Abstract
Objective: To analyze the clinical effectiveness and safety of Camrelizumab immunotherapy in patients with advanced esophageal carcinoma (aEC). Methods: A retrospective study was conducted on 142 aEC cases admitted between May 2020 to October 2022. The patients who received albumin-bound paclitaxel (ALB-bound PTX) and cis-platinum (DDP) were grouped into the control group (n=72), and the others received Camrelizumab immunotherapy in combination with ALB-bound PTX and DDP were grouped in to the research group (n=72). The clinical effectiveness, side effects (rash, nausea/vomiting, impaired liver function, leukopenia, thrombocytopenia, and alopecia), tumor marker levels (CEA, CA199, and CA125), immunoglobulin levels (IGA, IGM, and IGG), immune molecule levels (PD-1 and PD-L1), and the one-year survival rate were compared between the two groups. Furthermore, the risk factors affecting therapeutic effectiveness were identified by binary Logistic regression analysis. Results: Compared to the control group, the research group demonstrated a higher overall response rate, fewer side effects, and greater reductions in the levels of CEA, CA199, and CA125 after treatment. IgA, IgM, and IgG levels increased significantly in both groups after treatment, with a more pronounced improvement in the research group. PD-1 and PD-L1 levels decreased significantly after treatment, especially in the research group. The one-year survival rate was higher in the research group. Furthermore, treatment modality was a risk factor affecting therapeutic effectiveness in aEC patients. Conclusions: Camrelizumab immunotherapy is highly effective in treating aEC. It can increase the one-year survival rate, and elevate the levels of immunoglobulins and immune molecules while reducing the levels of tumor markers and incidence of side effects.
Keywords: Camrelizumab, immunotherapy, advanced esophageal carcinoma, clinical effectiveness, side effects of treatment
Introduction
Esophageal carcinoma (EC) is a prevalent malignancy in digestive system, characterized by its insidious onset, making early detection in patients challenging [1]. Many patients have already entered an advanced stage by the time they experience noticeable symptoms, which undoubtedly complicates treatment and poses a serious threat to their health and survival [2]. For advanced EC (aEC) patients, while radical surgery can directly remove the primary tumor, it is often difficult to eradicate all cancer cells, due to the possibility of cancer cells invading surrounding tissues or lymph node metastasis [3]. Chemotherapy plays a critical role in the postoperative treatment of aEC patients, effectively killing residual cancer cells and preventing further tumor development [4]. With various chemotherapy drugs available, selecting drugs suitable for patients is particularly important, as it is closely related to chemotherapy effectiveness and possible adverse reactions during the chemotherapy [5].
Currently, paclitaxel (PTX) and cis-platinum (DDP) are commonly used chemotherapy drugs for aEC treatment. The former can interfere with tumor cell division, while the latter can inhibit tumor cell replication [6]. However, PTX lacks selectivity for tumor tissues and may cause damage to other organs [7]. The emerging albumin (ALB)-bound PTX, which combines PTX with ALB, can more accurately target tumor tissues, improve efficacy, and reduce allergic reactions, with easy use [8]. Although these chemotherapy regimens can prolong survival, they can also damage normal cells, reduce immune function, and affect quality of life [9]. In recent years, immunotherapy has emerged as a promising approach in the treatment of advanced malignancies. Camrelizumab, a biologic drug specifically used in tumor immunotherapy, acts as an immunosuppressive regulator [10]. It can quickly and effectively inhibit excessive immune responses that may promote the growth and spread of tumors in some cases [11]. By modulating these immune response, Camrelizumab helps prevent tumor cell proliferation and metastasis, gaining valuable time for treatment [12]. When Camrelizumab enters the body, it can rapidly target the programmed death-1 (PD-1) receptor, an important protein on the surface of immune cells, playing a key role in the regulation of immune response [13]. By tightly binding to the PD-1 receptor, Camrelizumab effectively blocks its signal pathways, essentially “cutting off” the communication between tumor cells and the immune system [14]. This mechanism allows Camrelizumab gradually to restore the patient’s autoimmune system and enhance its ability to recognize and attack tumor cells [15]. Wu et al. demonstrated that the combination of Camrelizumab and Gemcitabine provided a new treatment option for gallbladder cancer with multiple abdominal lymph node metastases [16].
In this study, we included 142 aEC patients and comparatively analyzed the clinical effectiveness of Camrelizumab immunotherapy for aEC, validating the clinical advantages of this therapy.
Patients and methods
Clinical data collection
This study was approved by the Ethics Committee of Xiangya School of Pharmaceutical Sciences, Central South University. In this retrospective study, 142 aEC patients who received treatment at Xiangya School of Pharmaceutical Sciences, Central South University between May 2020 and October 2022 were selected. Based on their treatment regimen, the patients were classified into two groups: a control group received treatment with ALB-bound PTX and DDP (n=72), and a research group that received additional Camrelizumab immunotherapy on the basis of the treatment used in control group (n=72). The flowchart of this study is shown in Figure 1.
