Abstract
The use of medicinal plants in the management or prevention of diseases is one of the oldest human medicinal practices worldwide. Justicia carnea and Jatropha carcus are widely reported for their use in the management of blood disorders, hypertension, and diabetes. Objective: The study aimed at evaluating the effects of hydroethanolic leaf extracts of Justicia carnea and Jatropha carcus on the biochemical, hematological, and histological parameters of apparently healthy rats. Methodology: Thirty adult rats (n = 30) with an average weight of 153 g were randomly divided into six groups (A-F). Group A: negative control; Group B: positive control; Group C: Jatropha curcas (low dose); Group D: Jatropha curcas (high dose); Group E: Justicia carnea (low dose); and Group F: Justicia carnea (high dose). Standard and scientifically approved methods were used for sacrifice and laboratory diagnosis. Results: The study shows a significant increase in body weight across groups administered with the leaf extracts. Elevated levels of serum creatinine were recorded in rats administered with both extracts, indicating nephrotoxicity. The study also observed an increase in alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase across groups, indicating hepatotoxicity. Both extracts caused an increase in white blood cell count and hemoglobin concentration, a significant reduction in bleeding time, increased prothrombin time, and partial thromboplastin time at high dosages. Total iron binding capacity and serum ferritin values were increased in high doses and were statistically significant at P<0.05. Histomorphology of both extracts shows hepatorenal toxicity at high concentrations and none in the lungs or heart. Oral administration of Justicia carnea and Jatropha carcus extracts at high concentrations is not safe for the liver and kidneys. Conclusion: Biochemical parameters should be monitored regularly in humans exposed to both plants. Therefore, this study scientifically confirms and supports the traditional use of the leaves of Jatropha carcus and Justicia carnea to enhance hematological parameters.
Keywords: Jatropha, Justicia, therapeutic, toxicology, Kampala
Introduction
Medicinal products derived from plants are reported to be relatively safe compared to synthetic alternatives while offering profound therapeutic benefits, once standardized [1,2]. Research has reported increasing reliance on traditional herbs for medicinal purposes in developing nations [3]. This practice is still being contested due to the methods of preparation of medicinal herbs for consumption [4]. Jatropha carcus is a common plant in the southern part of Nigeria [5]. The leaf is consumed as a vegetable and as a natural remedy against diabetes and hypertension. It is also used for the management of blood disorders in some regions of West Africa [6]. Recent claims have it that the plant is not safe for use and that it could be toxic to organs in the body. Although, few reports on its pharmacological values and toxicological effects have been documented [7]. Justicia carnea (family: Acanthanceae) plants are consumed for different medicinal purposes in different regions of the world [8,9]. People use Justicia carnea and Jatropha carcus leaves to treat anemia and other conditions [10]. The research burden still lies in the manner and methods in which these plants are being consumed, which may confer adverse effects on the visceral organs and other physiological parameters. It is against this background that the researchers wish to evaluate the effects of hydroethanolic leaf extracts of Justicia carnea and Jatropha carcus on the biochemical, hematological, and histomorphological parameters of apparently healthy adult rats.
Materials and methods
Fresh leaves of Jatropha curcas and Justicia carnea were obtained from the Amassoma community of the Southern Ijaw Local Government Area of Bayelsa State, Nigeria, West Africa. The plants (leaves) were identified and voucher specimens were deposited in the herbarium by Professor Alade, G.O. of the Department of Pharmacognosy, Faculty of Pharmacy, Niger Delta University, in September 2022. The leaves were air dried at room temperature, and drying was completed in the oven at 40°C and pulverized to a fine powder. The dried material was macerated for 72 hours in ethanol (BDH Ltd., England) and distilled water (50:50) for seven days, respectively. A total of 400 g of the powdered leaf extracts was sieved using Whartman No. 1 filter paper and cellulose filter paper. The extraction was carried out using a vacuum rotor evaporator extractor (Caframo, vv2000, Ohio) with water as the solvent. The filtrate was dried at 300°C, and a yield of 172 g of the total weight was obtained and stored for use in an airtight container.
Experimental animals
Thirty (n = 30) healthy adult Sprague Dawley rats aged 2-3 months with an average weight of 135-170 g were purchased from the animal house of the Department of Pharmaceutical Sciences, University of Port-Harcourt, Rivers State, Nigeria. The adult rats were randomly divided into six groups of five (n = 5) rats each. The adult rats were housed in cages made of metal nesting and allowed to acclimatize for 2 weeks before experimentation, with a 12-hour light and dark cycle. All the adult rats were fed on a standard chow diet and allowed access to distilled water ad libitum. Experimental techniques and protocols used in this study were in line with the Guide to the Care and Use of Animals in Research and Teaching.
Experimental design
The duration of this study was thirty-five (35) days. The adult rats were randomly divided into six groups: A, B, C, D, E, and F, after 14 days of acclimatization. Administration was done orally using an orogastric tube for 21 days and allowed access to feed and water.
Group A: Negative Control.
Group B: positive control: 0.2 ml of hematinic (HB12).
Group C: 0.2 ml of a 1000 mg/kg hydroethanolic extract of Jatropha curcas (low dose).
Group D: 0.2 ml of a 1500 mg/kg hydroethanolic extract of Jatropha curcas (high dose).
Group E: 0.2 ml of a 1000 mg/kg hydroethanolic extract of Justicia carnea (low dose).
Group F: 0.2 ml of a 1500 mg/kg hydroethanolic extract of Justicia carnea (high dose).
Collection of blood samples for biochemical assay and histology
Sacrificing of experimental animals
After three weeks of administration of Justicia carnea and Jatropha curcas hydroethanolic leaf extracts, the experimental animals were sacrificed by adopting the American Veterinary Medical Association (AVMA) guideline for the euthanasia of animals in 2013. Eight (8) ml of sodium pentobarbital (PB) was given to each animal by intraperitoneal (IP) injection technique. The rats were allowed to undergo sedation and loss of consciousness. Blood samples were collected via cardiac puncture into pre-labeled EDTA and plain containers for biochemical and hematological assessment. The visceral organs (heart, liver, kidney, and lung) were harvested, washed with saline to remove excess blood, weighed, and fixed immediately with 10% buffered formalin for histological analysis using the method of [11].
