Abstract
Objective: The aim of the present study was to investigate the expression of erythroblast transformation specific-1 related gene (ERG) in patients with glioblastoma (GB) before and after bevacizumab (Bev) therapy as a predictive and prognostic biomarker. Methods: The present study used 58 GB tissues from 29 patients in 3 settings. Sixteen tumors were removed after neoadjuvant Bev administration (neoBev) and 13 represented newly diagnosed GB without previous Bev treatment (naïve Bev). Another 29 specimens of recurrence were obtained from salvage surgery or autopsy. Results: Immunohistochemical analysis showed both vessel density (VD) and ERG score were decreased in neoBev compared with naïve Bev. VD and ERG score tended to be lower at recurrence than at initial surgery (P=0.0026 and P=0.1338, respectively). In the naïve Bev and neoBev cohorts, overall survival (OS) with high and low expressions of ERG was comparable (P=0.7516 and P=0.3862, respectively). Conclusion: High expression of ERG in GB with naïveBev was significantly reduced with Bev, but not changed in refractoriness. Stratification of ERG expression levels might provide a useful predictive biomarker for GB treated with Bev.
Keywords: Endothelial cell, erythroblast transformation specific-1 related gene (ERG), glioblastoma, microvessel density, neoadjuvant bevacizumab, vascular normalization
Introduction
Tumor angiogenesis is essential for the growth of various solid tumors, including glioblastoma (GB). Vascular-targeted therapies, including anti-vascular endothelial growth factor (VEGF) antibodies such as bevacizumab (Bev), provide therapeutic growth control effects for malignant tumors.
According to previous reports, the mechanisms underlying the efficacy of Bev might decrease microvessel density and induction of tumor oxygenation in the tumor microenvironment (TME). In particular, Bev may act to “normalize” abnormal structures and functions of tumor vasculature before the tumor itself is destroyed, leading to improved delivery of both oxygen and drugs [1]. In our previous study, tumor hypoxia was recovered with a paradoxical decrease in microvessel density [2]. Bev thus acts not only to reduce the density of blood vessels, but also to modify endothelial properties. Reactivation of VEGF may not be initially involved in the acquisition of resistance to Bev, and other salvage angiogenic pathways than VEGF are likely induced under conditions of resistance to Bev therapy [3].
Predictive and prognostic biomarkers for Bev have not been identified in various cancer patients, including GB. In general, histological malignancy and clinical outcomes should correspond to the degree of differentiation of tumor cells and vascular proliferation. However, from the perspective of pathological diagnosis, the correlation between clinical outcome and degree of differentiation in vessel component cells including endothelial cells during therapy has been given little attention. How to predict whether vascular normalization will persist remains unclear.
Previous research has demonstrated both undifferentiated (CD31+/CD34-) and differentiated microvessels in renal cell carcinoma (RCC) [4]. A higher proportion of undifferentiated microvessels was associated with poor prognosis, whereas a higher population of differentiated microvessels was associated with favorable prognosis. Given this impact of tumor vascular differentiation on clinical outcome, the degree of vascular maturity in “vascular normalization” induced by Bev might provide a prognostic and predictive biomarker for GB treated with Bev.
To evaluate the maturity of microvessels in GB, we focused on the endothelial marker erythroblast transformation specific-1 related gene (ERG), an intranuclear transcriptional factor belonging to the ETS-1 family. ERG is expressed exclusively in the endothelium of matured and normal vessels, and contributes to homeostasis within endothelial cells [5-8]. ERG overexpression reduces vascular permeability and increases the vascular stability of vessels created during VEGF-dependent angiogenesis [9]. According to previous studies, low expression of ERG in tumor-associated endothelial cells was observed in a group of poor prognosis patients with RCC [10], lung cancer, breast cancer, and melanoma [6]. However, much less is known about the predictive role of ERG expression in GB microvessels.
We have experienced several unique cases of preoperative neoadjuvant Bev (neoBev) treatment for newly diagnosed GB, which significantly improved clinical symptoms and neuroradiographic shrinkage of the tumor volume via reduction of tumor vascularity and perifocal edema without particular adverse events [11-13]. When recurrences were identified after neoBev therapy, salvage surgery or autopsy was performed, allowing paired samples from different states of both Bev effectiveness and Bev refractoriness to be obtained from each patient. It is extremely rare to obtain tissue specimens measuring effectiveness of Bev, since tumor resection is usually not undergone during chemotherapy. Therefore, there are very few previous reports regarding histological analyses using paired samples from same patients before and after targeted therapy including Bev.
The present study aimed to compare expression levels of ERG in GB between effectiveness and refractoriness during Bev therapy, and to assess whether ERG would be useful as a predictive and prognostic biomarker for outcomes of patients with GB.
Materials and methods
Patient eligibility and entry procedures (Figure 1)
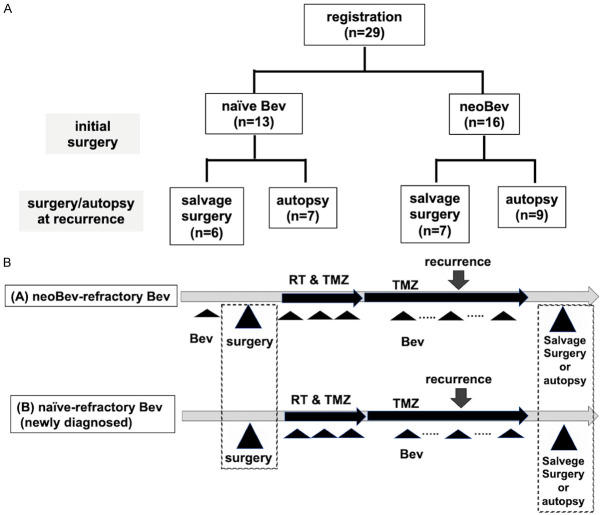
Figure 1.