Figure 1.
Flowchart of this study. aEC, advanced esophageal carcinoma; ALB, albumin; PTX, paclitaxel; DDP, cis-platinum.
Inclusion criteria: Clinical diagnosis of aEC; no prior chemotherapy; no history of drug allergies; good overall physical condition and tolerance to immunotherapy; clinical stage: IIIb-IV; first-time treatment; complete case records. Exclusion criteria: Liver or kidney dysfunction; abnormal coagulation function; other infectious diseases; referral to other hospitals during the treatment; other digestive system neoplastic diseases or contraindications to the study medication.
Treatment methods
The control group was given ALB-bound PTX plus DDP treatment. On the first day, the patient was given ALB-bound PTX (Hunan Kelun Pharmaceutical Co., Ltd., SFDA Approval No. H20203443) intravenously at a dose of 220 mg/m2; Additionally, DDP (Qilu Pharmaceutical, SFDA Approval No. H37021362) was given by intravenous drip at 25 mg/m2 from day 1 to day 3. Patients underwent two cycles of treatment, each lasting for 21 days.
In addition to the treatment described above, the patients in research group was injected with 200 mg of Camrelizumab (Jiangsu Hengrui Pharmaceuticals, SFDA Approval No. S20190027) dissolved in 100 mL 0.9% sodium chloride injection, once every 21 days. Patients received 2 cycles of chemotherapy, with 21 days per cycle.
Data extraction and patient sample size calculation
A total of 142 patients with aEC were screened from the medical record system. The extracted data included clinical effectiveness, side effects, tumor markers, immunoglobulins (Igs), immune molecules, and survival status, to assess and compare the clinical outcomes between the two groups. The patient sample size was determined to meet the minimum requirement of approximately 41 participants, based on the binomial proportion sample size estimation formula.
Outcome measures
We comparatively analyzed the clinical effectiveness, side effects (rash, nausea/vomiting, impaired liver function, leukopenia, thrombocytopenia, and alopecia), tumor markers [carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 199, and CA125], immunoglobulins (IgA, IgM, and IgG), immune molecules [PD-1 and programmed death receptor ligand-1 (PD-L1)], and the one-year survival rate of the two groups. Among these, clinical effectiveness, side effects, tumor markers, and Igs were the main observation indicators, and immune molecules and survival rate were secondary observation indicators.
(1) Clinical effectiveness. Complete response (CR): complete disappearance of the lesion on imaging, sustained for more than four weeks; Partial response (PR): tumor volume reduction of 30% or more, sustained for more than four weeks; Stable disease (SD): tumor volume reduction of less than 30% or an increase of less than 20%; Progressive disease (PD): tumor volume increase of 20% or more. The disease control rate (DCR) was calculated as the sum of the rates of CR, PR, and SD.
(2) Side effects. It was assessed mainly by observing and recording the occurrence of rash, nausea/vomiting, impaired liver function, leukopenia, thrombocytopenia, and alopecia during the treatment.
(3) Tumor markers. Before and after 6 weeks of treatment, 3 mL of fasting venous blood was drawn from both groups. The blood samples were centrifuged at 3,000 rpm for 15 minutes to separate the serum. Levels of CEA, CA199, and CA125 were determined using electrochemiluminescence.
(4) Immunoglobulins. Before and after the intervention, 3 mL of fasting blood samples were collected from patients in a calm state. The samples were then processed and analyzed for IgA, IgM, and IgG using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Enzyme-linked Biotechnology).
(5) Immune molecules. 4 mL of fasting venous blood was taken from each patient. The serum was separated by centrifugation, strictly completed within 15 minutes. The serum levels of PD-1 and PD-L1 levels were tested using ELISA kits (Sino Biological Inc., Beijing, SEKB10377, KIT10084).
(6) Survival rate. Patients were followed up for one year by telephone or outpatient visits, with all-cause death and the end of follow-up as the endpoint. The one-year survival rate of both groups was analyzed.
Statistical analysis
Continuous data were expressed as mean ± SEM, with between-group and within-group differences identified using independent sample t-tests and paired t-tests, respectively. Categorical variables were expressed by rate (percentage), and χ2 tests were employed for between-group comparisons. The one-year survival status of patients was analyzed using the Kaplan-Meier (K-M) method. Data analyses were made by SPSS 22.0. Statistical significance was defined as a P-value <0.05.
Results
Comparison of general information between the two groups
An analysis of general information, as shown in Table 1, revealed similar age, tumor diameter, body mass, Eastern Cooperative Oncology Group (ECOG) score, lesion site, and clinical stage between the control and research groups (all P>0.05).