Laboratory assessment
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), electrolytes/urea, and creatinine were analyzed using the enzymatic method (Randox diagnostic kits). An automated hematological analyzer (Sysmex XP-300) was used for hematological analysis.
Bleeding time was done using the Duke’s method. The animal’s tail was cleaned with 70% alcohol, and an incision of about 2.5 mm depth was made by pricking the tail with a lancet. A stopwatch was started immediately, and the blood was blotted every 15 seconds using filter paper until the bleeding ceased and the result was recorded. Activated partial thromboplastin time (APTT) was based on the principle that in citrated plasma, the addition of a platelet substitute, Factor XII activator, and calcium chloride allows for the formation of a stable clot. The time required for the formation of a stable clot is recorded in seconds and represents the actual APTT result.
Determination of serum total iron binding capacity and serum ferritin: Excess fe3+ was added to the sample to saturate serum transferrin; uncompleted fe3+ is precipitated with magnesium hydroxide carbonate; and the iron bonded to protein in the supernatant is then measured using a spectrometer.
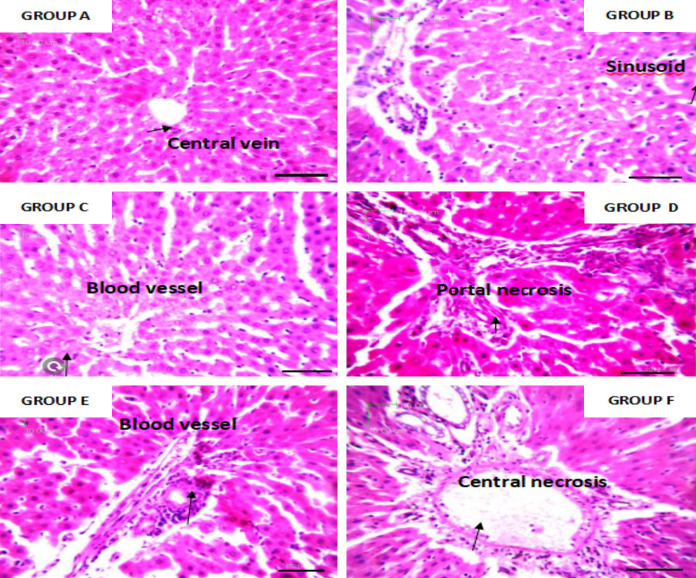
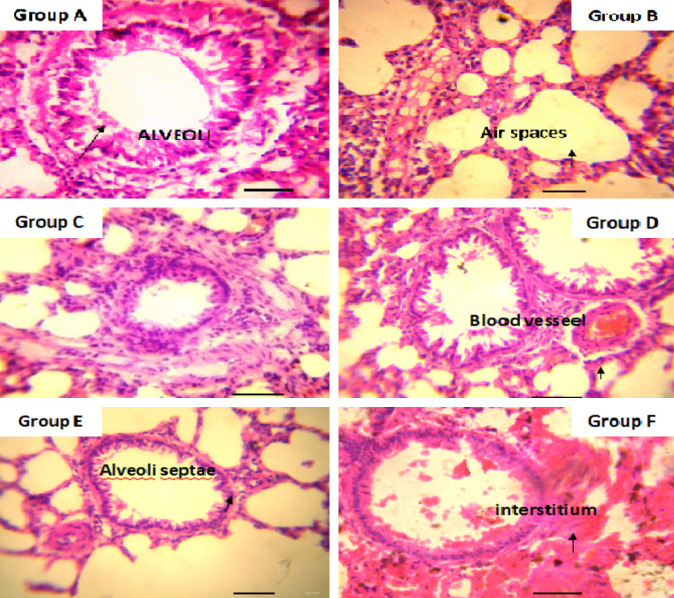
Routine tissue processing was done using an automatic tissue processor (LEICA TP 1020). Tissues were embedded using an embedding console (LE1CA EG 1160) and sectioned with LEICA P.M. 2125 RTS rotary microtome. The tissues were trimmed at 10 microns and sectioned at 4 microns in thickness. The sectioned tissues were attached to slides, dewaxed in xylene, and stained using hematoxylin and eosin according to the method of [11] for general tissue morphology examination. The stained slides were then examined using a compound digital light microscope at ×400 magnification (see Figures 8, 9 and 10).
Figure 8.
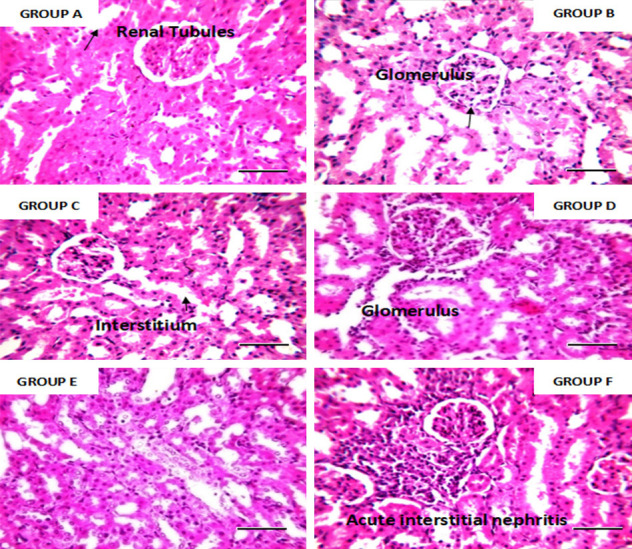
Photomicrograph of the kidney tissue of an adult rat. ×400 magnification and scale bar = 50 um. Group F shows acute interstitial nephritis while others are consistent with normal histology of the kidney. Conclusion: At high concentration, Justica carnea shows renal toxicity.
Figure 9.
Photomicrograph of liver tissue of adult rats stained with hematoxylin and eosin. High concentration of both leaf extracts shows morphological changes with inflammatory cell infiltrates while the others show normal histology. ×400 magnification and scale bar = 50 um. Conclusion: Both extracts show hepatotoxicity at high concentrations.
Figure 10.
Photomicrographs of lung tissue of adult rats stained with hematoxylin and eosin. Group A to Group F shows normal histology. ×400 magnification, Scale Bar = 50 um.
Statistical analysis
Results were presented as mean ± standard deviation (SD), and inferential statistical analysis was done using one-way ANOVA followed by a Turkey multiple comparison. Differences between means were considered significant at P≤0.05.
