Abstract
Objective: A multivariate logistic regression model was developed to identify the risk factors for postoperative bleeding in patients undergoing endoscopic submucosal dissection (ESD) for early esophageal cancer. Methods: The clinical data of 258 patients with early esophageal cancer who received ESD in Jiujiang Number One People’s Hospital from April 2019 to March 2022 were retrospectively analyzed. Patients with or without postoperative bleeding were included into a bleeding group and a control group, respectively, and general information with statistically significant difference between the two groups was included in the multivariate logistic regression model to screen the risk factors for postoperative bleeding in the patients. The risk factors were then used to construct a nomogram prediction model for postoperative bleeding, and internal (training set) and external (validation set) validation was performed. Results: (1) The incidence of post-ESD bleeding was 12.02% in the 258 patients with early esophageal cancer. (2) History of hypertension, lesion diameter, submucosal fibrosis, C-reactive protein (CRP), and albumin (ALB) were independent risk factors for postoperative bleeding after ESD in the patients (P<0.05). (3) The results of receiver operator characteristic curve (ROC) showed that the area under the curve (AUC) was 0.821 for the training set and 0.740 for the validation set. (4) The correction curve showed that the actual and predicted values of the training and validation sets were well fitted. Conclusion: Hypertension history, lesion diameter, submucosal fibrosis, CRP, and ALB are risk factors for postoperative bleeding in patients with early esophageal cancer undergoing ESD. The nomograms established based on these factors has good predictive value for postoperative bleeding in these patients.
Keywords: Early esophageal cancer, endoscopic submucosal section, bleeding, risk factors, nomograms
Introduction
Esophageal cancer is a common clinical malignancy of the digestive system that arises from the mucosal epithelium of the esophagus and has various pathologic forms, including squamous cell carcinoma and adenocarcinoma [1]. Typical clinical manifestations of esophageal cancer include progressive dysphagia, retrosternal pain, and persistent weight loss. In addition, the survival rate of patients with intermediate and advanced esophageal cancer is low, with poor prognosis and a survival rate of less than 20% within 5 years [2]. Therefore, treatment for early esophageal cancer (EEC) is one of the effective means to prevent the cancer from worsening and improves the recovery rate of patients. EEC refers to cancer infiltration confined to the mucosa and submucosa of the esophagus, with no clinical symptoms of lymph node metastasis. Most EEC and precancerous lesions can be eradicated by timely and effective endoscopic minimally invasive treatment, and the 5-year survival rate is as high as 95% [3,4].
Endoscopic submucosal dissection (ESD) is a type of endoscopic minimally invasive surgery, which was proposed by Japanese scholars as the first minimally invasive treatment for early gastric cancer, and is now widely used for early cancers and pre-cancerous lesions of the digestive tract, such as EEC [5]. ESD has the advantage of therapeutic resection of lesions without altering esophageal anatomy, as well as being cost-effective and less traumatic. In addition, during ESD, by injecting saline or other solutions, such as sodium hyaluronate, into the submucosal layer, the mucosa of the lesion is separated from the tissues underneath it, and after removing the entire piece of pathologic lesion tissue, its full volume can be obtained, which positively affects the final assessment of therapeutic efficacy [6,7].
In recent years, the therapeutic effect of ESD for EEC has improved greatly, and it can achieve a therapeutic effect comparable to surgery. However, ESD has certain requirements on the doctor’s skill level and has great operational difficulties. Especially when submucosal dissection cannot completely expose the submucosal layer, the operational difficulties are further increased, and pain, esophageal stenosis, gastrointestinal perforation, and bleeding may occur in the postoperative period [8,9]. Among these, bleeding is a common complication in patients undergoing ESD, which can prolong the patient’s hospital stay, elevate additional treatment costs, increase risks such as anemia, hypotension, and shock, or, in severe cases, even threaten the patient’s life [10]. According to statistics, the probability of bleeding after ESD in patients with EEC is about 5.1%, but depending on the patient group, the incidence may reach 10% to 20% [11]. Therefore, it is very important to identify risk factors for postoperative bleeding after ESD for EEC and provide rational interventions to reduce the risk of the postoperative bleeding.
However, it should be noted that there are few studies evaluating postoperative bleeding after ESD for EEC, and most of them only constructed logistic regression models, which is inferior to an easy-to-read bar graphical model [12]. In light of this, the present study constructed a bar graphical model of bleeding in regard to postoperative complications in order to provide an evaluation system for the prevention and treatment of bleeding in patients underwent ESD for EEC.