Table 1.
Comparison of general data between the two groups
| Indicator | Research group (n=71) | Control group (n=71) | χ2/t | P |
|---|---|---|---|---|
| Age (years) | 58.93±5.17 | 59.27±5.24 | 0.389 | 0.698 |
| Tumor diameter (cm) | 2.46±0.51 | 2.53±0.46 | 0.859 | 0.392 |
| Body mass (kg) | 60.58±5.76 | 61.22±5.81 | 0.659 | 0.511 |
| Eastern Cooperative Oncology Group score | 0.257 | 0.612 | ||
| 0 point | 30 (42.25) | 33 (46.48) | ||
| 1 point | 41 (57.75) | 38 (53.52) | ||
| Lesion site | 0.289 | 0.865 | ||
| Upper thoracic segment | 20 (28.17) | 18 (25.35) | ||
| Middle thoracic segment | 25 (35.21) | 24 (33.80) | ||
| Lower thoracic segment | 26 (36.62) | 29 (40.85) | ||
| Clinical staging | 0.753 | 0.386 | ||
| IIIb | 29 (40.85) | 24 (33.80) | ||
| IV | 42 (59.15) | 47 (66.20) |
Comparison of clinical effectiveness between the two groups
As shown in Table 2, the overall response rate was statistically higher in the research group than the control group (85.92% vs. 61.97%) (P<0.05).
Table 2.
Comparison of clinical effectiveness between the two groups
| Indicator | Research group (n=71) | Control group (n=71) | χ2 | P |
|---|---|---|---|---|
| Complete response | 0 (0.00) | 0 (0.00) | ||
| Partial response | 29 (40.85) | 11 (15.49) | ||
| Stable disease | 32 (45.07) | 33 (46.48) | ||
| Progressive disease | 10 (14.08) | 27 (38.03) | ||
| Total effective rate | 61 (85.92) | 44 (61.97) | 10.561 | 0.001 |
Analysis of factors influencing therapeutic effectiveness
Values were assigned to age, tumor diameter, body mass, ECOG score, lesion site, clinical staging, and treatment modality (Table 3). Subsequently, binary logistic regression analysis was carried out, and the results revealed that while age, tumor diameter, body mass, ECOG score, lesion site, and clinical staging did notsignificantly influence therapeutic effectiveness (all P>0.05), treatment modality emerged as a significant risk factor influencing therapeutic effectiveness in aEC patients (P<0.05, Table 4).
Table 3.
Assignment
| Indicator | Assignment |
|---|---|
| Age (years) | Continuous variable |
| Tumor diameter (cm) | Continuous variable |
| Body mass (kg) | Continuous variable |
| Eastern Cooperative Oncology Group score | 0 point =0 (n=63), 1 point =1 (n=79) |
| Lesion site | Upper thoracic segment =0 (n=38), middle thoracic segment =1 (n=38), lower thoracic segment =2 (n=38) |
| Clinical staging | IIIb (n=49) =0, IV (n=55) =1 |
| Treatment modality | Camrelizumab (n=71) =0, albumin-bound paclitaxel + cis-platinum (n=71) =1 |
Table 4.
Multivariate analysis of factors affecting therapeutic effectiveness
| Factor | β | SE | Wald | P | Exp (β) | 95% CI |
|---|---|---|---|---|---|---|
| Age (years old) | -0.007 | 0.035 | 0.044 | 0.834 | 0.993 | 0.926-1.064 |
| Tumor diameter (cm) | -0.339 | 0.219 | 2.399 | 0.121 | 0.712 | 0.464-1.094 |
| Body mass (kg) | 0.051 | 0.073 | 0.493 | 0.482 | 1.053 | 0.912-1.215 |
| Eastern Cooperative Oncology Group score | -0.118 | 0.427 | 0.076 | 0.783 | 0.889 | 0.385-2.053 |
| Lesion site | 0.332 | 0.266 | 1.554 | 0.212 | 1.394 | 0.827-2.348 |
| Clinical staging | 0.217 | 0.431 | 0.253 | 0.615 | 1.243 | 0.533-2.895 |
| Treatment modality | 1.466 | 0.444 | 10.905 | 0.001 | 4.331 | 1.815-10.338 |
Comparison of side effects between the two groups
As summarized in Table 5, the incidence of side effects including rash, nausea/vomiting, impaired liver function, leukopenia, thrombocytopenia, and alopecia was 12.68% in the research group, which was significantly lower than 28.17% in the control group (P<0.05).
Table 5.
