Abstract
Purpose: This study aimed to determine the optimal combination mode of intermittent pneumatic compression (IPC) when combined with hyperthermia (IPCH) at varying temperatures to prevent lower extremity deep vein thrombosis (DVT) following orthopedic surgery. Methods: Hemodynamic data from a previous experiment on the physiological state and the IPCH (40°C) group were utilized as the control group. Twenty adult male white rabbits, weighing 2.6±0.3 kg, underwent simulated hip surgery and were randomly assigned to the IPCH (42°C) group or the IPCH (45°C) group, with 10 rabbits in each group. IPCH treatment at 42°C and 45°C was administered to the respective groups on the first day post-operation. Hemodynamic data were assessed before and after treatment, followed by finite element hemodynamic analysis using analytical fluid dynamics. Results: In the IPCH (45°C) group, the blood flow velocity distribution vector, total deformation of the femoral vein and venous valve, and equivalent stress of the venous valve were significantly superior compared to the IPCH (40°C) and IPCH (42°C) groups. The increase in equivalent stress of the femoral vein was slightly less than in the IPCH (42°C) group, albeit not statistically significant. Conclusion: IPCH at 45°C demonstrates superior efficacy in enhancing deep venous blood return in the lower extremities compared to IPCH at 40°C and 42°C. Moreover, it maintains the femoral vein and venous valve closer to their physiologic state without elevating the risk of injury or rupture.
Keywords: Deep vein thrombosis, intermittent pneumatic compression, hyperthermia, hemodynamics, finite element analysis
Introduction
Deep vein thrombosis (DVT) is a prevalent complication in orthopedics, typically affecting the deep veins of the lower extremities [1]. Its incidence can reach 40%-60% in the absence of preventive interventions, often presenting no clinical symptoms during the initial stages [2,3]. Among the physical prophylactic measures used post-orthopedic surgery, intermittent pneumatic compression (IPC) stands out for its simplicity, minimal contraindications, and low risk of complication, thus it is widely adopted in clinical settings [4,5]. Conversely, hyperthermia has been identified as capable of enhancing local blood flow and dilating blood vessels, thereby promoting effective blood circulation and thwarting DVT development [6,7]. Our prior investigations, based on animal experiments and finite element hemodynamic analysis, delved into the initial efficacy of IPC combined with hyperthermia (IPCH). Results indicated that IPCH closely mimicked physiologic conditions regarding key hemodynamic features, such as blood flow velocity distribution, wall shear stress, total displacement of the femoral vein and venous valves, and equivalent stress of the femoral vein and venous valves. This reinforced the notion that IPCH outperformed IPC or hyperthermia in promoting blood return within the lower limb deep veins. Notably, the hemodynamic data of the 40°C IPCH group aligning with the physiological state are outlined in Table 1 [8]. Nonetheless, our IPCH treatment thus far has been limited to 40°C, leaving unexplored the optimal temperature for enhancing lower limb blood circulation. Kumaran et al. [9] highlighted the effectiveness of moderate temperature increments in boosting cellular metabolism, with heat-induced vasodilation further amplifying local tissue blood circulation. Notably, when local tissue temperatures range between 36°C and 45°C, there is a rapid and substantial increase in blood perfusion, reaching peak significance at 42°C-45°C [10-12]. Consequently, varying IPCH temperatures may induce diverse hemodynamic changes, with a moderate temperature elevation, possibly leading to enhanced lower limb blood circulation. This study sought to undertake IPCH treatment at different temperatures to identify the most effective temperature for facilitating the return of deep veins within the lower limbs. By elucidating the optimal IPCH configuration, this research aimed to offer new and improved preventive strategies for clinical DVT management.
Table 1.
Hemodynamic outcomes of physiological state and IPCH (40°C) group
| Hemodynamic outcome | Physiological state | IPCH (40°C) |
|---|---|---|
| Blood flow velocity distribution vector (m·s^-1) | 0.55 | 0.33 |
| Wall shear stress (Pa) | 21.04 | 132 |
| Total deformation of the femoral vein (μm) | 7.33 | 22.69 |
| Total deformation of the venous valve (μm) | 34.24 | 15.47 |
| Equivalent stress of femoral vein (M Pa) | 0.0084 | 0.0373 |
| Equivalent stress of venous valve (M Pa) | 0.117 | 0.087 |
IPCH: intermittent pneumatic compression combined with hyperthermia.