Data and methods
General data
This is a retrospective study. Two hundred and fifty-eight patients with EEC admitted to Jiujiang Number One People’s Hospital for ESD between April 2019 and March 2022, including 149 males and 109 females, were selected for the study. Inclusion criteria: (1) patients aged 18 years or older with complete clinical data; (2) patients diagnosed with EEC, including stages 0 to II (stage 0: tumor is limited to the intraepithelial layer and has not invaded the fundal membrane; stage I: tumor has invaded the mucosal or submucosal layer and may invade the muscular layer, but no lymph node metastases and no distant metastases; stage II: the tumor invades the esophageal epithelium or has some lymph node metastases, but still no distant metastases) [13]; (3) patients underwent ESD for the first time; and (4) patients who met the indications for ESD and successfully completed the surgery. Exclusion Criteria: (1) patients who had undergone ESD for esophageal cancer; (2) patients with infections or other gastrointestinal diseases; (3) patients with psychiatric disorders; (4) patients with other serious underlying complications; (5) patients with serious hepatic or renal failure. This study was approved by the Ethics Committee of Jiujiang No. 1 People’s Hospital.
Investigation methods
General clinical data collection: basic patient information was collected through the hospital information system, and a two-person entry was used to collect and check the clinical data. The recorded data included demographic data of patients with EEC (age, gender, body mass index (BMI), history of smoking (total cigarette inhalation ≥180 cigarettes was considered to have a history of smoking), family history of esophageal cancer), medical history (history of hypertension and diabetes mellitus), data of surgical treatment (diameter of the lesion, location of the lesion, depth of the lesion’s infiltration, resection of the lesion, duration of the operation, pathologic classification, whether the submucosal layer was fibrotic or not, and the type of submucosal injection), and hematological indices (C-reactive protein (CRP), serum albumin (ALB), serum procalcitonin (PCT), interleukin-6 (IL-6), and serum amyloid (SAA)). Among the infiltration depths: M1 was the inner epithelial layer, M2 was the lamina propria of the mucosa, M3 was the muscularis mucosae, and SM1 was the upper 1/3 of the submucosa.
Hematologic parameters: Three mL of venous blood was collected from the patients who fasted for 24 h after the surgery. The blood samples were centrifuged at 4,000 rpm for 5 min to obtain serum. CRP and PCT were determined by immunoturbidimetric method, ALB by colorimetric method (AU-5800 automated biochemical analyzer), IL-6 and SAA by enzyme-linked immunosorbent assay (ELISA).
Postoperative bleeding was indicated by the occurrence of vomiting, black or bloody feces, a decrease in hemoglobin ≥20 g/L or associated hemodynamic instability in the period from 24 hours to 30 days after surgery, and the need of further endoscopic intervention, hemostatic or transfusion procedures [14].
Grouping: Patients with postoperative hemorrhage were included in a bleeding group (BG), and those without were in a control group (CG).
External verification
Prospectively, 83 patients with EEC who visited our hospital for ESD from January 2023 to December 2023 were selected, and whether they had postoperative bleeding or not was used as the external validation set of the nomograms model. A confusion matrix was constructed to validate the accuracy of the nomograms model, and the sensitivity, specificity, and accuracy were calculated.
Sensitivity = (True Positives (TP)/TP + False Negatives (FN)) × 100%; Specificity = (True Negatives (TN)/TN + False Positives (FP)) × 100%; Accuracy = (TP+TN/83) × 100%.
Statistical methods
Data analysis was performed using Statistical Package for the Social Sciences (SPSS) 22.0 statistical software. Quantitative data conforming to normal distribution were expressed as mean ± standard deviation (x̅±s), and processed using t-test. Count data were expressed as n (%), and one-way intergroup comparisons were performed using the chi-square test, and statistically significant factors were included in logistic regression analysis to screen the risk factors for bleeding. R (R4.1.2) software and rms package were selected to build a thermogram model of postoperative bleeding after ESD, and the internal validation of the model was performed by Bootstrap repeated sampling (sample size = 500). Hosmer-Lemeshow goodness-of-fit test was employed to validate the fit effect of the model. Degree test and calibration line were used to validate the accuracy of the model. Receiver operating characteristic (ROC) curve and decision curve were plotted to validate the discriminability and the clinical application value of the model, respectively. Area under the curve (AUC) = 0.50 and a statistically significant difference at P<0.05 indicate no predictive effect.
Results
Occurrence of hemorrhage after ESD
Postoperative bleeding occurred in 31 out of the 258 EEC patients treated with ESD, with an incidence of 12.02%.
Univariant analysis
A one-way statistical analysis of the general clinical data of 258 patients with EEC who received ESD showed that there were no significant differences between the CG and the BG in gender, BMI, smoking history, family history of esophageal cancer, diabetes history, lesion focus, depth of invasion, nidus resection, pathologic classification, submucosal injection volume, PCT, IL-6, and SAA levels 24 hours after ESD (P>0.05). However, significant differences were identified in terms of age, history of hypertension, duration of surgery, lesion diameter, submucosal fibrosis, CRP level 24 hours after surgery, and ALB level 24 hours after surgery (P<0.05) (Table 1).