Patient registration and treatment protocol. A. Patient registration and distribution of naïve Bev and neoBev (effective Bev). B. Schema of treatment protocol. Paired samples were obtained via initial surgery at new diagnosis and salvage surgery or autopsy at recurrence.
The present study was conducted at The Jikei University Kashiwa Hospital. Tumor resection was carried out at two collaborating institutes (The Jikei University Kashiwa Hospital and Kagawa University Hospital, Kagawa, Japan) between January 2015 and December 2021.
A total of 58 GB paired tissues were obtained from 29 patients under 3 groups as follows: (1) Sixteen tumors were removed after neoBev therapy, i.e., during a state of effective to Bev. (2) Thirteen tumors were removed as newly diagnosed GB patients without any previous treatment including Bev (naïve Bev). (3) Twenty-nine recurrent tumors after Bev administration (refractory Bev) (Figure 1A). The refractory Bev group included 16 specimens obtained at autopsy and 13 specimens of recurrent tumors resected during salvage surgery.
Importantly, refractory Bev cases in all 13 naïve Bev cases included 7 autopsy specimens and 6 salvage surgery specimens and refractory Bev cases in all 16 neoBev cases included 7 autopsy specimens and 9 salvage surgery specimens. These tissues were obtained as paired specimens from the same patient.
Treatment protocol
Patients in the neoBev group were treated with preoperative Bev at a dose of 10 mg/kg on day 0 and temozolomide (TMZ) at a dose of 150 mg/m2 on days 1-5. Two weeks after neoBev, MRI was performed. The residual tumor was removed 3-4 weeks after neoBev. Radiation (RT) and TMZ were administered more than two weeks after surgery. Maintenance treatment with TMZ began more than four weeks after completion of RT at a starting dose of 150 mg/m2 for five consecutive days of a 28-day cycle. All newly diagnosed GB patients without preoperative chemotherapy including Bev (naïve Bev) were treated with concomitant Stupp regimen and Bev after surgical resection [14,15] (Figure 1B).
Study oversight
The study protocol was approved by the ethics committees of The Jikei University School of Medicine Kashiwa Hospital and Kagawa University Hospital and by the institutional review boards of The Jikei University Kashiwa Hospital (approval nos. JKI18-052, 26-334) and Kagawa University Hospital (approval no. 2022-107). The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrolment.
Assessment of neuroradiological response and clinical outcome
Neuroradiological response before and after preoperative neoBev was assessed as previously described [3,12,13]. Progression free survival (PFS) was defined from initial therapy to progression. Overall survival (OS) was defined from initial therapy to death.
Immunohistochemistry
To assess changes in endothelial markers during Bev therapy, expressions of CD34 and ERG were analyzed using 4-μm sections of formalin-fixed, paraffin-embedded tissues. Procedures were followed according to the protocols of the manufacturers. Briefly, antigen was retrieved using a microwave method in 10-mM citrate buffer (pH 6.0). After blocking with 2.5% normal horse serum (ImmPress Detection Systems; Vectorlabs, Burlingame, CA, USA) for 60 min, sections were incubated overnight at 4°C with anti-CD34 (1:100, M7165; abcam, Cambridge, UK), anti-ERG (1:1000, EPR3864; abcam) antibodies. Immunoreactivity was visualized by the peroxidase-diaminobenzidine reaction. Expression levels of endothelial markers were assessed in tumor tissue under five representative high-power fields (HPFs). All experiments were assessed as the consensus decisions of four authors (AI, NF, MM and TT).
Assessment of CD34 and ERG
To clarify the loss of ERG staining, Initially, CD34-stained sections were screened in a low-power field (×40) and five hot spots were selected. The number of positive luminal structures in these areas were counted in a HPF (×400, 0.47 mm2). ERG scoring was assessed at the same spots of CD34-positivity. The ERG score was defined as combined values of the labeling index in endothelial cells (0, none; 1, ≤25%; 2, 25-50%; or 3, >50%) and staining intensity (0, none; 1, weak; 2, moderate; 3, strong), as previously described [10].
Assessment of bioinformatics from CGGA dataset and TCGA research network
Based on median expression values of ERG, the GB cohort from the Chinese Glioma Genome Atlas (CGGA) dataset, obtained from GlioVis [16] (http://gliovis.bioinfo.cnio.es), were divided into high-expression (top 50%) and low-expression (bottom 50%) groups. Similarly, the Cancer Genome Atlas (TCGA) Research Network (https://www.cancer.gov/tcga) dataset was used to analyze prognosis stratified by median ERG expression.
Statistical analysis
Continuous data are described as mean ± standard deviation and categorical data are presented as numbers and percentages. The Mann-Whitney U test was used for comparisons of continuous data between two independent groups, and the Wilcoxon signed-rank test was used for comparisons of continuous data between two paired groups. Fisher’s exact test was used for categorical data. Overall survival stratified by various parameters was analyzed using the Kaplan-Meier method and significance was determined using the log-rank test. We divided the subgroups as follows to compare the expression of CD34 and ERG: at initial and recurrence, into naïve Bev and neoBev. Furthermore, at recurrence, we distinguished between autopsy samples and reoperation samples. Statistical analyses were performed using STATA18 (Stata Corp. LP, College Station, TX, USA) and GraphPad Prism version 10 (GraphPad Software, Boston, MA, USA). All p-values were two-sided, with the level of significance set at P<0.05.
