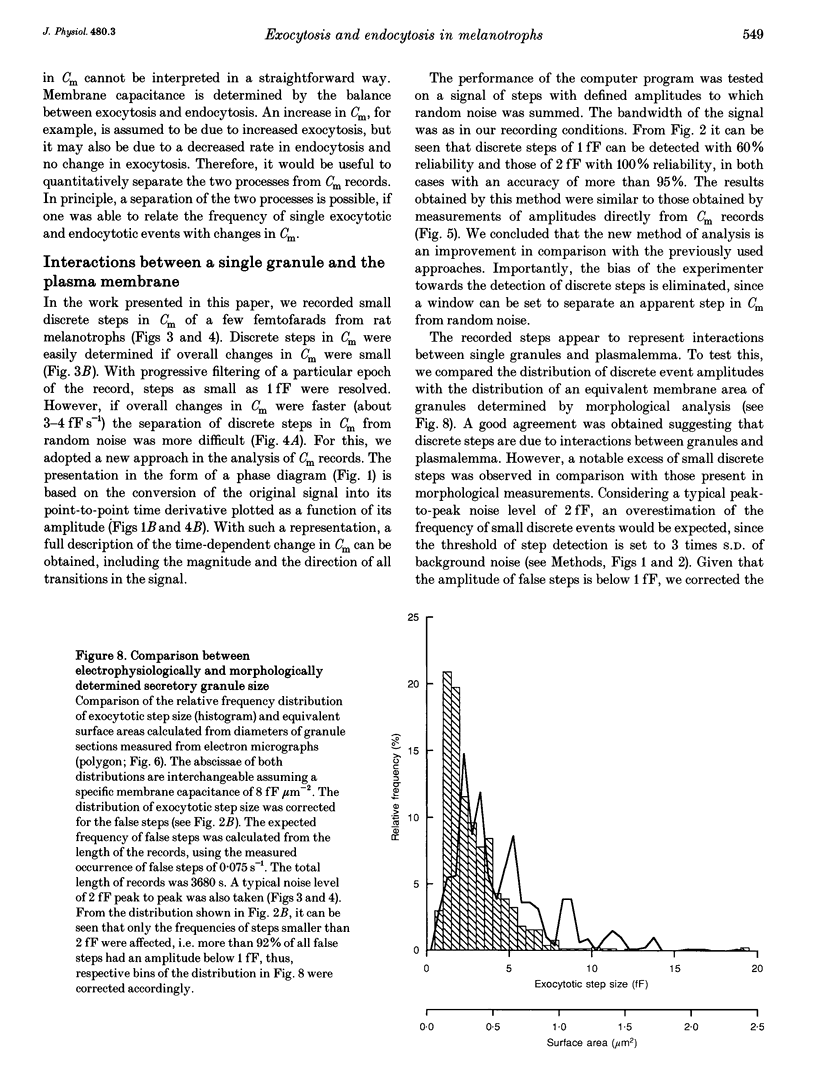
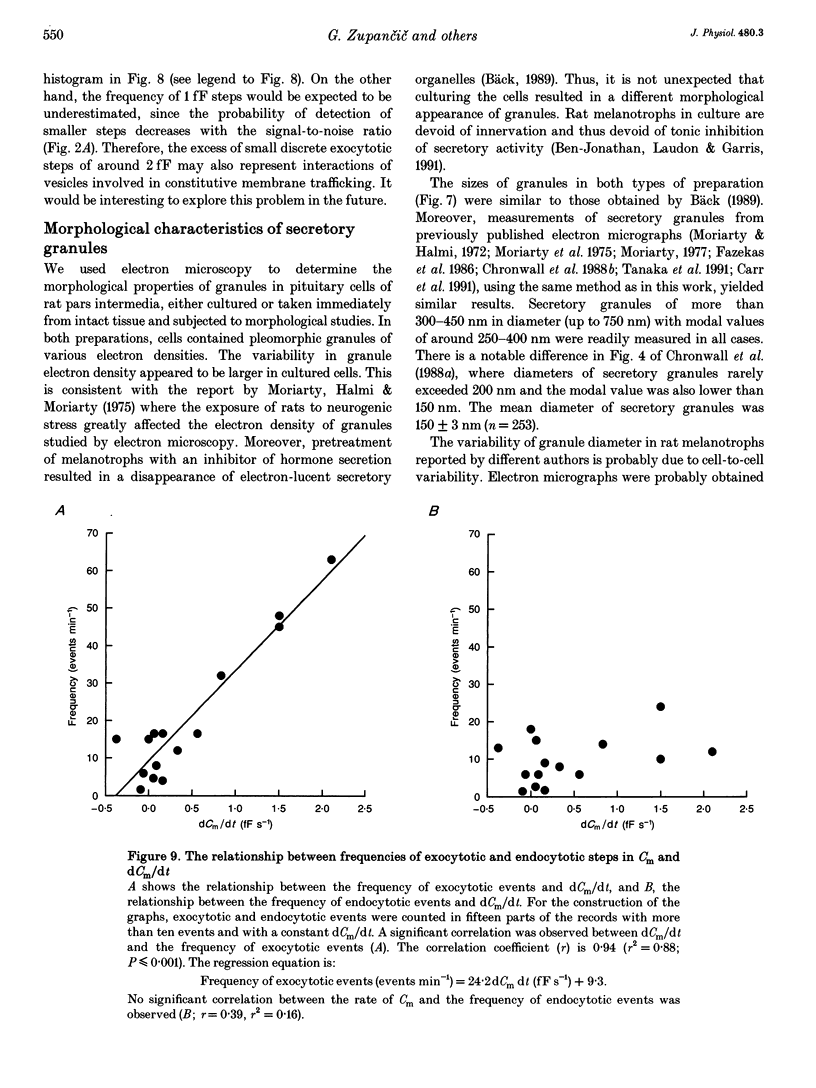


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Bäck N., Soinila S., Virtanen I. Endocytotic pathways in the melanotroph of the rat pituitary. Histochem J. 1993 Feb;25(2):133–139. doi: 10.1007/BF00157985. [DOI] [PubMed] [Google Scholar]
- Bäck N. The effect of bromocriptine on the intermediate lobe of the rat pituitary: an electron-microscopic, morphometric study. Cell Tissue Res. 1989 Feb;255(2):405–410. doi: 10.1007/BF00224124. [DOI] [PubMed] [Google Scholar]
- Carr J. A., Saland L. C., Samora A., Benavidez S., Krobert K. In vivo effects of serotonergic agents on alpha-melanocyte-stimulating hormone secretion. Neuroendocrinology. 1991 Dec;54(6):616–622. doi: 10.1159/000125968. [DOI] [PubMed] [Google Scholar]
- Chronwall B. M., Bishop J. F., Gehlert D. R. Rat intermediate lobe in culture: a histological and biochemical characterization. Peptides. 1988;9 (Suppl 1):169–180. doi: 10.1016/0196-9781(88)90241-0. [DOI] [PubMed] [Google Scholar]
- Chronwall B. M., Hook G. R., Millington W. R. Dopaminergic regulation of the biosynthetic activity of individual melanotropes in the rat pituitary intermediate lobe: a morphometric analysis by light and electron microscopy and in situ hybridization. Endocrinology. 1988 Oct;123(4):1992–2002. doi: 10.1210/endo-123-4-1992. [DOI] [PubMed] [Google Scholar]
- Fazekas I., Rappay G., Bácsy E., Medzihradszky-Schweiger H., Gyévai A., Gaál G. Dissimilar responsiveness of cultured corticotrophs and melanotrophs to tripeptide aldehydes. Histochemistry. 1986;84(4-6):418–422. doi: 10.1007/BF00482972. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Gillis K. D., Misler S. Single cell assay of exocytosis from pancreatic islet B cells. Pflugers Arch. 1992 Jan;420(1):121–123. doi: 10.1007/BF00378654. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Lindau M., Gomperts B. D. Techniques and concepts in exocytosis: focus on mast cells. Biochim Biophys Acta. 1991 Dec 12;1071(4):429–471. doi: 10.1016/0304-4157(91)90006-i. [DOI] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Synthesis and secretion of corticotropins, melanotropins, and endorphins by rat intermediate pituitary cells. J Biol Chem. 1979 Aug 25;254(16):7885–7894. [PubMed] [Google Scholar]
- Maruyama Y. Ca2+-induced excess capacitance fluctuation studied by phase-sensitive detection method in exocrine pancreatic acinar cells. Pflugers Arch. 1986 Nov;407(5):561–563. doi: 10.1007/BF00657517. [DOI] [PubMed] [Google Scholar]
- Moriarty G. C., Halmi N. S. Adrenocorticotropin production by the intermediate lobe of the rat pituitary. An electron microscopic-immunohistochemical study. Z Zellforsch Mikrosk Anat. 1972;132(1):1–14. doi: 10.1007/BF00310292. [DOI] [PubMed] [Google Scholar]
- Moriarty G. C., Halmi N. S., Moriarty M. The effect of stress on the cytology and immunocytochemistry of pars intermedia cells in the rat pituitary. Endocrinology. 1975 Jun;96(6):1426–1436. doi: 10.1210/endo-96-6-1426. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsse O., Lindau M. The dynamics of exocytosis in human neutrophils. J Cell Biol. 1988 Dec;107(6 Pt 1):2117–2123. doi: 10.1083/jcb.107.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rupnik M., Zorec R. Cytosolic chloride ions stimulate Ca(2+)-induced exocytosis in melanotrophs. FEBS Lett. 1992 Jun 1;303(2-3):221–223. doi: 10.1016/0014-5793(92)80524-k. [DOI] [PubMed] [Google Scholar]