Table 1.
Univariant analysis of factors affecting hemorrhage after ESD for EEC
| Element | n | BG | CG | χ2/t | P |
|---|---|---|---|---|---|
| (n = 31) | (n = 227) | ||||
| Male [n (%)] | 149 | 18 (58.06) | 131 (57.70) | 0.001 | 0.970 |
| Age | 58.19±20.68 | 49.54±18.28 | 2.433 | 0.016 | |
| BMI (kg·m2) | 25.90±4.47 | 25.35±4.24 | 0.679 | 0.498 | |
| Smoking history | 170.32±76.45 | 165.64±96.49 | 0.259 | 0.796 | |
| Family history of esophageal cancer [n (%)] | 10 (32.26) | 73 (32.16) | 0.000 | 0.991 | |
| Hypertension history [n (%)] | 19 (61.29) | 68 (29.96) | 11.982 | 0.001 | |
| Diabetes history [n (%)] | 8 (25.81) | 43 (18.94) | 0.810 | 0.368 | |
| Position of lesions [n (%)] | 0.018 | 0.991 | |||
| Upper esophagus | 14 (45.16) | 103 (45.37) | |||
| Middle esophagus | 10 (32.26) | 75 (33.04) | |||
| Lower esophageal | 7 (22.58) | 49 (21.59) | |||
| Infiltrative depth [n (%)] | 0.015 | 0.902 | |||
| M1-M2 | 20 (64.52) | 149 (65.64) | |||
| M3-SM1 | 11 (35.48) | 78 (34.36) | |||
| Lesion resection [n (%)] | 0.094 | 0.760 | |||
| Complete resection | 24 (77.42) | 170 (74.89) | |||
| Partial resection | 7 (22.58) | 57 (25.11) | |||
| Duration of surgery (min) | 50.05±3.30 | 49.05±2.32 | 2.133 | 0.034 | |
| Lesion diameter (cm) | 3.05±1.21 | 2.56±0.86 | 2.782 | 0.006 | |
| Pathological types [n (%)] | 4.945 | 0.084 | |||
| Carcinoma in situ | 15 (48.39) | 87 (38.33) | |||
| Intramucosal carcinoma | 6 (19.35) | 90 (39.65) | |||
| Submucosal carcinoma | 10 (32.26) | 50 (22.02) | |||
| Submucosal injection solution [n (%)] | 0.043 | 0.836 | |||
| Glycerin fructose | 17 (54.84) | 120 (52.86) | |||
| Normal saline | 14 (45.16) | 107 (47.14) | |||
| Submucosal fibrosis [n (%)] | 12 (38.71) | 45 (19.82) | 5.652 | 0.017 | |
| CRP (mg·L-1) | 52.13±6.58 | 48.80±5.43 | 3.127 | 0.002 | |
| ALB (g·L-1) | 26.41±2.62 | 28.86±4.06 | 3.271 | 0.001 | |
| PCT (μg·mL-1) | 2.70±0.94 | 2.53±0.84 | 1.089 | 0.277 | |
| IL-6 (ng·mL-1) | 18.39±1.60 | 18.04±1.74 | 1.046 | 0.296 | |
| SAA (mg·L-1) | 31.30±2.63 | 31.49±2.63 | 0.378 | 0.706 |
ESD: Endoscopic submucosal dissection; EEC: early esophageal cancer; BG: bleeding group; CG: control group; BMI: body mass index; M1: the inner epithelial layer; M2: the lamina propria of the mucosa; M3: the muscularis mucosae; SM1: the upper 1/3 of the submucosa; CRP: C-reactive protein; ALB: serum albumin; PCT: serum procalcitonin; IL-6: interleukin-6; SAA: serum amyloid.
Multivariant logistic regression analysis
Logistic regression was conducted using presence of bleeding as dependent variable (yes = 1, no = 0) and significant factors in univariant analysis as independent variables (age, history of hypertension, duration of surgery, lesion diameter, submucosal fibrosis, CRP and ALB). The values assigned to the independent variables are presented in Table 2. Logistic regression analysis showed that comorbid hypertension, lesion diameter, submucosal fibrosis, CRP, and ALB were independent risk factors for bleeding after ESD in patients with EEC (P<0.05) (Table 3).
Table 2.
Assignment table of argument variables
| Independent variable | Assignment |
|---|---|
| Age | Original values |
| History of hypertension | Nil = 0; find = 1 |
| Duration of surgery | Original values |
| Lesion diameter | Original values |
| Submucosal fibrosis | No = 0; yes = 1 |
| CRP | Original values |
| ALB | Original values |
CRP: C-reactive protein; ALB: serum albumin.