Results
Clinical characteristics (Table 1)
Table 1.
Patient characteristics
| Overall n=29 | p value | ||
|---|---|---|---|
|
| |||
| Naïve-refractory | Effective-refractory | ||
| n=13 | n=16 | ||
| Mean age (SD) (years) | 56.8 (9.3) | 66.9 (10.1) | 0.0123a |
| Sex, male/female | 9 (77.0)/4 (23.0) | 12 (75.0)/4 (25.0) | 1b |
| Tumor location | |||
| Frontal | 3 (23.1) | 6 (37.5) | 0.536b |
| Temporal | 6 (46.1) | 6 (37.5) | |
| Parietal | 3 (23.1) | 2 (12.5) | |
| Occipital | 0 (0.0) | 2 (12.5) | |
| Cerebellar | 1 (7.7) | 0 (0.0) | |
| Mean PFS (SD) (months) | 10.6 (11.3) | 9.53 (4.2) | 0.167c |
| Mean OS (SD) (months) | 29.4 (23.3) | 16.4 (7.5) | 0.0562c |
| Refractory sample | |||
| Salvage surgery | 6 (54.0) | 7 (56.0) | 1b |
| Autopsy | 7 (46.0) | 9 (44.0) | |
OS, overall survival; PFS, progression-free survival; SD, standard deviation. Date are presented as n (%) unless otherwise indicated.
Mann-Whitney U test;
Fisher’s exact test;
log-rank test.
Clinical characteristics and the results of immunohistochemical analyses for the 29 cases are summarized in Table 1. The neoBev group was significantly older than the naïve group (P=0.0123). No significant differences in sex or tumor location were seen (Table 1). In addition, distribution of samples at refractoriness of Bev derived from salvage surgery and autopsy between naïve and neoBev was not significantly different (Table 1).
Histological findings and immunohistochemistry (Figure 2)
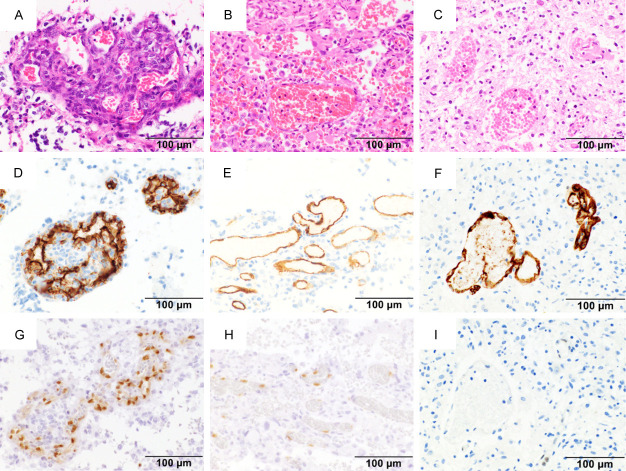
Figure 2.
Histological findings of hematoxylin and eosin and immunohistochemistry. Photomicrograph of hematoxylin and eosin (A-C), and immunohistochemical staining with CD34 (D-F) and ERG (G-I). Strong positive staining for ERG is seen in naïve Bev (G). Intermediate positive staining for ERG in neoBev (H). Negative staining for ERG in refractory Bev (I). Magnification: ×400. Bar: 100 µm.
Typical endothelial proliferation with a glomeruloid structure and palisading necrosis was observed in naïve Bev (Figure 2A; naïve Bev case). In contrast, vessels showed dilatation and collapse of glomeruloid structure in neoBev (Figure 2B; neoBev case). Also, in Bev refractoriness, the glomeruloid microvasculature was indistinct (Figure 2C; autopsy sample from a naïve-Bev refractory case). Thrombosis and hyalinization were observed in both naïve and neoBev groups.
Expression of ERG was detected in CD34-positive cells located in the endothelial cell monolayer along the vessel wall in neoBev and refractory Bev as well as the glomeruloid microvasculature in naïve Bev (Figure 2D-F). Approximately 70% of CD34-positive cells showed strong expression of ERG (Figure 2G), with a 40% frequency of moderate positive (Figure 2H) or negative (Figure 2I).
Comparison of CD34 and ERG expressions during Bev therapy (Figure 3)
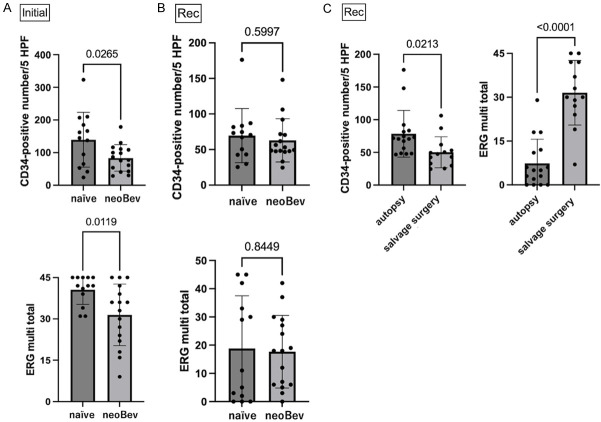
Figure 3.
Comparison of CD34 and ERG expression between naïve and neoBev at initial surgery (A) and salvage surgery or autopsy at recurrence (B). Comparison of CD34 and ERG expressions between autopsy and salvage surgery at recurrence (C).