Comparison of side effects between the two groups
| Indicator | Research group (n=71) | Control group (n=71) | χ2 | P |
|---|---|---|---|---|
| Rash | 1 (1.41) | 4 (5.63) | ||
| Nausea/vomiting | 3 (4.23) | 5 (7.04) | ||
| Impaired liver function | 1 (1.41) | 3 (4.23) | ||
| Leukopenia | 1 (1.41) | 2 (2.82) | ||
| Thrombocytopenia | 1 (1.41) | 1 (1.41) | ||
| Alopecia | 2 (2.82) | 5 (7.04) | ||
| Total | 9 (12.68) | 20 (28.17) | 5.243 | 0.022 |
Comparison of tumor marker levels between the two groups
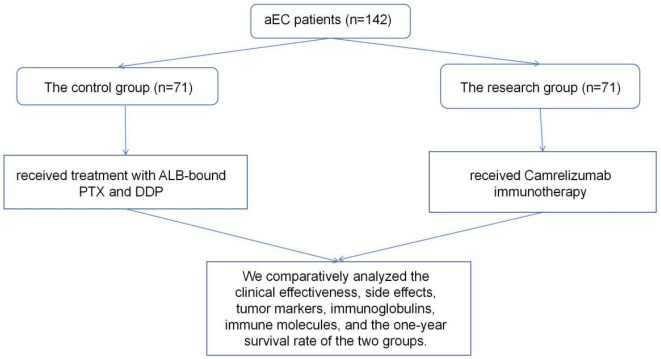
Tumor markers CEA, CA199, and CA125 were evaluated before and after the treatment, with the results shown in Figure 2. There were no significant inter-group differences in tumor markers before treatment (all P>0.05). Both groups exhibited marked reductions in CEA, CA199, and CA125 levels after treatment, with significantly lower levels in the research group (all P<0.05).
Figure 2.
Comparison of pre- and post-treatment CEA, CA199, and CA125 levels between the two groups. A. Pre- and post-treatment CEA levels; B. Pre- and post-treatment CA199 levels; C. Pre- and post-treatment CA125 levels. Note: **P<0.01 vs. before treatment; aP<0.05 vs. Control. CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199; CA125, carbohydrate antigen 125.
Comparison of Ig levels between the two groups
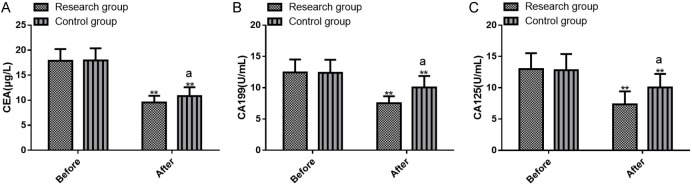
The immunoglobulins IgA, IgM, and IgG were assessed, and the results are displayed in Figure 3. The two groups did not differ statistically in pre-treatment Ig levels (all P>0.05). After treatment, both groups experienced significant increases in IgA, IgM, and IgG levels, with a greater rise in the research group than the control group (P<0.05).
Figure 3.
Comparison of pre- and post-treatment IgA, IgM, and IgG levels between the two groups. A. Pre- and post-treatment IgA levels; B. Pre- and post-treatment IgM levels; C. Pre- and post-treatment IgG levels. Note: **P<0.01 vs. before treatment; aP<0.05 vs. Control. IgA, immunoglobulins A; IgM, immunoglobulins M; IgG, immunoglobulins G.
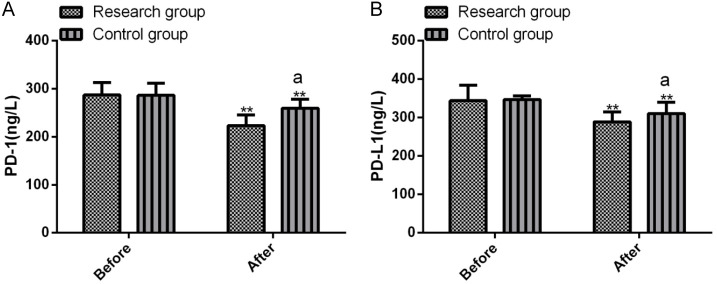
Comparison of immune marker levels between the two groups
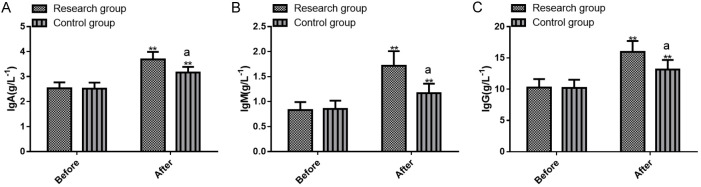
PD-1 and PD-L1 levels were analyzed before and after treatment, with results presented in Figure 4. There were no significantinter-group differences in pre-treatment PD-1 and PD-L1 levels (all P>0.05). PD-1 and PD-L1 declined in both groups after treatment, with greater reductions in the research group (all P<0.05).