Ethical approval
Ethical approval to conduct this study was sought and obtained from the ethical committee of the Faculty of Basic Medical Sciences, College of Health Sciences, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria.
Results
The results are presented in tables, graphs, and photomicrographs below.
Effect of hydroethanolic leaf extracts on the body and organ weight (g)
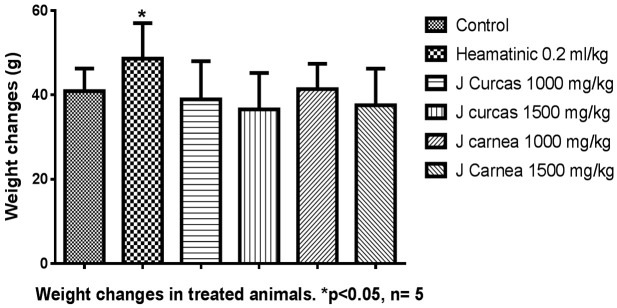
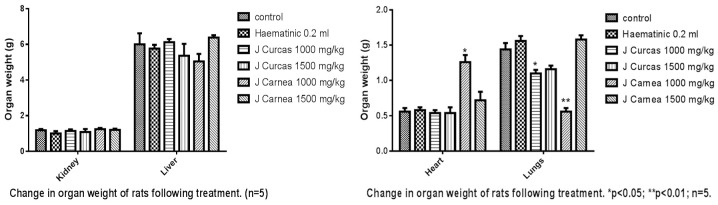
The leaf extracts had significant changes in body weight across the groups, as presented in Figure 1. Groups C and D, administered with Jatropha carcus, and Group B, a positive control administered with HB12, showed a reduction in body weight. Group C (6.1 ± 1.18) and Group F (6.5 ± 0.14) showed slight hypertrophy of the liver, while other groups showed mild changes. The changes in the liver weight were grossly insignificant (P<0.05) compared with the control. Justicia carnea exhibited significant cardiomyopathy in groups E and F, while Jatropha carcus exhibited mild atrophy in groups C and D compared with positive and negative controls as presented in the group presented in Figure 2. There was a reduction in the weight of the lungs in groups administered with both extracts compared with controls.
Figure 1.
The effect of hydroethanolic leaf extracts on the adult rats’ body weight shows a statistically significant increase in group B administered with standard drug.
Figure 2.
The image shows the effect of hydroethanolic leaf extracts on the organ weights of the adult rats studied. The increase in the heart and lungs was statistically significant in the group administered with low dose of the Jatropha carcus and Justica carnea. Other weight changes were not significant.
Effect of hydroethanolic leaf extracts on electrolytes, renal function, liver function biochemical parameters
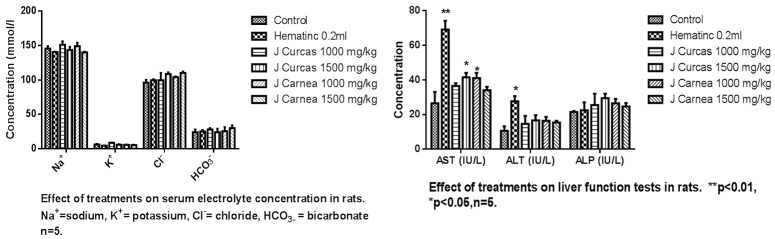
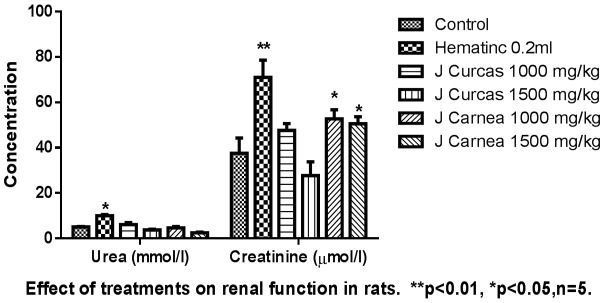
A statistically significant increase in liver aspartate aminotransferase (AST) was recorded, and an insignificant increase in alanine aminotransferase (ALT) and alkaline phosphatase (ALP) was found, at P<0.05, across groups administered with extracts, as presented in Figure 3. ALT was increased in groups treated with low and high doses of Jatropha carcus (14.6 ± 4.6; 16.6 ± 7.0) and Justicia carnea low (23.6 ± 0.0) and high dose (17.8 ± 1.4) compared with control groups (26.5 ± 6.5). Increased values of aspartate aminotransferase (AST) were recorded from both low (36.5 ± 11.5) and high (41.5 ± 17.5) doses of Jatropha carcus and low (59.0 ± 0.0) and high (29.5 ± 3.5) doses of Justicia carnea when compared with the control (26.5 ± 6.5). Also, increased levels of alkaline phosphatase were recorded with low (2618.3 ± 64.9) and high (3012.5 ± 259.0) doses of Jatropha carcus and low (2917.3 ± 0.0) and high (2565.2 ± 13.7) doses of Justicia carnea when compared with the control (2228.9 ± 48.8). The urea level of the group treated with Jatropha carcus-low dose (1000 mg/kg) (4.6 ± 0.9), and high dose (1500 mg/kg) (3.8 ± 0.3); Justicia carnea-low dose (4.7 ± 0.6), and high dose (2.5 ± 0.3) showed reduced values when compared to control (5.1 ± 0.2). Justicia carnea showed a significant increase in serum creatinine (Jatropha Carcus - low 47.6 ± 16.0 and high 77.7 ± 2.5), Justicia Carnea (low dose 71.8 ± 2.0 and high dose 70.6 ± 6.7) compared to control (37.55 ± 8.3). Extracts showed no statistically significant difference in the serum electrolytes evaluated as presented in Figure 4.
Figure 3.
The image shows the effect of the hydroethanolic leaf extracts on the electrolyte levels of the adult rats. The effects of the extracts on the potassium (k+), Sodium (Na+), chloride (Cl-), and bicarbonate (HCO3-) were not statistically significant at P>0.05.
Figure 4.
The image shows the effect of the hydroethanolic leaf extracts on urea and creatinine on the adult rats. The extracts had no significant effect on the urea level except upon the group administered with standard drug which shows a significant difference. The extracts show a significant difference in creatinine level.