Expression levels of CD34 and ERG in naïve Bev and neoBev from initial surgery at new diagnosis were compared (Figure 3A). Vascular density was significantly lower in neoBev than in naïve Bev (P=0.0265). ERG score was also lower in neoBev than in naïve Bev (P=0.0119).
Expression levels of CD34 and ERG in naïve Bev and neoBev from salvage surgery and autopsy at recurrence were explored (Figure 3B). No significant differences in vascular density or ERG score were seen in either group (P=0.5997 and P=0.8449, respectively).
Expression levels of CD34 and ERG from salvage surgery and autopsy at recurrence of both naïve and neoBev were separately explored (Figure 3C). Expression of CD34 was higher in the autopsy group than in the salvage surgery group (P=0.0213). In contrast, ERG score was significantly higher with the salvage surgery group than with the autopsy group (P<0.0001).
Alteration of CD34 and ERG expressions during Bev therapy in paired samples from same patients (Figures 4 and 5; Supplementary Tables 1 and 2)
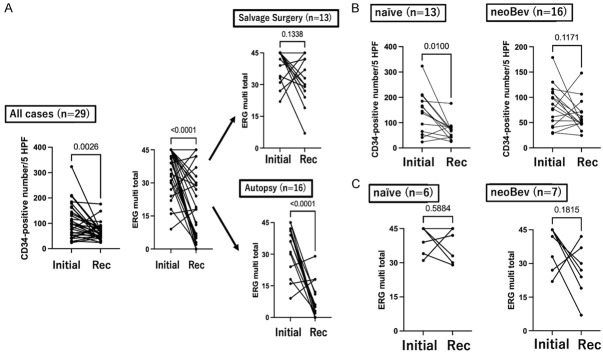
Figure 4.
Comparison of CD34 and ERG expressions between initial surgery and recurrence in paired samples from same patients (A). Comparison of CD34 between initial and recurrent surgery in naïve and neoBev populations (B). Comparison of ERG between initial and recurrent surgeries in naïve and neoBev cases (C).
Figure 5.
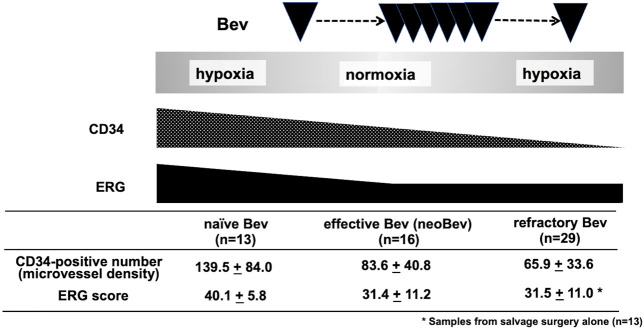
Schema representing alteration of CD34 and ERG scores in naïve, effective, and refractory Bev.
Expression levels of CD34 and ERG in paired samples derived from each patient at initial surgery and recurrence were also quantitated (Supplementary Tables 1 and 2) and compared (Figure 4A; left and middle panels). Expressions of CD34 and ERG score were significantly lower at recurrence than in the initial surgery group (P=0.0026 and P<0.0001, respectively). To explore the possibility that staining was affected by autopsy sample conditions, including fixation, expression of ERG in paired samples obtained from reoperation and autopsy were compared separately (Figure 4A; right panels). ERG score tended to be lower in samples obtained from reoperation at recurrence than in samples from initial surgery, but no significant difference was identified (P=0.1338). Using autopsy samples, however, ERG score was significantly lower in samples at recurrence than at initial surgery (P<0.0001). These results suggest that differences in immunohistochemical staining robustness for ERG might exist between specimens derived from resection surgery and those obtained at autopsy.
To explore alterations of CD34 in paired samples obtained from each patient at the initial surgery and recurrence, expression levels of CD34 were compared separately in the naïve and neoBev groups (Supplementary Tables 1 and 2; Figure 4B). Expression of CD34 at recurrence was significantly lower in the naïve Bev group than in the initial surgery group (P=0.0100). Expression of CD34 in the neoBev group also tended to be lower at recurrence than at initial surgery, but the difference was not significant (P=0.1171).
To explore the possibility of staining being affected by autopsy sample conditions, including fixation, expression levels of ERG in paired samples derived from each patient at initial surgery and recurrence were also compared separately in naïve and neoBev groups (Supplementary Tables 1 and 2; Figure 4C). ERG scores tended to be decreased at recurrence in the neoBev group, but no significance differences were seen in either the naïve or the neoBev group (P=0.5884 and P=0.1815, respectively).
In summary, alterations in CD34 and ERG expressions were seen during Bev therapy (Figure 5). During the period of Bev effectiveness, expression levels of both ERG and CD34 decreased. While the suppression of ERG level was not persistent, CD34 remained decreased during Bev therapy, even when the tumor became refractory to Bev (Figure 5).
Comparison between neuroradiological reduction rate and ERG score (Supplementary Table 2; Supplementary Figures 1 and 2)
After the first Bev dose in the neoBev group, the volume reduction rate was evaluated on T1-weighted imaging with gadolinium enhancement (T1Gd) and fluid-attenuated inversion recovery (FLAIR) (Supplementary Table 2). The relationship between volume reduction rate on MRI and ERG score is shown in Supplementary Figures 1 and 2. Volume reduction on FLAIR tended to be poorer in the group with low ERG expression, but no significant difference was identified.