Table 3.
Logistic regression analysis of factors affecting hemorrhage after ESD for EEC
| Variable | β | SE | Wald χ2 | P | OR | Cut-off value | 95% CI | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower limit | Upper limit | |||||||
| Constant | -11.215 | 5.668 | 3.915 | 0.048 | ||||
| Age | 0.021 | 0.012 | 3.068 | 0.080 | 1.021 | 0.998 | 1.046 | |
| History of hypertension | 1.187 | 0.438 | 7.363 | 0.007 | 3.278 | 1.391 | 7.728 | |
| Duration of surgery | 0.122 | 0.094 | 1.692 | 0.193 | 1.130 | 0.940 | 1.358 | |
| Lesion diameter | 0.539 | 0.250 | 4.635 | 0.031 | 1.714 | 3.45 | 1.050 | 2.800 |
| Submucosal fibrosis | 0.925 | 0.462 | 4.005 | 0.045 | 2.523 | 1.019 | 6.245 | |
| CRP | 0.086 | 0.039 | 4.882 | 0.027 | 1.090 | 58.10 | 1.010 | 1.177 |
| ALB | -0.168 | 0.064 | 6.821 | 0.009 | 0.845 | 23.85 | 0.745 | 0.959 |
SE: standard error; OR: odds ratio; CI: confidence interval; CRP: C-reactive protein; ALB: serum albumin.
A nomogram risk prediction model for hemorrhage after ESD for EEC
The 5 independent risk factors for postoperative bleeding obtained from logistic regression analysis were imported into the R program. For training and validation, 30% of the patient data were randomly selected as validation set, and the rest were used as training set.
Establishment of nomogram model
Based on the training set data, a bar chart prediction model was constructed using the 5 independent risk factors (Figure 1), and we calculated the regression equation of the model Logit (P) = 1.187 × history of hypertension + 0.539 × lesion diameter + 0.925 × submucosal fibrosis + 0.086 × CRP - 0.168 × ALB. As seen in the columnar model in this study, EEC patients with a history of hypertension, larger lesion diameter, and development of submucosal fibrosis, combined with more severe changes in CRP and ALB parameters, had a total score of 269 in the columnar risk prediction model, which was associated with postoperative bleeding. This corresponded to a bleeding risk of ≥90% (Figure 1).
Figure 1.
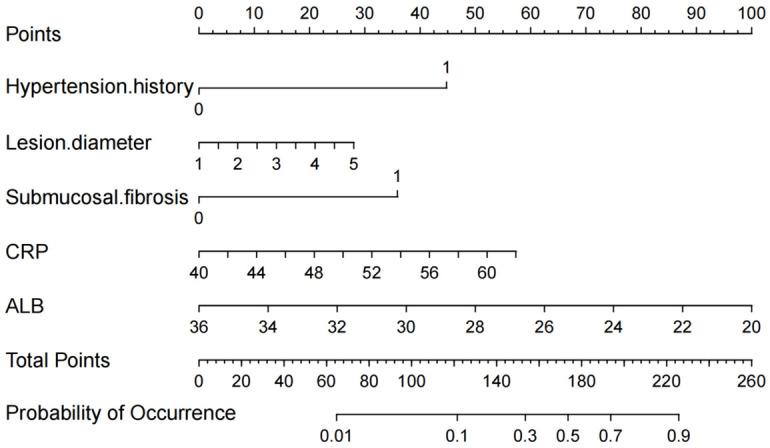
Prediction of hemorrhage risk after ESD in EEC patients. ESD: endoscopic submucosal dissection; EEC: early esophageal cancer; CRP: C-reactive protein; ALB: serum albumin.
Evaluation of the model
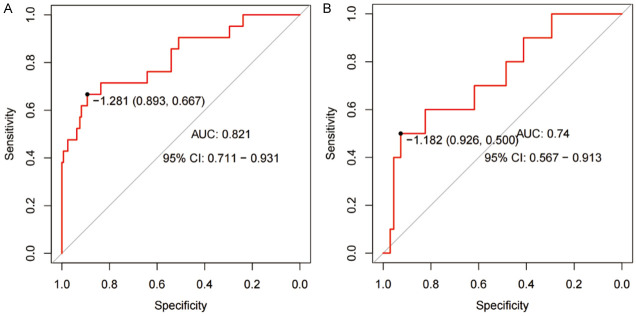
The internal validation of the nomogram model in the training set for predicting postoperative bleeding showed an AUC of 0.821 (95% confidence interval (CI) = 0.711-0.931), a sensitivity of 89.3%, a specificity of 66.7%, and a maximum Denyon index of -1.128 (Figure 2A). Prediction in the verification set showed an AUC of 0.740 (95% CI = 0.567-0.913), sensitivity of 92.6%, specificity of 50.0%, and a maximum Denyon index of -1.182 (Figure 2B).