Effect of hydroethanolic leaf extracts on complete blood count
The results showed an increase in white blood cell count in groups C (17.0 ± 0.9) and D (13.6 ± 4.6) administered with low and high doses of Jatropha curcas compared with negative and positive control (7.2 ± 0.2) and (9.7 ± 1.3) respectively. Also, groups E and F rats administered with Justicia carnea (low and high doses) showed an increase in WBC count (20.4 ± 0.4) and (12.1 ± 1.9) respectively. The red blood cell count was increased in groups C, D, and E rats. Group F rats, administered with a high dose of Justicia carnea, showed reduced RBC (6.3 ± 0.1) compared with the negative control (7.3 ± 0.9). The mean differences were statistically insignificant at P<0.05. Group B rats (13.7 ± 0.6) were administered with hematinic, Group C (13.2 ± 0.5), and Group D (13.45 ± 0.3) were administered with low and high doses of Jatropha curcas, and Group E (13.1 ± 1.2), administered with a low dose of J. carnea, showed an increase in hemoglobin concentration compared with the negative control. Group F, administered with a high dose of Justicia carnea, showed a reduced hemoglobin concentration. Packed cell volume was increased in group B rats (13.7 ± 0.6), group C rats (43.5 ± 0.5), and group D rats (47.5 ± 1.5) compared with group A rats (41.0 ± 5.0), while groups E and F administered with Justicia carnea (high and low doses) showed reduced packed cell volume compared with group A. Jatropha curcas shows blood-building properties when compared with group B rats administered with standard hematinic. Group B rats (hematinic) and group D rats (high dose of Jatropha curcas) all showed an increase in mean cell volume, while others showed a reduction in MCV compared with the negative control. The high dose of Justicia carnea showed an increase in platelet count in group F (770.5 ± 97.5), and group D (729 ± 167.0), and the low dose showed a statistically significant reduction in platelet count (542.5 ± 50.5) at P<0.05 when compared with the group. The mean concentration in lymphocytes shows an increased count and decrease in neutrophils and monocytes in both extracts compared with the negative control (See Table 1).
Table 1.
Effects of hydroethanolic leaf extracts on complete blood count
| Parameters | Group A | Group B | Group C | Group D | Group E | Group F |
|---|---|---|---|---|---|---|
| WBC × 103/L | 7.2 ± 0.2 | 9.7 ± 1.3 | 17.0 ± 0.9 | 13.6 ± 4.6 | 20.4 ± 0.4 | 12.1 ± 1.9 ns |
| RBC × 1012/L | 7.3 ± 0.9 | 8.1 ± 0.6 | 7.8 ± 0.2 | 8.4 ± 0.5 | 7.4 ± 0.9 | 6.3 ± 0.1 ns |
| Hb (g/dl) | 11.3 ± 1.2 | 13.7 ± 0.6 | 13.2 ± 0.5 | 13.45 ± 0.3 | 13.1 ± 1.2 | 11.45 ± 0.2 ns |
| PCV | 41.0 ± 5.0 | 47.0 ± 2.0 | 43.5 ± 0.5 | 47.5 ± 1.5 | 40.5 ± 6.5 | 34.5 ± 0.5 ns |
| MCV (%) | 57.5 ± 0.1 | 59.1 ± 1.9 | 56.25 ± 0.1 | 57.1 ± 1.4 | 55.6 ± 2.5 | 54.2 ± 0.4 ns |
| MCH (Pg) | 15.8 ± 0.2 | 17.1 ± 0.4 | 17.0 ± 0.4 | 16.0 ± 0.6 | 17.7 ± 0.5 | 17.6 ± 0.1 ns |
| MCHC (g/dl) | 27.4 ± 0.3 | 28.8 ± 0.3 | 30.4 ± 0.8 | 28.0 ± 0.3 | 31.9 ± 2.3 | 32.5 ± 0.0 ns |
| PLT × 1012/L | 714.0 ± 1.0 | 668 ± 91 | 705.5 ± 205 | 729 ± 167 | 542.5 ± 50.5 | 770.5 ± 97.5 ns |
| Lymphocytes | 77.0 ± 7.0 | 78.5 ± 10.5 | 83.5 ± 3.5 | 79 ± 8.0 | 82.0 ± 6.0 | 90.0 ± 0.0 ns |
| Monocytes | 10.0 ± 3.5 | 6.0 ± 3.0 | 6.5 ± 1.5 | 8.0 ± 4.0 | 9.0 ± 2.0 | 7.0 ± 1.0 ns |
| Neutrophils | 14 ± 4.0 | 16.0 ± 8.0 | 10.0 ± 5.0 | 13.0 ± 4.0 | 9.0 ± 4.0 | 3.0 ± 1.0 ns |
Key: Group A = Negative control; Group B = Positive control (haematinic) (0.02 ml/kg); Group C = Low dose of Jatropha curcas (1000 mg/kg); Group D = High dose of Jatropha curcas (1500 mg/kg); Group E = Low dose of Justicia carnea (1000 mg/kg); Group F = High dose of Justicia carnea (1500 mg/kg).
Effects of hydroethanolic leaf extracts on bleeding time and prothrombin time
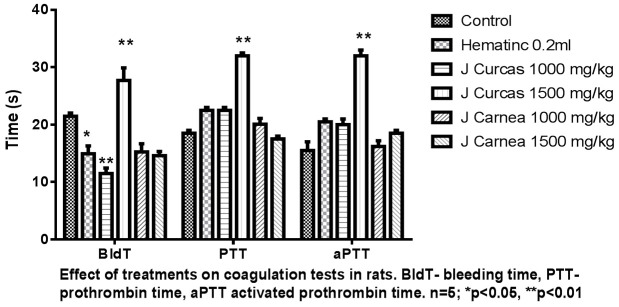
Rats in Group B administered with 0.02 ml/kg body weight of hematinic showed reduced bleeding time when compared with group A (negative control) rats. The mean differences between groups A and B were not statistically significant (21.46 ± 5.4 versus 14.91 ± 1.3) in groups A and B, respectively. Group C (1000 mg/kg of J. curcas), Group E (1000 mg/kg of J. carnea), and Group F (1500 mg/kg of J. carnea) showed reduced bleeding time. Group D rats (1500 mg/kg of Jatropha curcas) showed an increased bleeding time. Prothrombin time values were increased in Group A (22.5 ± 0.5) rats administered with hematinic, Group C rats (33.0 ± 0.0) rats administered with a low dose of Jatropha curcas, Group D (32.5 ± 12.5) rats administered with a high dose of Jatropha curcas, Group E (29.0 ± 3.0) rats administered with a low dose of Justicia carnea; while there was a mild reduction in Group F, with high dose of Justicia carnea, compared with group A (18.5 ± 0.5) and normal negative control. The differences in mean values were not statistically significant at P<0.05 as presented in Figure 5.