Comparison of OS by vessel density and expression levels of ERG (Figure 6)
Figure 6.
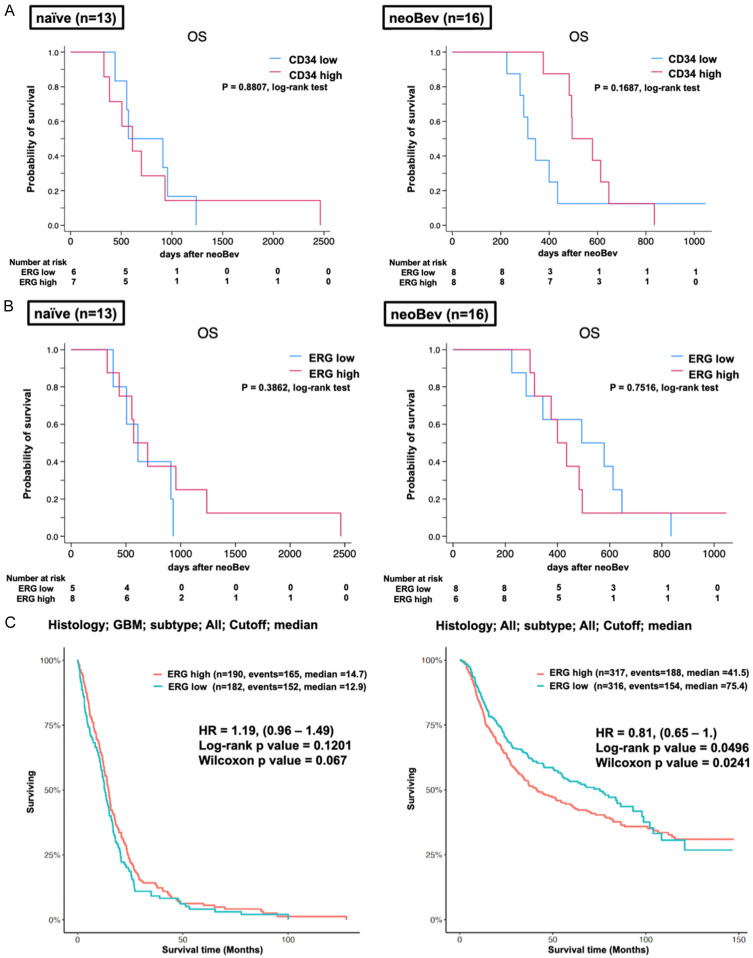
Kaplan-Meier analysis log-rank test. A. Comparison of overall survival (OS) between high and low expression levels of CD34 in naïve (left panel) and neobev (right panel). B. Comparison of OS between high and low expression levels of ERG in naïve (left panel: P=0.3862; log-rank test) and neobev (right panel: P=0.7516; log-rank test). C. Left panel: TCGA data set; comparison of OS between low and high expressions of ERG in GBM cohort (P=0.1201, log-rank test; P=0.067, Wilcoxon test). Right panel: CGGA data set; comparison of OS between low and high expressions of ERG in the glioma cohort (P=0.0498, log-rank test; P=0.0241, Wilcoxon test).
Given that downregulations of CD34 and ERG expression levels were observed during Bev therapy, we evaluated OS with stratification by expression levels of both factors in the naïve Bev and neoBev groups. In the neoBev group, OS tended to be more favorable in patients with high CD34 expression than in those with low expression, but the difference was not significant (Figure 6A; P=0.1687, log-rank test). OS in the naïve Bev and neoBev groups was almost identical (Figure 6A; P=0.8807, log-rank test). In addition, OS stratified by the level of ERG expression in naïve Bev and neoBev groups was evaluated. In each naïve and neoBev cohort, OS with high and low expressions of ERG was comparable with no significant difference (Figure 6B; P=0.7516, P=0.3862, log-rank test). The TCGA database showed that the difference in OS for GB cases did not differ significantly between high and low ERG expression (Figure 6C; P=0.1201, log-rank test; P=0.067, Wilcoxon test). On the other hand, the CGGA database showed a significantly better prognosis for patients with low ERG expression in all histological types of brain tumor (P=0.0498, log-rank test; P=0.0241, Wilcoxon test).
Discussion
Tumor angiogenesis in brain tumors assessed by endothelial markers
In general, microvascular density and VEGF expression levels are considered to correspond to the histological grade of malignancy and prognosis for glioma [17]. Endothelial cells with growth potential play a pivotal role in GB. However, benign tumors such as pilocytic astrocytoma, with WHO grade I, are also highly angiogenic with endothelial proliferation evident on histological examination [18].
To evaluate tumor vascularity histologically, the density of cells positive for endothelial markers such as CD31 and CD34 is frequently used for immunohistochemical analysis (Figure 2D-F). However, CD34 is also known as a marker of multipotent stem cells with expression observed in not only endothelial cells, but also brain tumor cells [19,20]. In addition, glioma stem cells could differentiate to endothelial cells under exposure to angiogenic factors including VEGF [21,22]. Calabrese et al. demonstrated that CD34-positive cells located in the perivascular niche decreased after Bev treatment in an animal model [23]. This result suggests that Bev might inhibit growth of not only endothelial cells, but also multipotential cancer stem cells.
The degree of CD34 positivity does not always reflect clinical outcome and reliable biomarkers providing indications for Bev therapy are therefore needed. Endothelium-specific markers that reflect the degree of differentiation might prove useful to accurately evaluate the therapeutic efficacy of Bev.