Figure 2.
ROC curves. A: Training set. B: Verification set. ROC: receiver operator characteristic; AUC: area under the curve; CI: confidence interval.
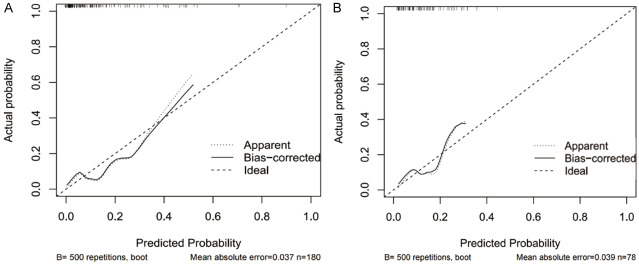
The calibration curves showed that the overall trend for the training (Figure 3A) and validation sets (Figure 3B) was basically in line with the ideal curve, which means that the model has good fit and good predictive effect. The results of Hosmer-Lemeshow goodness-of-fit test were χ2 = 8.602, P = 0.377 (>0.05), which means that the model has high goodness-of-fit, and there was no significant difference between the probability predicted by the model and the actual observed frequency, so there is no obvious systematic bias in the model data. Some of the most important factors are summarized below.
Figure 3.
Calibration curves. A: Training set. B: Verification set.
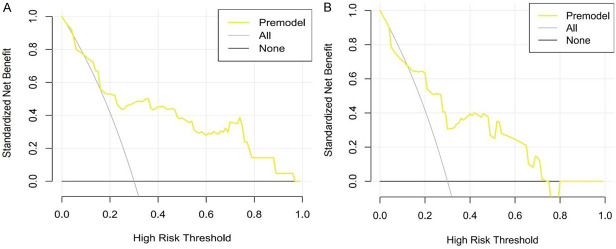
The judgment curves in the training set were in the range of black and gray curves for thresholds ranging from 0.1 to 1.0 (Figure 4A), and the judgment curves in the validation set were in the range of black and gray curves for thresholds ranging from 0.1 to 0.78 (Figure 4B), indicating that the model prediction results in this study are accurate.
Figure 4.
Decision curves. A: Training set. B: Verification set.
External validation showed (Figure 5) that the nomograms prediction model developed in this study had a sensitivity of 80.00%, a specificity of 94.52%, and an accuracy of 92.77%, which is a high degree of accuracy.
Figure 5.
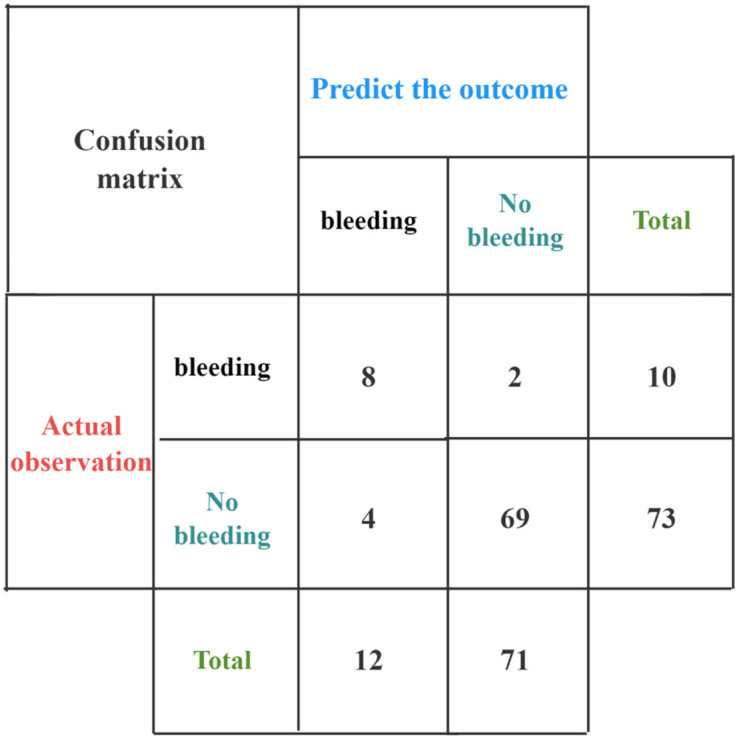
External validation of the model.