- Sikdar S. K., Zorec R., Brown D., Mason W. T. Dual effects of G-protein activation on Ca-dependent exocytosis in bovine lactotrophs. FEBS Lett. 1989 Aug 14;253(1-2):88–92. doi: 10.1016/0014-5793(89)80936-6. [DOI] [PubMed] [Google Scholar]
- Sikdar S. K., Zorec R., Mason W. T. cAMP directly facilitates Ca-induced exocytosis in bovine lactotrophs. FEBS Lett. 1990 Oct 29;273(1-2):150–154. doi: 10.1016/0014-5793(90)81072-v. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Nomizu M., Kurosumi K. Intracellular sites of proteolytic processing of pro-opiomelanocortin in melanotrophs and corticotrophs in rat pituitary. J Histochem Cytochem. 1991 Jun;39(6):809–821. doi: 10.1177/39.6.1851777. [DOI] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Almers W. Millisecond studies of secretion in single rat pituitary cells stimulated by flash photolysis of caged Ca2+. EMBO J. 1993 Jan;12(1):303–306. doi: 10.1002/j.1460-2075.1993.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman S. D., Terry B. R., Findlay G. P. Multiple conductances in the large K+ channel from Chara corallina shown by a transient analysis method. Biophys J. 1992 Mar;61(3):736–749. doi: 10.1016/S0006-3495(92)81878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillez P., René F., Plante M., Hindelang C., Klein M. J., Félix J. M., Stoeckel M. E. Differentiation of the melanotrophic cells of rat pituitary primordium in organotypic culture in defined medium. Cell Tissue Res. 1992 Jan;267(1):169–183. doi: 10.1007/BF00318702. [DOI] [PubMed] [Google Scholar]
- Zorec R., Sikdar S. K., Mason W. T. Increased cytosolic calcium stimulates exocytosis in bovine lactotrophs. Direct evidence from changes in membrane capacitance. J Gen Physiol. 1991 Mar;97(3):473–497. doi: 10.1085/jgp.97.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]