Figure 4.
Comparison of pre- and post-treatment PD-1 and PD-L1 levels between the two groups. A. Pre- and post-treatment PD-1 levels; B. Pre- and post-treatment PD-L1 levels. Note: **P<0.01 vs. before treatment; aP<0.05 vs. Control. PD-1, programmed death-1; PD-L1, programmed death receptor ligand-1.
Comparison of 1-year survival rate between the two groups
Of the 71 patients in the research group, 9 died within one year, with a one-year survival rate of 87.32%. Among the 71 patients in the control group, 26 died within one year, with a one-year survival rate of 63.38%. The K-M curves drawn showed a higher survival rate in the research group compared to the control group (P<0.05), as shown in Figure 5.
Figure 5.
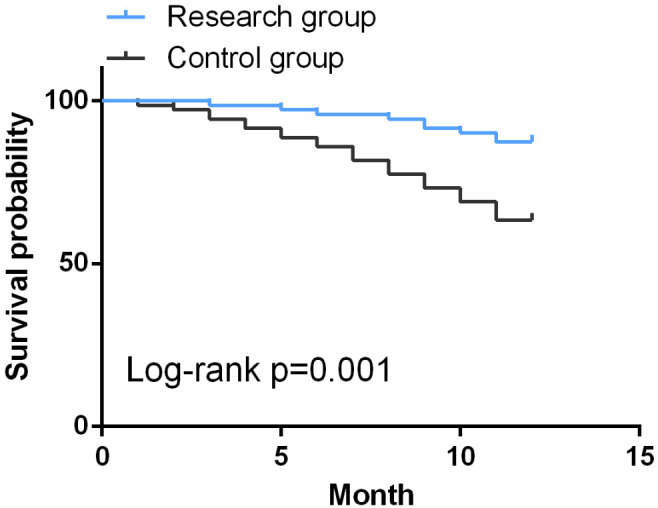
Kaplan-Meier curves of 1-year survival.
Discussion
Esophageal carcinoma (EC) is associated with alcohol abuse and smoking. Early detection of EC is challenging, and the disease is often diagnosed at an advanced stage, at which point chemoradiotherapy or surgery becomes necessary [17]. While chemoradiotherapy is effective in killing cancer cells, it can also inadvertently injure normal cells, leading to weakened immunity and other side effects, such as vomiting and hair loss [18]. Moreover, the overall prognosis remains poor for many patients, since the advanced nature of the disease often limits the effectiveness of treatment, resulting in shortened survival times. Elderly patients, who frequently have other comorbidities and frailty, are not suitable candidates for surgery, making conservative management preferred in many cases [19]. Camrelizumab, a PD-1 immunosuppressant, offers a targeted approach to treating tumors by harnessing the body’s immune system to eliminate cancer cells more precisely without inducing resistance, a common problem of chemoradiotherapy [4]. In addition, Camrelizumab can strengthen the immune system and produce long-term immune memory, providing a lasting effect in patients [20]. In this study, we applied immunotherapy with Camrelizumab in aEC patients, and validated its advantages, providing useful clinical evidence for the prevention and treatment of aEC.
Camrelizumab is a humanized PD-1 monoclonal antibody that acts as an immunosuppressant by binding to PD-1 receptors and blocking the connection between PD-1 and PD-L1, thus improving the body’s immunity against tumors [21]. This is the basic principle of cancer immunotherapy, which strengthens the body’s natural defense to more effectively combat cancer [22]. Previous studies have shown that stereotactic radiosurgery (SRS) in combination with Ranibizumab intervention for advanced non-small cell lung cancer (NSCLC) can effectively inhibit angiogenesis, tumor growth and metastasis and significantly improve patients’ quality of life, with a good synergistic effect [23]. In our study, the overall response rate was significantly higher in the research group than the control group (85.92% vs. 61.97%), suggesting the advantages of the Camrelizumab-based immunotherapy regimen in improving curative effect. We also identified the risk factors influencing therapeutic effectiveness in aEC patients and found that the treatment modality was a risk factor, further confirming the efficacy of Camrelizumab immunotherapy. Previous studies have also shown that Camrelizumab in combination with chemotherapy and antiangiogenic agents for advanced NSCLC with malignant pleural effusion can improve cancer-related features, reduce tumor size in primary and metastatic cancers, and remove tumor cells from patients [24]. Although the ALB-bound PTX + DDP therapy has a definite anti-tumor therapeutic effect, it inevitably causes damage to other healthy cells, tissues, and organs while exerting a negative impact on patient immunity. While Camrelizumab can block tumor cell signaling pathways, it may also affect the normal function of tissues and organs, leading to complications such as fever, cough, and infection. However, patients