Discussion
The logistic regression analysis in this study showed that a history of hypertension, lesion diameter >3.45 cm (optimal cutoff value = 3.45 cm), submucosal filament fibrosis, CRP>58.10 mg·L-1 (optimal cutoff value = 58.10 mg·L-1), and ALB<23.85 mg·L-1 (optimal cutoff value = 23.85 mg·L-1) were the risk factors for bleeding after ESD for EEC (P<0.05). In many clinical procedures, the occurrence of postoperative bleeding in patients is closely related to their suffering from hypertension. While developing a nomogram model to predict the risk of postoperative bleeding, previous studies have found that hypertension is associated with coagulation dysfunction and poor patient prognosis, and is a risk factor for postoperative bleeding [15,16]. Hotca et al. concluded that a history of hypertension was a risk factor for bleeding after ESD for EEC [17]. The results of the present study are consistent with the findings of previous studies. The possible reasons are: (1) Hypertension can expose vessel walls to prolonged high pressure, reducing their elasticity and increasing their fragility. This makes them more susceptible to damage during endoscopic procedures, thereby raising the risk of bleeding. (2) Organ hypoplasia and abnormal clotting mechanisms associated with hypertension may further increase the likelihood of bleeding [18].
In the nomogram constructed by Zheng, tumor size was an independent risk factor for intraoperative blood loss or postoperative bleeding [19]. Besides, in the treatment of patients with esophageal cancer, bleeding rates were found to be positively correlated with lesion diameter, with smaller lesions associating with lower bleeding rates [20], which is consistent with the results of this study. The possible reasons are: (1) Larger lesions increase the resection volume, which may extend operative time, complicate the surgery, and result in greater trauma, thereby enlarging the area at risk for postoperative bleeding. (2) Larger esophageal lesions may be more prone to trauma during ESD, especially if the vasculature is complex and richly supplied, making it difficult to predict or control the vascular bed adequately [21].
In addition, Draganov et al. suggest that submucosal fibrosis is a risk factor for hemorrhage after ESD for EEC [22], which is consistent with the results of this study. Submucosal fibrosis may deform and expose vascular structures, making these vessels more susceptible to injury during ESD. The increased stiffness of fibrous tissue can complicate dissection and heighten the risk of mechanical vascular injury during surgical manipulation. Additionally, the presence of vascularized tissue can hinder intraoperative hemostasis, with traditional techniques (e.g., electrocoagulation, hemostatic clips) often proving ineffective in controlling bleeding. Post-ESD bleeding events in EEC may also alter levels of inflammatory mediators. Researchers have identified CRP and ALB as clinical predictors that improve the accuracy of diagnosing coagulation dysfunction, as determined through multifactorial logistic regression analysis when creating a nomogram [23,24]. Previous studies have shown that when the incidence of postoperative bleeding is low, the inflammatory response in the patient is less likely to be activated, leading to a decrease in serum inflammatory markers CRP levels, and conversely, an increase in ALB levels [25,26]. In the nomogram of this study, CRP and ALB were also of predictive value for postoperative bleeding after ESD in patients with EEC. The study’s findings aligned with previous research, showing that CRP levels decreased and ALB levels increased when postoperative bleeding occurred. The possible reasons for these observations are as follows: (1) CRP is an acute phase protein typically produced by the liver at in response to inflammation or infection, resulting in elevated serum levels. (2) ALB is an important protein synthesized by the liver, playing a key role in maintaining the colloid osmotic pressure of the blood, transporting various substances, and regulating inflammatory responses in the body. A sustained decrease in ALB levels during treatment may signal disease progression and increased hepatocellular injury.
Health care providers can work well on targeted nursing countermeasures for patients with EEC undergoing ESD, according to the nomograms created in this study. (1) For patients with a history of hypertension, preoperative blood pressure control can be performed. Intraoperatively, delicate surgical techniques can be used to reduce damage to surrounding tissues to reduce the likelihood of bleeding. The patient’s blood pressure and vital signs should be closely monitored after surgery to promptly detect and treat any signs of bleeding. (2) For patients with lesion diameter >3.45 cm and submucosal fibrosis, the overall health status should be assessed in detail, including the size, location and depth of the lesion, as well as the patient’s coagulation function, etc. Intraoperatively, appropriate lesion resection methods should be applied, and precise hemostatic techniques, such as electrocoagulation and hemostatic clips, need to be used to control possible bleeding points. Postoperatively, the patient’s vital signs and endoscopic wound should be monitored closely to detect and deal with bleeding promptly. Also, based on the patient’s specific situation, medications such as proton pump inhibitors can be administered to prevent bleeding by reducing the secretion of gastric acid, thereby minimizing irritation to the postoperative wound and lowering the risk of bleeding. For patients with significant changes in blood inflammation makers such as CRP and ALB, immediate endoscopic hemostasis may be necessary. This can include techniques such as spraying the wound with ice-cold saline containing norepinephrine. Care should be taken to avoid aggravating the inflammatory response. It is also crucial to closely monitor the patient’s serum inflammation markers to assess the degree of inflammation and the systemic response [27,28].