Figure 5.
The image shows the effect of the hydroethanolic leaf extracts on bleeding time, prothrombin time, and activated prothrombin time. Jatropha carcus shows a significant difference in bleeding time, prothrombin time, and activated prothrombin time. Justicia Carnea showed no effect on the coagulation profile.
Effect of hydroethanolic leaf extracts on total iron binding capacity and serum ferritin
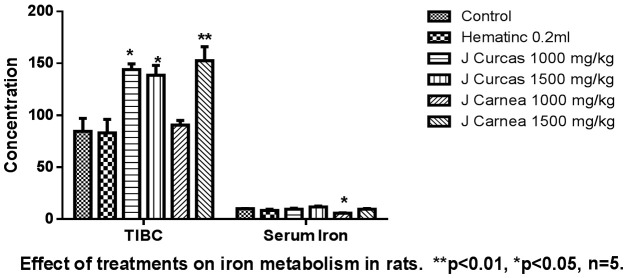
The leaf extract on total iron binding capacity (TIBC) and serum ferritin in adult rats shows an increase in TIBC among groups administered with plant extract compared with the positive control (Group B = 83.0 ± 13.0) and negative control (Group A = 84.51 ± 2.5). The mean ± standard error of mean for Groups C, D, E, and F were 144.0 ± 56.0, 138.5 ± 29.5, 90.5 ± 4.5, and 152.5 ± 13.5, respectively. Serum ferritin for adult rats in the group administered with hematinic was reduced (8.4-1.1). Group E, administered with a low dose of Justicia carnea (5.8 ± 0.3), Groups C and D, administered with a low and high dose of Jatropha carnea, and Group F, administered with a high dose of Justicia carnea, all showed an increase in serum ferritin. The difference among groups in total iron binding capacity and serum ferritin was not statistically significant at P<0.05 as presented in Figure 6.
Figure 6.
The image shows the effect of the hydroethanolic leaf extracts on iron metabolism (total iron binding capacity-TIBC, and serum Iron). TIBC was affected by both Jatropha carcus and Justica carnea. The serum iron level was affected by Justicia carnea.
Discussion
Plants with medicinal capacity are important targets for drug development. Some medicinal plants are rich sources of natural phytochemicals with antioxidant activity that are mainly phenolic compounds [12-14]. Due to emerging and re-emerging diseases, because of modernization and stressful life conditions, people are inclined towards the use of herbal products to keep various diseases at bay and boost their health status. Medicinal plants are believed to be important in promoting good health because of their nutritive and pharmacological activities [15-17]. Therapeutic and toxicological assessment of hydroethanolic leaf extracts of Jatropha curcas and Justicia carnea on the biochemistry, hematology, and histology of apparently healthy rats was investigated.
The leaf extracts show a reduction in body weight, except for the high dose of Justicia carnea. This may be due to the presence of berberine, which can suppress adipocyte differentiation mainly by suppressing cAMP response element-binding protein (CREB) activity, leading to a decrease in CCAAT/enhancing-binding protein beta (C/EBP)-triggered transcriptional cascades. This study is in line with another study [18], which administered high doses of ethanol extract of Jatropha curcas (50, 100, and 200 mg/kg) orally and intraperitoneally and had a significant reduction in body weight. According to the results, the reduction in body weight might be due to a combination of some factors, such as tannins, which significantly reduced food intake [19,20]. This study is also in agreement with other research [21], that reported that an increase in the intake of medicinal herbs among men causes a decrease in their body weight.
Traditional herbal medicine practitioners often administer crude preparations without regard to their possible adverse effects [22,23] and therefore there is a need for a study of this nature. This study revealed that low doses of Jatropha carcus and Justicia carnea caused nonsignificant changes in sodium ion (Na+), chloride, or bicarbonate ions and a reduction in potassium ion levels among the rats studied. This study is not in alignment with other work [24-26], which reported a significant increase in electrolytes in rats administered with a medicinal plant (Vitex doniana). Urea and creatinine are waste products of protein metabolism that need to be excreted by the kidney. Urea levels can be increased by many factors, such as dehydration, antidiuretic drugs, and diet, while creatinine is more specific to the kidney since kidney damage is the only significant factor that increases serum creatinine [27]. However, a marked increase in serum urea andcreatinine levels depicts damage to the kidney [27] as noted in the study. This indicates adverse effects on renal function with use of both plants, which is contrary to another report [28].
The currently reported increase in the level of alkaline phosphatase agrees with additional research [31,32] and is contrary to the findings of other studies [28]. The current study shows that high doses (crude extracts) of Justicia carnea and Jatropha carcus (Hospital too far) caused portal and central necrosis of the liver. The findings of this study are contrary to other work [9] that reported the extracts have no toxicity potential in the liver. Also, [31] more research suggests that leaf extracts have non-toxic properties for visceral organs. However, this study observed that the extracts exhibited a non-toxic effect in both the lung and heart of experimental rats, which agrees with numerous other studies [8,33-36]. Liver enzymes such as AST, ALT, and ALP are marker enzymes for liver function and integrity [29]. These enzymes are usually elevated in acute hepatotoxicity or mild hepatocellular injury but tend to decrease with prolonged intoxication due to liver damage [29]. When the liver cell membrane is damaged, varieties of enzymes normally located in the cytosol are released into the bloodstream [30].