treated with Camrelizumab experienced a much lower incidence of adverse effects than those receiving traditional chemotherapy approaches [25]. Similarly, we found that the total incidence of adverse reactions in the research group was significantly lower compared to the control group (12.68% vs. 28.17%). This suggests that Camrelizumab immunotherapy for aEC is helpful to prevent the occurrence of adverse reactions such as rash, nausea/vomiting, impaired liver function, leukopenia, thrombocytopenia, and alopecia to some extent. Preoperative serum CEA levels have been shown to predict resectability in EC patients and can serve as an influencing factor for the objective response rate of PTX plus DDP regimen combined with PD-1 inhibitor neoadjuvant therapy for EC [26]. In this study, CEA, CA199, and CA125 levels were markedly reduced in the research group more than the control group, suggesting that immunotherapy with Camrelizumab can effectively improve the local tumor control effect, reduce the levels of tumor markers, and thus delay the disease progression in patients. Other studies have shown that the development of immunotherapy, particularly immune checkpoint inhibitors targeting PD-1/PD-L1, has improved the prognosis of cancer patients, and that Camrelizumab immunotherapy has prolonged survival in EC patients, possibly due to improved anti-tumor immunity [27]. Subsequently, Ig detection results revealed markedly elevated IgA, IgM, and IgG levels in the research group more than the control group after the treatment, underscoring the role of Camrelizumab immunotherapy in enhancing patients’ immunity. By activating and enhancing the patient’s immune system, this therapy helps the body better recognize and attack tumor cells, thus enhancing anti-tumor immunity while also reducing the risk of infection and complications. The PD-1/PD-L1 signaling pathway plays a crucial role in suppressing T cell activation, accelerating tumor-specific T cell death, and fostering immune evasion. This pathway is highly active in the tumor microenvironment, leading to immune evasion and immunosuppression. Our study showed that compared to the control group, the PD-1 and PD-L1 in the research group were significantly lower after treatment, suggesting that Camrelizumab, as a specific IgG4 monoclonal antibody, has strong binding affinity with PD-1. This drug can bind closely with PD-1 receptors on the surface of CD4+ and CD8 cells in the human body to effectively block PD-1 pathways, relieve the T cell suppression and quickly activate the immune response, demonstrating robust anti-tumor effects. Previous evidence has shown that Camrelizumab immunotherapy combined with apatinib offers a favorable safety profile and strong efficacy in patients with unresectable recurrent or metastatic bone and soft-tissue sarcoma, improving patient survival [28]. The results of this study showed that the one-year survival rate of the research group was 87.32%, significantly higher than that of the control group (63.38%). Camrelizumab thus has great advantages in significantly extending the survival of aEC patients.
The therapeutic mechanism of Camrelizumab immunization for aEC operates through several pathways: (1) The combination of immunotherapy and chemotherapy can synergistically improve the tumor microenvironment, enhance the sensitivity of tumor cells to drugs, thus making the anti-tumor treatment more effective [29]. (2) The Camrelizumab-based regimen not only offers good therapeutic effect, but also presents fewer side effects compared to traditional chemotherapy. More importantly, it achieves a strong synergistic effect when combined with other forms of immunotherapy [30].
In summary, Camrelizumab immunotherapy has significant clinical efficacy in the treatment of advanced esophageal carcinoma, effectively reducing side effects while improving the levels of tumor markers, immune cells, PD-1 and PD-L1.
Disclosure of conflict of interest
None.