Conclusion
This study established an easy-to-read and intuitive nomogram to predict bleeding after ESD in patients with EEC. The nomogram showed that the AUC of the internal validation of the model group was 0.821, and the AUC of the external validation was 0.740. The actual values of the internal validation and external validation were well fitted with the predicted values, indicating that the nomogram had good accuracy and consistency. However, it is worth noting that there are some limitations in this study. For example, the prediction model has insufficient individualization and insufficient sample size, which affects the accuracy of the evaluation system. Meanwhile, whether lesion diameter >3.45 cm, CRP>58.10 mg·L-1, and ALB<23.85 g·L-1 are strong independent risk factors for hemorrhage after ESD for EEC requires further investigation in actual clinical practice.
Disclosure of conflict of interest
None.
References
- 1.Wu J, Wang Y, Cheng Y, Cheng L, Zhang L. Comprehensive landscape and future perspectives of non-coding RNAs in esophageal squamous cell carcinoma, a bibliometric analysis from 2008 to 2023. Pathol Oncol Res. 2024;30:1611595. doi: 10.3389/pore.2024.1611595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiao R, Jiang W, Xu K, Luo Q, Wang L, Zhao C. Lipid metabolism analysis in esophageal cancer and associated drug discovery. J Pharm Anal. 2024;14:1–15. doi: 10.1016/j.jpha.2023.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakanaka K. Treatment strategy for early-stage esophageal cancer. Jpn J Radiol. 2024;42:677–684. doi: 10.1007/s11604-024-01547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatta W, Koike T, Uno K, Asano N, Masamune A. Management of superficial esophageal squamous cell carcinoma and early gastric cancer following non-curative endoscopic resection. Cancers (Basel) 2022;14:3757. doi: 10.3390/cancers14153757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabrillac E, Dupret-Bories A, Vairel B, Woisard V, De Bonnecaze G, Vergez S. Narrow-band imaging in oncologic otorhinolaryngology: state of the art. Eur Ann Otorhinolaryngol Head Neck Dis. 2021;138:451–458. doi: 10.1016/j.anorl.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Cheng YQ, Wang GF, Zhou XL, Lin M, Zhang XW, Huang Q. Early adenocarcinoma mixed with a neuroendocrine carcinoma component arising in the gastroesophageal junction: a case report. World J Gastrointest Oncol. 2024;16:563–570. doi: 10.4251/wjgo.v16.i2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motomura D, Bechara R. Complete circumferential endoscopic submucosal dissection for early Barrett’s neoplasia. Gastrointest Endosc. 2024;99:337–345. doi: 10.1016/j.gie.2023.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Tahara T, Shijimaya T, Nishimon S, Kobayashi S, Matsumoto Y, Nakamura N, Okazaki T, Takahashi Y, Tomiyama T, Honzawa Y, Fukata N, Fukui T, Naganuma M. Injury to the muscle layer and risk of non-cardiac chest pain after endoscopic submucosal dissection for esophageal cancer. J Gastrointestin Liver Dis. 2024;33:25–29. doi: 10.15403/jgld-5133. [DOI] [PubMed] [Google Scholar]
- 9.Gao SG, Qi ZP, Qi YJ, Hou YY, Liu YW, Li MX, Li B, Sun D, Shi Q, Cai SL, Zhou PH, Zhong YS. Porphyromonas gingivalis predicts local recurrence after endoscopic submucosal dissection of early esophageal squamous cell carcinoma or precancerous lesion. BMC Cancer. 2023;23:43. doi: 10.1186/s12885-022-10469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Luo K, Zhou X, Yang S. Effect of endoscopic mucosal resection and endoscopic submucosal dissection on postoperative wound bleeding-related complications in patients with superficial esophageal cancer: a meta-analysis. Int Wound J. 2024;21:e14702. doi: 10.1111/iwj.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libânio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc. 2016;84:572–586. doi: 10.1016/j.gie.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Hatta W, Koike T, Abe H, Ogata Y, Saito M, Jin X, Kanno T, Uno K, Asano N, Imatani A, Masamune A. Recent approach for preventing complications in upper gastrointestinal endoscopic submucosal dissection. DEN Open. 2021;2:e60. doi: 10.1002/deo2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furue Y, Yoda Y, Hori K, Nakajo K, Kadota T, Murano T, Shinmura K, Ikematsu H, Yano T. Outcomes of repeated endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma on endoscopic resection scar. Dis Esophagus. 2024;37:doae018. doi: 10.1093/dote/doae018. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka I, Tarasawa K, Saito H, Hirasawa D, Fujimori K, Fushimi K, Matsuda T. Is proton-pump inhibitor effective in preventing postoperative bleeding after esophageal endoscopic submucosal dissection? Dis Esophagus. 2024;37:doad060. doi: 10.1093/dote/doad060. [DOI] [PubMed] [Google Scholar]