Assessment of hematological parameters and coagulation profiles can be used to determine the extent of deleterious effects on blood constituents [37]. The increased percentage of packed cell volume (in groups B, C, and D), red blood cell count (in groups C, D, and E), and hemoglobin concentration (in groups B, C, D, and E) observed in this study showed that the leaf extracts, especially Jatropha curcas, exhibit blood-boosting effects. This might be attributed to the fact that the extracts may be rich in bioactive constituents that promote the activities of hematopoiesis. The presence of antioxidants and phytochemicals like terpenoids and tannins in the aqueous extracts may be responsible for the hemopoietic-stimulating effects. This agrees with research findings which revealed that prophylactic and therapeutic oral administration of anti-oxidant supplements of plant extracts significantly increased cells of hemopoietic origin in animals exposed to potentially lethal doses of radiation [38]. Erythrocytes have also been found to be protected from oxidative damage by flavonoids, tannins, and terpenes [39].
The difference in white blood cell count (WBC) between groups C and D (low and high doses of Jatropha curcas) and groups E and F (low and high doses of Justicia carnea) when compared with the negative and positive controls shows that the extracts have immune-building properties such as alkaloids and flavonoids (secondary metabolic compounds) that exhibit a wide range of biological activities like anti-inflammatory, anti-allergic, anti-microbial activities, promotion of lymphocyte activity, increased phagocytosis, induction of interferon production, and are also toxic against cells of foreign activities. These activities have been widely studied for their potential use in the elimination and reduction of human cancer cell lines [40]. Administration of these extracts exhibits a stimulatory effect on the effector cells of the immune system. Immune boosters are usually recommended to strengthen and harmonize degenerative body systems and assist the immune system in fighting against invading agents such as bacteria and viruses [41]. The significant reduction of the platelet count (thrombocytopenia) observed from this study might be due to decreased production of platelets from the bone marrow or increased destruction of platelets due to an immune reaction or infection [42-44].
Both plant extracts demonstrated high coagulation properties. The significant reduction in the bleeding time observed in this study shows that both extracts possess coagulation properties that do not have the potential to improve hemostasis or inhibit platelet aggregation. This agrees with the findings of additional research [45], which reported that Jatropha extract possesses both procoagulant and anticoagulant activities. It is therefore advisable to be cautious when the extracts are used together with antiplatelet drugs such as aspirin or non-steroidal anti-inflammatory drugs (NSAIDs), particularly in excessive doses. The increased prothrombin time and partial thromboplastin time observed in this study, show that the leaf extracts act on both the intrinsic and extrinsic pathways and inhibit the tissue factor pathway, which relies on the presence of factor VII, which in turn relies on activation by vitamin K. Hence, patients with known bleeding tendencies or hemophilia disease are to take into consideration the interaction potential of these plants with the natural blood clotting system. The presence of warfarin and other vitamin K antagonists is known to lead to prolonged PT [46-48]. An increase in APTT is indicative that the inhibitory effect is via the contact coagulation pathway, as reported in the present study.
Iron in the normal range is necessary for the metabolic pathways in the body. The significant increase in both serum ferritin and total iron binding capacity compared with the positive control group shows that the leaf extracts do not possess iron-chelating potential. This might be due to the absence or inadequate level of some secondary metabolites (phenol, flavonoid), which have the potential to enhance iron-chelating activities. Iron chelators can bind with metal ions such as calcium and magnesium, which are vital ions for living organisms. The prolonged use of plant chelators with high concentrations may decrease calcium and magnesium levels. The increase in total iron binding capacity and serum ferritin might also be due to the dosage of the leaf extracts and the hematinic administered (0.1 ml low dose of the extract, 0.2 ml high dose) of the plant and 0.02 ml of hematinic, respectively. Photomicrographs of the heart tissue of rats stained with H&E are shown at ×400 magnification. The histology is consistent with the normal cellular anatomy of the heart (see Figure 7). Leaf extracts exhibited non-toxic properties at different concentrations and durations. Photomicrograph of the histology of the kidneys of adult rats stained with H&E are shown at ×400 magnification. Group F shows acute interstitial nephritis, while others are consistent with normal histology of the kidney (see Figure 8). A high dose of Justica carnea shows renal toxicity. Photomicrographs of the liver tissue of adult rats stained with H&E are shown at ×400 magnification. High doses of both extracts show morphological changes with inflammatory cell infiltrates, while the others show normal histology (see Figure 9).
Figure 7.
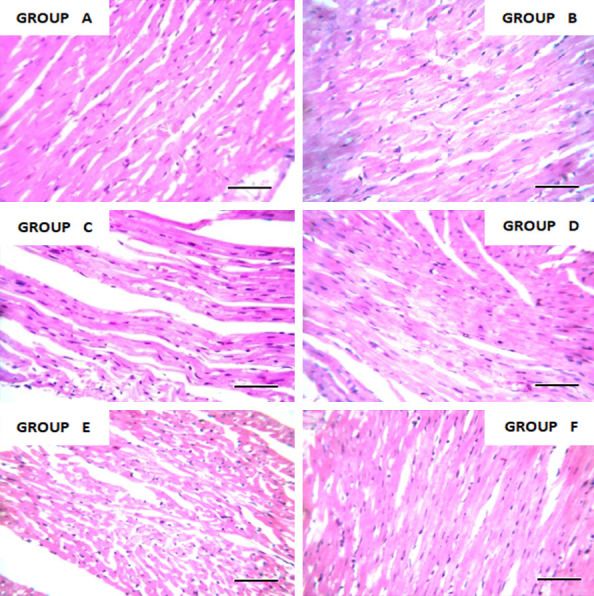
Photomicrograph of heart tissue of adult rat stained with hematoxylin and eosin. ×400 magnifications and scale bar = 50 um. Histology is consistent with the normal histology of the heart. Leaf extract shows nontoxic properties at concentration and duration exposed.
Conclusion
Oral administration of Justicia carnea and Jatropha carcus crude extracts at high concentrations is not safe. Biochemical parameters should be monitored regularly in humans exposed to both plants. Jatropha carcus and Justicia carnea contain flavonoids and alkaloids that are non-toxic to the lungs and heart tissues. However, serious caution about the dose should be taken into consideration when using these herbal remedies. The findings of this study also indicate that the aqueous extracts of Jatropha curcas and Justicia carnea possess blood-boosting and replenishing properties, and this validates claims by local consumers that they possess anti-anemic effects that boost blood levels in anemic patients and pregnant women and replenish blood loss via menstruation when consumed in an adequate amount. However, visceral organs like the liver and kidney should be monitored during administration.