References
- 1.Bai L, Yan L, Guo Y, He L, Sun Z, Cao W, Lu J, Mo S. Perineural invasion is a significant indicator of high malignant degree and poor prognosis in esophageal cancer: a systematic review and meta-analysis. Front Oncol. 2022;12:816270. doi: 10.3389/fonc.2022.816270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu H, Ma X, Ye T, Wang H, Wang Z, Liu Q, Zhao K. Esophageal cancer in China: practice and research in the new era. Int J Cancer. 2023;152:1741–1751. doi: 10.1002/ijc.34301. [DOI] [PubMed] [Google Scholar]
- 3.Zhang XF, Liu PY, Zhang SJ, Zhao KL, Zhao WX. Principle and progress of radical treatment for locally advanced esophageal squamous cell carcinoma. World J Clin Cases. 2022;10:12804–12811. doi: 10.12998/wjcc.v10.i35.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, Wang F, Feng S, Peng F, Wang X, Chen S, He M, Zhang N, Wang H, Zeng B, Liu Z, Kidane B, Seder CW, Koyanagi K, Shargall Y, Luo H, Peng S, Cheng C. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10:e003497. doi: 10.1136/jitc-2021-003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang H, Wang H, Fang Y, Zhu JY, Yin J, Shen YX, Zeng ZC, Jiang DX, Hou YY, Du M, Lian CH, Zhao Q, Jiang HJ, Gong L, Li ZG, Liu J, Xie DY, Li WF, Chen C, Zheng B, Chen KN, Dai L, Liao YD, Li K, Li HC, Zhao NQ, Tan LJ. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol. 2023;34:163–172. doi: 10.1016/j.annonc.2022.10.508. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, Shen L, Shu Y, Wang C, Dai T, Mao T, Chen L, Guo Z, Liu B, Pan H, Cang S, Jiang Y, Wang J, Ye M, Chen Z, Jiang D, Lin Q, Ren W, Wang J, Wu L, Xu Y, Miao Z, Sun M, Xie C, Liu Y, Wang Q, Zhao L, Li Q, Huang C, Jiang K, Yang K, Li D, Liu Y, Zhu Z, Chen R, Jia L, Li W, Liao W, Liu HX, Ma D, Ma J, Qin Y, Shi Z, Wei Q, Xiao K, Zhang Y, Zhang Y, Chen X, Dai G, He J, Li J, Li G, Liu Y, Liu Z, Yuan X, Zhang J, Fu Z, He Y, Ju F, Liu Z, Tang P, Wang T, Wang W, Zhang J, Luo X, Tang X, May R, Feng H, Yao S, Keegan P, Xu RH, Wang F. Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell. 2022;40:277–288. e273. doi: 10.1016/j.ccell.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, Zeng Z, Zhu J, Hou Y, Du M, Jiao J, Jiang H, Gong L, Li Z, Liu J, Xie D, Li W, Lian C, Zhao Q, Chen C, Zheng B, Liao Y, Li K, Li H, Wu H, Dai L, Chen KN. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs. neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg. 2021;156:444–451. doi: 10.1001/jamasurg.2021.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W, Zhao J, Kang L, Li C, Xu Z, Li J, Zhang M. Fluoroscopy-guided salvage photodynamic therapy combined with nanoparticle albumin-bound paclitaxel for locally advanced esophageal cancer after chemoradiotherapy: a case report and literature review. Cancer Biother Radiopharm. 2022;37:410–416. doi: 10.1089/cbr.2020.4595. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Li Q, Chen B, Xi M, Makelike K, Liu S, Hu Y, Zhu Y. Phase I study of cisplatin and nanoparticle albumin-bound-paclitaxel combined with concurrent radiotherapy in locally advanced esophageal squamous cell carcinoma. Cancer Med. 2023;12:15187–15198. doi: 10.1002/cam4.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Feng J, Niu Z, Ba Y, Zhang H, Liu Y, Zhang L, Min X, Huang J, Cheng Y, Wang D, Shen Y, Yang Q, Zou J, Xu RH ESCORT-1st Investigators. Effect of camrelizumab vs. placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, Liu S, Chen B, Xi M. Recent advances in combination of immunotherapy and chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancers (Basel) 2022;14:5168. doi: 10.3390/cancers14205168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Sohal D. Current state of the art: immunotherapy in esophageal cancer and gastroesophageal junction cancer. Cancer Immunol Immunother. 2023;72:3939–3952. doi: 10.1007/s00262-023-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G, Su X, Huang Y, Luo G, Wang Z, Cai P, Zheng Y, Bei T, Huang M, Bai Y, He H, Xiang J, Cai M, Zhong J, Guo Q, Zhang X. Intensive cycles of neoadjuvant camrelizumab combined with chemotherapy in locally advanced esophageal squamous cell carcinoma: a single-arm, phase II trial. J Transl Med. 2023;21:411. doi: 10.1186/s12967-023-04273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Zhu L, Cheng Y, Liu Z, Cai X, Shao J, Zhang M, Liu J, Sun Y, Li Y, Yi J, Yu B, Jiang H, Chen H, Yang H, Tan L, Li Z. Three-arm phase II trial comparing camrelizumab plus chemotherapy versus camrelizumab plus chemoradiation versus chemoradiation as preoperative treatment for locally advanced esophageal squamous cell carcinoma (NICE-2 Study) BMC Cancer. 2022;22:506. doi: 10.1186/s12885-022-09573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, Cheng Y, Yuan X, Xiao J, Tai Y, Wang L, Zou J, Zhang Y, Shen L. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2021;27:3069–3078. doi: 10.1158/1078-0432.CCR-20-4691. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Wang Z, Li J, Peng XH, Tang YC, Huang XB, He YG. Camrelizumab combined with gemcitabine and albumin-bound paclitaxel for neoadjuvant therapy in the treatment of progressive gallbladder cancer: a case report. Front Oncol. 2022;12:818626. doi: 10.3389/fonc.2022.818626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stabellini N, Chandar AK, Chak A, Barda AJ, Dmukauskas M, Waite K, Barnholtz-Sloan JS. Sex differences in esophageal cancer overall and by histological subtype. Sci Rep. 2022;12:5248. doi: 10.1038/s41598-022-09193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand U, Dey A, Chandel AKS, Sanyal R, Mishra A, Pandey DK, De Falco V, Upadhyay A, Kandimalla R, Chaudhary A, Dhanjal JK, Dewanjee S, Vallamkondu J, Perez de la Lastra JM. Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2022;10:1367–1401. doi: 10.1016/j.gendis.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moletta L, Pierobon ES, Capovilla G, Zuin IS, Carrillo Lizarazo JL, Nezi G, Lonardi S, Murgioni S, Galuppo S, Zanchettin G, Salvador R, Provenzano L, Valmasoni M. Short- and long-term outcomes in elderly patients with resectable esophageal cancer: upfront esophagectomy compared to surgery after neoadjuvant treatments. J Clin Med. 2024;13:4271. doi: 10.3390/jcm13144271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang P, Zhou X, Yang X, Wang Y, Sun T, Feng S, Ma X. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol. 2021;19:333. doi: 10.1186/s12957-021-02446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, Zhao J, Er P, Zhang T, Chen X, Wang Y, Jiang Y, Wang Q, Zhang B, Qian D, Wang J, Zhou D, Ren X, Yu Z, Zhao L, Yuan Z, Wang P, Pang Q. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist. 2021;26:e1110–e1124. doi: 10.1002/onco.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Gao D, Li J, Hu G, Liu J, Liu D. The predictive value of systemic inflammatory factors in advanced, metastatic esophageal squamous cell carcinoma patients treated with camrelizumab. Onco Targets Ther. 2022;15:1161–1170. doi: 10.2147/OTT.S382967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J, Guo J, Cheng B, Sun X, Zhang H, Ma J. Synergistic effect of stereotactic radiotherapy combined with karelizumab on patients with advanced NSCLC. J Healthc Eng. 2022;2022:7875627. doi: 10.1155/2022/7875627. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Xie XH, Shen PX, Wu JH, Qiu GH, Lin XQ, Xie ZH, Qin YY, Zheng B, Liu M, Zhou CZ. Recurrent pleural effusion as a rare manifestation after prolonged PD1 inhibitor (camrelizumab)-based immunotherapy: a case report. Hum Vaccin Immunother. 2023;19:2240689. doi: 10.1080/21645515.2023.2240689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua Y, Huang X, Li C, Gao N. An epulis-like camrelizumab related reactive cutaneous capillary endothelial proliferation (RCCEP) in the oral cavity: a case report. Oral Oncol. 2023;140:106369. doi: 10.1016/j.oraloncology.2023.106369. [DOI] [PubMed] [Google Scholar]
- 26.Ma R, Yuan D, Mo C, Zhu K, Dang C, Zhang Y, Yin J, Li K. Factors affecting the ORR after neoadjuvant therapy of TP regimen combined with PD-1 inhibitors for esophageal cancer. Sci Rep. 2023;13:6080. doi: 10.1038/s41598-023-33038-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Wang P, Huang X, Han Y, Zhang P. Biomarkers for immunotherapy in esophageal cancer. Front Immunol. 2023;14:1117523. doi: 10.3389/fimmu.2023.1117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Li M, Zhang B, Yang C, Wang Y, Zheng S, Tang L, Zhou C, Qian G, Huang Y, Yu W, Li H, Wang Y, He A, Shen Z, Zhang J, Li X, Yang Q, Hu H, Yao Y. A pilot study of multi-antigen stimulated cell therapy-I plus camrelizumab and apatinib in patients with advanced bone and soft-tissue sarcomas. BMC Med. 2023;21:470. doi: 10.1186/s12916-023-03132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang NN, Zheng H, Hu JX, Cui K, Si PP, Ge W. Camrelizumab in combination with neoadjuvant chemotherapy in resectable locally advanced esophageal squamous carcinoma cancer: results from a retrospective study. Kaohsiung J Med Sci. 2024;40:291–295. doi: 10.1002/kjm2.12793. [DOI] [PubMed] [Google Scholar]
- 30.Meng X, Wu T, Hong Y, Fan Q, Ren Z, Guo Y, Yang X, Shi P, Yang J, Yin X, Luo Z, Xia J, Zhou Y, Xu M, Liu E, Jiang G, Li S, Zhao F, Ma C, Ma C, Hou Z, Li J, Wang J, Wang F. Camrelizumab plus apatinib as second-line treatment for advanced oesophageal squamous cell carcinoma (CAP 02): a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:245–253. doi: 10.1016/S2468-1253(21)00378-2. [DOI] [PubMed] [Google Scholar]