Alterations of ERG in TME as a parameter for “vascular normalization” during Bev therapy
Previous studies have demonstrated that the TME changes from hypoxic to normoxic accompanied by a decrease in CD34- and nestin-positive cells following Bev administration in patients with newly diagnosed GB [11]. Tumor oxygenation and decreases in both vascular density and stemness were maintained during the period in which Bev therapy was effective. In contrast, the normoxic TME returned to a hypoxic condition accompanied by decreased microvessel density and stemness with “paradoxical” suppression of VEGF expression when Bev recurred (Figure 5) [2]. Based on these results, “vascular normalization” is considered to be maintained during the period of Bev effectiveness in accordance with suppression of angiogenesis and stem cell infiltration under a normoxic TME.
A factor involved in maintaining “vascular normalization” would logically represent a candidate biomarker for VEGF-targeted therapy. ERG is essential for postnatal vascular development and is involved in tumor angiogenesis and growth through the Wnt/β-catenin signal pathway [9]. In addition, overexpression of ERG reduces vascular permeability and increases the vascular stability of VEGF-dependent angiogenesis [9], therefore ERG might be considered a parameter of “vascular normalization” induced by Bev therapy. Since Bev induces “vascular normalization” during its period of effectiveness, we speculated that ERG is a potential marker of “vascular normalization”, in other words “vascular differentiation”, might be upregulated after neoBev compared with naïve Bev. We therefore focused on ERG as a known marker of normal and well-differentiated endothelium, which might be available as a biomarker for therapeutic response and clinical outcome after Bev therapy, and may also help to elucidate mechanisms of Bev response or resistance.
In the present study, ERG was unexpectedly seen to be significantly decreased in neoBev compared with naïve Bev (Figure 3A, 3B). Vessel density probably decreased as VEGF was downregulated after neoBev, which might have led to declines in ERG expression paralleling decreases in endothelial cells possessing ERG. Our results appear compatible with previous evidence that the ETS-dependent transcription pathway including ERG is regulated downstream of VEGF signaling [8,24].
Interestingly, the endothelial-mesenchymal transition was induced by downregulation of ERG [6]. After Bev therapy, levels of VEGF expression decreased under conditions of both Bev effectiveness and Bev refractoriness [11], altering the vascular morphology. In addition, epithelial mesenchymal transition is supposed to be frequently observed at refractoriness after initial Bev therapy [25] and was found to be induced when Bev treatment persisted for a long period.
We asked the question whether levels of ERG expression might be different between effectiveness after neoBev therapy and refractoriness. Theoretically, ERG should be upregulated during Bev effectiveness, whereas ERG should be downregulated during Bev refractoriness (Figure 4). ERG might thus be useful as a reliable biomarker for predicting therapeutic responses and clinical outcomes in patients with GB treated using Bev.
Comparison of ERG and CD34 expression levels between the naïve Bev group and neoBev group
In the present study, ERG expression level was high in naïve Bev, and ERG score was lower in the neoBev group than in the naïve Bev group (P=0.0119) (Figure 3A). Thus, expression of ERG can be decreased by administering Bev from the naïve state. Given that VEGF and ERG are essential in vascular development, regulation of ERG and VEGF might be coordinated. Induction of VEGF might be one mechanism for ERG upregulation; however, these alterations in the TME were not observed during refractoriness in the present study.
Decreased vascular density as evaluated by CD34 immunohistochemistry and regression of tumor volume with perifocal edema according to the neuroradiographic response to neoBev were prominent as previously described [12], which might not contribute to favorable clinical outcomes.
Most previous studies have demonstrated an association between high levels of CD34 in naïve tumor specimens and poor prognosis. No studies have explored associations between degree of therapeutic response during Bev effectiveness and clinical outcomes including OS.
Given comparative analyses of ERG expression between well and poorly differentiated RCC, it might be difficult to determine whether persistence of ERG expression in GB might affect clinical outcomes. Probably because, effects could be potentially variable and inconsistent, depending on blood supply demands of each tumor.
Alterations of ERG and clinical outcome during Bev therapy
Loss of ERG expression was associated with poor prognosis in patients with clear cell RCC. In contrast, expression levels of ERG did not correspond to the degree of malignancy in the central nervous system tumors, including lower or high-grade glioma, GB, schwannoma, meningioma, hemangiopericytoma/solitary fibrous tumor, and metastatic brain tumors [20]. As previously described, both endothelial and smooth muscle cells are involved in the microvascular proliferation leading to vascular hyperplasia within glial tumors [26]. However, the availability of biomarkers for therapeutic response and clinical outcome after chemotherapy including Bev was not investigated in detail.
The present study showed that the high expression of ERG in GB during naïve status was significantly reduced with Bev, but no significant change in refractoriness was seen compared with the period of Bev effectiveness. This may mean that at the time of recurrence, patients are in a state of resistance acquisition due to other factors that cannot be controlled by Bev. The blood vessels originally within GB might be relatively mature vessels that express ERG. Although Bev may temporarily suppress tumor growth by impairing the growth of blood vessels and reducing the ERG score, the tumor may eventually recur because its ability to grow cannot be controlled by suppressing the growth of blood vessels alone. Thus, in GB, the mere expression of ERGs does not reflect tumor malignancy, and the level of ERG expression cannot be concluded to be related to tumor prognosis.