Acknowledgements
We acknowledge Mr. Agu Charles, Department of Histopathology, Niger Delta University Teaching Hospital, Okolobiri, Bayelsa State, Nigeria, for his assistance with tissue processing and the staff of the Pharmacognosy Department, Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria, for their active role in the identification of the leaves and extraction.
Disclosure of conflict of interest
None.
References
- 1.Iwu MM. Food as medicine: functional food plants of Africa. CRC Press; 2016. [Google Scholar]
- 2.Legbosi NL, Ibor OY. Subchronic study of Nauclea latifolia Leaf extract on valproic acid-induced toxicity in liver, lung and kidney of rats. EC Pharmacol Toxicol. 2019;7:347–363. [Google Scholar]
- 3.O’Hara MW, Wisner KL. Perinatal mental illness: definition, description and etiology. Best Pract Res Clin Obstet Gynaecol. 2014;28:3–12. doi: 10.1016/j.bpobgyn.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwalewa EO, Adewunmi CO, Omisore NO, Adebanji OA, Azike CK, Adigun AO, Adesina OA, Olowoyo OG. Pro- and antioxidant effects and cytoprotective potentials of nine edible vegetables in southwest Nigeria. J Med Food. 2005;8:539–544. doi: 10.1089/jmf.2005.8.539. [DOI] [PubMed] [Google Scholar]
- 5.Akhigbe AO, Idu M, Orhue ES, Ataman JE, Ehimwenman SO. Effect of Jatropha tanjorensis Jl Ellis and Soroja leaves in rabbits: biochemistry and ultrasonography. Research Journal of Medicinal Plant. 2009;3:29–33. [Google Scholar]
- 6.Olayiwola G, Iwalewa EO, Omobuwajo OR, Adeniyi AA, Verspohi EJ. The antidiabetic potential of Jatropha tanjorensis leaves. Niger J Nat Prod Med. 2004;8:55–58. [Google Scholar]
- 7.Ehimwenma SO, Osagie AU. Phytochemical Screening and anti-anemia effects of Jatropha tanjorensis leaf in protein malnourishment rats. Plant Anch. 2007;7:509–516. [Google Scholar]
- 8.Emmanuel U. Oxidative and biochemical parameters analysis of alloxan-induced diabetic rats administered methanol leaf and fruit extracts of Kiglia africana. London Journal of Research in Science: Natural and Formal. 2019 [Google Scholar]
- 9.Anthonia OC, Uroko RI, Njoku OU, Ezeanyika LUS, Sunday U. Nutritive properties of aqueous extract Justicia carnea leaves and its effects on haematological and some biochemical indices of anaemia induced male Wistar albino rats. Biomed Res. 2019;30:645–654. [Google Scholar]
- 10.Akintimehin ES, Karigidi KO, Omogunwa TS, Adetuyi FO. Safety assessment of oral administration of ethanol extract of Justicia carnea leaf in healthy Wistar rats: hematology, antioxidative and histology studies. Clin Phytosci. 2021;7:2. [Google Scholar]
- 11.Corrêa GM, de-Alcântara AFC. Chemical constituents and biological activities of species of justicia: a review. Braz J Pharm. 2011;22:220–238. [Google Scholar]
- 12.Beredugo S, Eric EU, Oboma YI, Sadjere OC. Histomorphological evaluation of the combined therapeutic potential of Carica papaya and Musa paradisiaca on indomethacin induced gastric injury. World Journal of Advanced Science and Technology. 2022;02:012–017. [Google Scholar]
- 13.Atrooz OM. The antioxidant activity and polyphenolic contents of different plant seeds extracts. Pak J Biol Sci. 2009;12:1063–1068. doi: 10.3923/pjbs.2009.1063.1068. [DOI] [PubMed] [Google Scholar]
- 14.Azubike NC, Okwuosa CN, Achukwu PU, Maduka TC, Chike O. Acute toxicity and histopathological effects of crude aqueous extract of Jatropha curcas leaves in mice. Res J Med Plant. 2015;9:340–346. [Google Scholar]
- 15.Amadi BA, Ibegbulem CO, Egbebu AC. Assessment of the effect of aqueous extract of (Asimina triloba) root on organ weights and liver function of albino rats. Int J Natl Appl Sci. 2016;2:79–81. [Google Scholar]
- 16.Dahiya P, Kamal R, Gupta R, Bhardwaj R, Chaudhary K, Kaur S. Reactive oxygen species in periodontitis. J Indian Soc Periodontol. 2013;17:411–416. doi: 10.4103/0972-124X.118306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kone WM, Koffi AG, Bomisso EL, Bi FHT. Ethnomedical study and iron content of some medicinal herbs used in traditional medicine in cote d’ivoire for the treatment of anaemia. African Journal of Traditional Complementary and Alternative Medicines. 2012;9:81–7. doi: 10.4314/ajtcam.v9i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EJ, Chen Y, Huang JQ, Li KM, Razmovski-Naumovski V, Poon J, Chan K, Roufogalis BD, McLachlan AJ, Mo SL, Yang D, Yao M, Liu Z, Liu J, Li GQ. Evidence-based toxicity evaluation and scheduling of Chinese herbal medicines. J Ethnopharmacol. 2013;146:40–61. doi: 10.1016/j.jep.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Koker A, Samai M, Abiri OT, Bawoh M, Kwanashie HO. Haematinics and weight reduction properties of ethanol extract of Jatropha curcas seeds in rats. Sierra Leone J Biomed Res. 2015;7:49–56. [Google Scholar]
- 20.Igbinosa OO, Igbinosa IH, Chigor VN, Uzunuigbe OE, Oyedemi SO, Odjadjare EE, Okoh AI, Igbinosa EO. Polyphenolic contents and antioxidant potential of stem bark extracts from Jatropha curcas (Linn) Int J Mol Sci. 2011;12:2958–2971. doi: 10.3390/ijms12052958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooda J, Shah A, Zhang L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients. 2014;6:1080–1102. doi: 10.3390/nu6031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter. 2017;22 doi: 10.1111/hel.12330. [DOI] [PubMed] [Google Scholar]