- 15.Hao J, Dang P, Quan X, Chen Z, Zhang G, Liu H, Shi T, Yan Y. Risk factors, prediction model, and prognosis analysis of myocardial injury after acute upper gastrointestinal bleeding. Front Cardiovasc Med. 2022;9:1041062. doi: 10.3389/fcvm.2022.1041062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Peng D, Liu D, Li R. The feasibility of endoscopic resection for colorectal laterally spreading tumors. Updates Surg. 2023;75:2235–2243. doi: 10.1007/s13304-023-01650-0. [DOI] [PubMed] [Google Scholar]
- 17.Hotca AE, Jacobi A, Bloom JR, Hsieh K, Cherry DR, Sheu R, Runnels J, Moshier E, Fu W, Sahni G, Goodman KA. The role of coronary artery calcium score to assess risk of cardiovascular disease in irradiated esophageal cancer patients. Int J Radiat Oncol Biol Phys. 2023;117:E302. [Google Scholar]
- 18.Lin TY, Su TH. Progression of portal hypertension after atezolizumab plus bevacizumab for hepatocellular carcinoma-report a case and literature review. J Formos Med Assoc. 2024;123:916–919. doi: 10.1016/j.jfma.2024.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Zheng H, Wu Z, Wu Y, Mo S, Dai W, Liu F, Xu Y, Cai S. Laparoscopic surgery may decrease the risk of clinical anastomotic leakage and a nomogram to predict anastomotic leakage after anterior resection for rectal cancer. Int J Colorectal Dis. 2019;34:319–328. doi: 10.1007/s00384-018-3199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Yu J, Yao N, Li X, Yang Y, Wang L, Sun M, Guo Y, Wang H, Yan S, Li B. Efficacy and safety of four different endoscopic treatments for early esophageal cancer: a network meta-analysis. J Gastrointest Surg. 2022;26:1097–1108. doi: 10.1007/s11605-022-05276-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Shan L, Peng C, Cong B, Zhao X. Learning curve for minimally invasive oesophagectomy of oesophageal cancer and survival analysis. J Cardiothorac Surg. 2021;16:328. doi: 10.1186/s13019-021-01712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draganov PV, Aihara H, Karasik MS, Ngamruengphong S, Aadam AA, Othman MO, Sharma N, Grimm IS, Rostom A, Elmunzer BJ, Jawaid SA, Westerveld D, Perbtani YB, Hoffman BJ, Schlachterman A, Siegel A, Coman RM, Wang AY, Yang D. Endoscopic submucosal dissection in North America: a large prospective multicenter study. Gastroenterology. 2021;160:2317–2327. e2. doi: 10.1053/j.gastro.2021.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Xia S, Lin Y, Chen Y, Xian L, Yang Y, Qiu X, Xu L, Xingshu Z, Chen D, Xia X, Zuo Y, Wang S. The role of coagulopathy and subdural hematoma thickness at admission in predicting the prognoses of patients with severe traumatic brain injury: a multicenter retrospective cohort study from China. Int J Surg. 2024 doi: 10.1097/JS9.0000000000001650. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang Y, Xia S, Chen J, Ke J, Lin S, Lin Q, Tang X, Huang H, Zheng N, Wang Y, Chen F. Construction of a prediction model for rebleeding in patients with acute upper gastrointestinal bleeding. Eur J Med Res. 2023;28:351. doi: 10.1186/s40001-023-01349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Jiang H, Ming CY, Wang Y, Mao D, Wei YF. Influence of different kinds of surgical resection on operation-related clinical indexes, inflammatory cytokines and complications in elderly patients with esophageal cancer. Pak J Med Sci. 2020;36:532–537. doi: 10.12669/pjms.36.3.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang QY, Lu F, Li AM. The clinical value of high mobility group box-1 and CRP/Alb ratio in the diagnosis and evaluation of sepsis in children. Eur Rev Med Pharmacol Sci. 2022;26:6361–6366. doi: 10.26355/eurrev_202209_29662. [DOI] [PubMed] [Google Scholar]
- 27.Yoshinami Y, Nishimura E, Hosokai T, Yamamoto S, Matsuda S, Nomura M, Kawakubo H, Kato K, Kitagawa Y. Rare malignant neoplasm of the esophagus: current status and future perspectives. Jpn J Clin Oncol. 2024;54:111–120. doi: 10.1093/jjco/hyad144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brigden T, Mitchell C, Redrup Hill E, Hall A. Ethical and legal implications of implementing risk algorithms for early detection and screening for oesophageal cancer, now and in the future. PLoS One. 2023;18:e0293576. doi: 10.1371/journal.pone.0293576. [DOI] [PMC free article] [PubMed] [Google Scholar]