Moreover, therapeutic intensity such as the number of cycles of Bev might influence the persistence of ERG expression, which might in turn play a role in the persistence of therapeutic efficacy from Bev. In fact, the neoBev group included patients with larger tumor volume along with more robust edema compared with the naïve Bev group, resulting in poor preoperative performance scale. Further, patients in the neoBev group were significantly older (P=0.0123) with poorer prognosis than patients in the naïve group (P=0.0562) (Table 1). These results suggest that selection bias for patients who received neoBev therapy could not be ruled out. Alteration of ERG during the period of naïve effectiveness and expression levels might thus have significance as predictive and prognostic biomarkers.
Previous GB studies have shown that Bev improves PFS but does not significantly improve OS [14,15,27]. Regarding the utility of ERGs as biomarkers, we were unable to obtain data that showed a significant difference in OS (Figure 6) between high and low ERG expression, but PFS tended to be better for the neoBev group, which showed a lower ERG score (Supplementary Figure 1). Based on the PFS results, a decrease in ERG score might mean that Bev worked well. ERG thus may well represent a marker reflecting Bev efficacy and may be involved in tumor growth and angiogenesis during Bev effectiveness.
Alterations of ERG and tumor volume after neoBev
To evaluate the relationship between Bev effectiveness and ERG, we analyzed the relationship between the volume reduction rate on T1Gd/FLAIR and level of ERG expression after neoBev therapy. However, no difference was seen in T1Gd reduction rate; rather, patients with low ERG tended to be poor responders on FLAIR (P=0.0914) (Supplementary Figure 2). Although these results may seem contradictory, previous studies have shown that only the volume reduction rate on T1Gd is associated with OS after neoBev therapy, while the volume reduction rate on FLAIR is not associated with prognosis [12,13]. ERG score did not reflect volume reduction rates on T1Gd/FLAIR, but it is consistent with the fact that the reduction rate on FLAIR does not imply good prognosis.
From the perspective that ERG is a marker reflecting the effect of Bev treatment, the level of ERG expression in specimens obtained from recurrent surgery may be a factor in estimating the efficacy of continued treatment. It remains unclear whether ERG expression level at the time of recurrence might reflect treatment effectiveness of Bev. Hopefully, further analyses will clarify this issue.
To the best of our knowledge, the present study is the first to report analyses using patient samples exploring the status of ERG expression along with VEGF and CD34 by in situ observation in GB during treatment with multiple modalities, including RT, TMZ, and Bev. Alteration of ERG expression in the same patient under different conditions such as Bev naïve, effective, and refractory conditions was meaningful to analyze by multiple modalities during treatment.
Limitations
Some limitations to the present study need to be kept in mind when interpreting the results. First, this study was performed retrospectively with a limited number of patients. Further studies with larger cohorts are thus needed in the future. The timing of obtaining samples and the therapeutic intensity of TMZ and Bev (i.e., the number of cycles) were inconsistent until re-operation or autopsy during refractoriness in each patient. Second, re-operation at recurrence was seldom performed due to the general condition of patients and the difficulty getting approval from patients and their families, particularly when KPS was poor. Obtaining paired samples from the same patients in this manner was thus extremely difficult. Autopsy samples might be more available, but variations in time to fixation may have affected results [28]. These combined factors limit the generalizability of the study conclusions to broader patient populations, so the findings need to be interpreted with caution and validation in future prospective studies is needed.
Conclusions
The present study demonstrated high expression of ERG in GB with naïve status, and Bev markedly reduced ERG score. This result was unexpected given previous reports of low ERG expression as a poor prognostic factor. In addition, no significant changes were seen in ERG expression with refractory status compared with effective status, or in OS between high and low ERG expressions.
Acknowledgements
The authors are grateful to Ms. Eri Honzawa, Ms. Akemi Yashiro, and Ms. Mamiko Ohwada from the Division of Diagnostic Pathology, the Jikei University School of Medicine Kashiwa Hospital, for technical assistance with laboratory work and sample preparation. The authors also thank FORTE Science Communications (https://www.forte-science.co.jp/) for English language editing. This work was supported by JSPS KAKENHI (Grant Nos. 21K09161 and 23K14573).
Disclosure of conflict of interest
None.