- 23.Abebe W. An overview of herbal supplement utilization with particular emphasis on possible interactions with dental drugs and oral manifestations. J Dent Hyg. 2003;77:37–46. [PubMed] [Google Scholar]
- 24.Faiza R, Waqas KK, Adeel M, Muhammad G. Detection of bioactive fractions of Justicia adhatoda leaves. Canadian J Appl Sci. 2013;1:388–398. [Google Scholar]
- 25.Hamzah RU, Egwim EC, Kabiru AY, Muazu MB. Phytochemical and invitro antioxidant properties of the methanolic extract of fruits of blighia sapida, vitellaria paradoxa and vitex doniana. Oxid Antioxid Med Sci. 2013;2:215–221. [Google Scholar]
- 26.Ekhuemelo DO, Kiyam AS, Abu VE. Antitermitic properties of Vitellaria paradoxa (CF Gaertn.) stem bark extracts on Daniellia oliveri (Rolfe) Hutch. & Dalziel AND Vitex doniana Sw. woods. Journal of Research in Forestry, Wildlife and Environment. 2020;12:145–153. [Google Scholar]
- 27.Audu R, Amin AB, Sadiq MS, Tijjani A, Babangida L, Abdullahi I. Chemical composition of African Black Plum (vitex doniana) leaf ensiled with urea and broiler litter. Nigerian Journal of Animal Production. 2022;49:145–153. [Google Scholar]
- 28.Garba SH, Adelaiye AB, Mshelia LY. Histopathological and biochemical changes in the rats kidney following exposure to a pyrethroid based mosquito coil. J Appl Sci Res. 2007;3:1788–1793. [Google Scholar]
- 29.Marcellinus AE, Sylvanus B, Peter BE. Biochemical and histomorphological evaluation of ageratum conyzoides on aluminium chloride induced hepatotoxicity in a rat model. World J Pharm Med Res. 2022;8:163–170. [Google Scholar]
- 30.Onyije FM, Beredugo S, Ilegbedion IG, Enaowho MT, Tio O. Anti-anti-inflammatory, protective, and necrotic reversal potentials of Aspilia Africana in aluminium chloride hexahydrate induced hepatic and renal degenerative changes. Afr J Cellular Path. 2015;5:55–61. [Google Scholar]
- 31.Nwadiogbu OV, Ikechukwu UR, Ikechukwu ES, Obiora A. Hepatoprotective and healthy kidney promoting potentials of methanol extract of Nauclea latifolia in alloxan induced diabetic male Wistar Albino rats. Asian J Biochem. 2017;12:71–78. [Google Scholar]
- 32.Anarado CE, Ajiwe VIE, Anarado CJO, Obumselu OF, Umedum NL, Okafor SE. The phytochemistry, ethnomedicinal and pharmacology uses of justicia carnea lindl used in traditional medicine in Nigeria-a review. South Asian Res J Nat Prod. 2021;4:85–93. [Google Scholar]
- 33.Onyeabo C, Achi NK, Ekeleme-Egedigwe CA, Ebere CU, Okoro CK. Haematological and biochemical studies on Justicia carnea leaves extract in phenylhydrazine induced-anemia in albino rats. Acta Sci Pol Technol Aliment. 2017;16:217–230. doi: 10.17306/J.AFS.0492. [DOI] [PubMed] [Google Scholar]
- 34.Igbinaduwa PO, Kabari KM, Chikwue TC. Phytochemical and anti-anaemic properties of ethanol leaf extract of Justicia carneavahl (acantheceae) Nigerian J Pharm Appl Sci Res. 2019;8:55–61. [Google Scholar]
- 35.Lumese FE, Onoagbe IO, Eze GI, Omoruyi FO. Safety assessment of Uvaria chamae root extract: acute and subchronic toxicity studies. J Afr Assoc Physiol Sci. 2016;4:53–60. [Google Scholar]
- 36.Aladodo RA, Muhammad NO, Balogun EA. Effects of aqueous root extract of hematological indices in Alloxan-induced diabetic rats. Fountain J Nat Appl Sci. 2013;2:52–58. [Google Scholar]
- 37.Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, Ko YH, Sayers CM, Baran M, Ware JH, Kennedy AR. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat Res. 2008;169:384–396. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grassmann J. Terpenoids as plant antioxidants. Vitam Horm. 2005;72:505–535. doi: 10.1016/S0083-6729(05)72015-X. [DOI] [PubMed] [Google Scholar]
- 39.Mujumdar AM, Misar AV. Anti-inflammatory activity of Jatropha curcas roots in mice and rats. J Ethnopharmacol. 2004;90:11–15. doi: 10.1016/j.jep.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Nadkarni AK. Dr. K.M. Nadkarni’s Indian Materia Medica. In: Nadkarni KM, editor. Bombay: Popular Prakashan; 1976. p. 705. [Google Scholar]
- 41.Prasad DMR, Izam A, Khan MMR. Jatropha curcas: plant of medical benefits. J Med Plants Res. 2012;6:2691–2699. [Google Scholar]
- 42.Al-Mamary MA Jr. Antioxidant activity of commonly consumed vegetables in Yemen. Malays J Nutr. 2002;8:179–189. [PubMed] [Google Scholar]
- 43.Jensen WB. The origin of the Soxhlet extractor. J Chem Educ. 2007;84:1913. [Google Scholar]
- 44.Lindahl TL, Ramström S. Methods for evaluation of platelet function. Transfus Apher Sci. 2009;41:121–125. doi: 10.1016/j.transci.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Nurden AT. Qualitative disorders of platelets and megakaryocytes. J Thromb Haemost. 2005;3:1773–1782. doi: 10.1111/j.1538-7836.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- 46.Osoniyi O, Onajobi F. Coagulant and anticoagulant activities in Jatropha curcas . J Ethnopharmacol. 2003;89:101–105. doi: 10.1016/s0378-8741(03)00263-0. [DOI] [PubMed] [Google Scholar]
- 47.Soetan KO, Akinrinde AS, Ajibade TO. Preliminary studies on the haematological parameters of cockerels fed raw and processed guinea corn (Sorghum bicolor) Proceedings of the 38th Conference of the Nigerian Society for Animal Production. 2013;2013:49–52. [Google Scholar]
- 48.Spolarich AE, Andrews L. An examination of the bleeding complications associated with herbal supplements, antiplatelet and anticoagulant medications. J Dent Hyg. 2007;81:67. [PubMed] [Google Scholar]