Abbreviations
- Bev
bevacizumab
- ERG
erythroblast transformation specific-1 related gene
- FLAIR
fluid-attenuated inversion recovery
- GB
glioblastoma
- HPFs
high-power fields
- neoBev
neoadjuvant bevacizumab
- OS
overall survival
- PFS
progression free survival
- RT
radiation
- RCC
renal cell carcinoma
- T1Gd
T1-weighted imaging with gadolinium enhancement
- TMZ
temozolomide
- TME
tumor microenvironment
- VEGF
vascular endothelial growth factor
- WHO
World Health Organization
Supporting Information
References
- 1.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y, Tamura R, Tanaka T, Ohara K, Tokuda Y, Miyake K, Takei J, Akasaki Y, Yoshida K, Murayama Y, Sasaki H. “Paradoxical” findings of tumor vascularity and oxygenation in recurrent glioblastomas refractory to bevacizumab. Oncotarget. 2017;8:103890–103899. doi: 10.18632/oncotarget.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezaki T, Tanaka T, Tamura R, Ohara K, Yamamoto Y, Takei J, Morimoto Y, Imai R, Kuranai Y, Akasaki Y, Toda M, Murayama Y, Miyake K, Sasaki H. Status of alternative angiogenic pathways in glioblastoma resected under and after bevacizumab treatment. Brain Tumor Pathol. 2024;41:61–72. doi: 10.1007/s10014-024-00481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao X, Qian CN, Zhang ZF, Tan MH, Kort EJ, Yang XJ, Resau JH, Teh BT. Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clin Cancer Res. 2007;13:161–169. doi: 10.1158/1078-0432.CCR-06-0774. [DOI] [PubMed] [Google Scholar]
- 5.Kanki Y, Nakaki R, Shimamura T, Matsunaga T, Yamamizu K, Katayama S, Suehiro JI, Osawa T, Aburatani H, Kodama T, Wada Y, Yamashita JK, Minami T. Dynamically and epigenetically coordinated GATA/ETS/SOX transcription factor expression is indispensable for endothelial cell differentiation. Nucleic Acids Res. 2017;45:4344–4358. doi: 10.1093/nar/gkx159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai N, Ohguchi H, Nakaki R, Matsumura Y, Kanki Y, Sakai J, Aburatani H, Minami T. Downregulation of ERG and FLI1 expression in endothelial cells triggers endothelial-to-mesenchymal transition. PLoS Genet. 2018;14:e1007826. doi: 10.1371/journal.pgen.1007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AV, Birdsey GM, Randi AM. Regulation of endothelial homeostasis, vascular development and angiogenesis by the transcription factor ERG. Vascul Pharmacol. 2016;86:3–13. doi: 10.1016/j.vph.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wythe JD, Dang LT, Devine WP, Boudreau E, Artap ST, He D, Schachterle W, Stainier DY, Oettgen P, Black BL, Bruneau BG, Fish JE. ETS factors regulate Vegf-dependent arterial specification. Dev Cell. 2013;26:45–58. doi: 10.1016/j.devcel.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birdsey GM, Shah AV, Dufton N, Reynolds LE, Osuna Almagro L, Yang Y, Aspalter IM, Khan ST, Mason JC, Dejana E, Göttgens B, Hodivala-Dilke K, Gerhardt H, Adams RH, Randi AM. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling. Dev Cell. 2015;32:82–96. doi: 10.1016/j.devcel.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han E, Kim J, Jung MJ, Chin S, Lee JH, Won KY, Moon A. ERG and nestin: useful markers of immature vessels and novel prognostic markers in renal cell carcinoma. Int J Clin Exp Pathol. 2021;14:116–125. [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura R, Tanaka T, Miyake K, Tabei Y, Ohara K, Sampetrean O, Kono M, Mizutani K, Yamamoto Y, Murayama Y, Tamiya T, Yoshida K, Sasaki H. Histopathological investigation of glioblastomas resected under bevacizumab treatment. Oncotarget. 2016;7:52423–52435. doi: 10.18632/oncotarget.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takei J, Fukasawa N, Tanaka T, Yamamoto Y, Tamura R, Sasaki H, Akasaki Y, Kamata Y, Murahashi M, Shimoda M, Murayama Y. Impact of neoadjuvant bevacizumab on neuroradiographic response and histological findings related to tumor stemness and the hypoxic tumor microenvironment in glioblastoma: paired comparison between newly diagnosed and recurrent glioblastomas. Front Oncol. 2022;12:898614. doi: 10.3389/fonc.2022.898614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka T, Tamura R, Takei J, Morimoto Y, Teshigawara A, Yamamoto Y, Imai R, Kuranari Y, Tohmoto K, Hasegawa Y, Akasaki Y, Murayama Y, Miyake K, Sasaki H. An exploratory prospective phase II study of preoperative neoadjuvant bevacizumab and temozolomide for newly diagnosed glioblastoma. J Neurooncol. 2024;166:557–567. doi: 10.1007/s11060-023-04544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowman RL, Wang Q, Carro A, Verhaak RG, Squatrito M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro Oncol. 2017;19:139–141. doi: 10.1093/neuonc/now247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhry IH, O’Donovan DG, Brenchley PE, Reid H, Roberts IS. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology. 2001;39:409–415. doi: 10.1046/j.1365-2559.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 18.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 20.Haber MA, Iranmahboob A, Thomas C, Liu M, Najjar A, Zagzag D. ERG is a novel and reliable marker for endothelial cells in central nervous system tumors. Clin Neuropathol. 2015;34:117–127. doi: 10.5414/NP300817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 22.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Fish JE, Cantu Gutierrez M, Dang LT, Khyzha N, Chen Z, Veitch S, Cheng HS, Khor M, Antounians L, Njock MS, Boudreau E, Herman AM, Rhyner AM, Ruiz OE, Eisenhoffer GT, Medina-Rivera A, Wilson MD, Wythe JD. Dynamic regulation of VEGF-inducible genes by an ERK/ERG/p300 transcriptional network. Development. 2017;144:2428–2444. doi: 10.1242/dev.146050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, Park M, Bergers G. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wesseling P, Vandersteenhoven JJ, Downey BT, Ruiter DJ, Burger PC. Cellular components of microvascular proliferation in human glial and metastatic brain neoplasms. A light microscopic and immunohistochemical study of formalin-fixed, routinely processed material. Acta Neuropathol. 1993;85:508–514. doi: 10.1007/BF00230490. [DOI] [PubMed] [Google Scholar]
- 27.Chinot OL, de La Motte Rouge T, Moore N, Zeaiter A, Das A, Phillips H, Modrusan Z, Cloughesy T. AVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28:334–340. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 28.Pikkarainen M, Martikainen P, Alafuzoff I. The effect of prolonged fixation time on immunohistochemical staining of common neurodegenerative disease markers. J Neuropathol Exp Neurol. 2010;69:40–52. doi: 10.1097/NEN.0b013e3181c6c13d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.