Materials and methods
Experimental animals
Given that the experimental data for the physiological state and IPCH (40°C) groups have been previously documented, our current study focused solely on including experimental animals in the IPCH (42°C) and IPCH (45°C) groups. A cohort of 20 healthy male New Zealand Large White rabbits, with 10 rabbits allocated to each group, weighing 2.64±0.42 kg, were acquired from the Animal Experimentation Center of Xinjiang Medical University (No. SCXK (Xin) 2018-0002). The rabbits were individually housed in single cages to ensure independent feeding, maintaining a circadian rhythm balance indoors at a room temperature of 22-24°C and humidity levels between 50% and 60%. They were fed once in the morning and once in the evening with free access to water. Prior to commencing the study, ethical approval was secured from the Animal Experiment Center of Xinjiang Medical University.
Drugs and devices
The anesthetics used were Xylazine Hydrochloride Injection (Dunhua Shengda Animal Drugs Co., Ltd.; batch no. 20201101, 2 ml/bottle) and Zoletil®50 (Vickers Ltd., France; batch no. 83888105; 250 mg/bottle). The intermittent pneumatic compression device employed was from the Guangzhou Longzhijie company (LGT-2200HN expert type). A temperature-controlled heating plate (custom-made heating soft plate) with a temperature setting range of 40 to 65°C was utilized. Additionally, a color Doppler blood flow imaging device (GE LOGIQ E9, ML6-15 probe, operating at frequencies of 6 to 15 MHz) was used, with the angle between the speed of sound and blood flow being less than 60°.
Modeling software
The modeling software utilized in this study comprised Mimics 21.0 (Materialise, Belgium), Geomagic Studio 2014 (3D Systems, USA), Unigraphics NX 10.0 (Siemens, Germany), and ANSYS Workbench 2019 (ANSYS, USA). The computations were performed on a Dell Precision T7600 desktop workstation equipped with an E5-2643 Central Processing Unit, 32 GB of RAM, a Professional Graphics card (NVIDIA Quadro 4000), and operated on a Windows 7 Flagship 64-bit operating system.
Modeling methods
Twenty male Great White rabbits underwent simulated hip surgery under anesthesia. An initial anesthetic mixture of Zoletil®50 and Xylazine Hydrochloride Injection at a dose of 0.1 ml/kg was administered intramuscularly in the rabbits’ dorsal region. Once successfully anesthetized, the rabbits were positioned in the right lateral recumbent stance. The fur surrounding the left hip region was depilated and thoroughly cleansed with water, with the area sterilized. A sterile cloth was placed, and a 3 cm incision was made along the lateral aspect of the left hip. The muscle tissue was gently dissected towards the proximal area of the left femur, with the insertion of a titanium alloy self-tapping locking screw (1.6 mm length, 0.6 mm diameter) following local drilling and hemostasis. The incision was meticulously sutured, and gauze was applied to secure the screws. Color Doppler Flow Imaging (CDFI) was utilized to assess hemodynamic parameters of the left femoral vein pre- and post-modeling, focusing on blood flow rate, cardiac output, heart rate, and general blood flow conditions. Modeling success was determined by a significant reduction in hemodynamic parameters post-procedure, and a notable difference was observed compared to pre-modeling values.
Therapeutic programs
Following the operative procedures, each group’s experimental protocol was executed on a subsequent day: employing a random number table method, the 20 rabbits were allocated to the IPCH (42°C) and IPCH (45°C) groups. The IPCH (42°C) group underwent a regimen of IPC combined with hyperthermia at 42°C, while the IPCH (45°C) group received a similar intervention at 45°C. The IPC pressure setting was 60 mmHg, maintained for 20 minutes. Subsequent to the treatment regimen, hemodynamic evaluations were conducted on the experimental animals in each group using CDFI.
Acquisition of hemodynamic parameters
Following the treatment, CDFI was utilized to assess various hemodynamic parameters of the femoral vein within 1 minute post-treatment, including mean flow velocity, peak flow velocity, blood flow rate, vascular diameter, heart rate, resistance index, and other relevant metrics. Subsequent to the experimental procedures and completion of the CDFI evaluation in each group, rabbits were euthanised by air embolisation. The rabbits received a dorsal intramuscular injection of an anesthetic solution composed of Zoletil®50 and Xylazine Hydrochloride Injection at a dosage of 0.1 ml/kg. Once adequately anesthetized, the area behind the auricle was gently cleansed with moistened gauze to expose the large marginal ear vein. A syringe containing 10 ml of air was then inserted parallel to the marginal ear vein, and the air was gradually administered into the vein. The absence of breathing and heartbeat indicated successful euthanasia of the rabbit.
Finite element hemodynamic analysis
Finite element hemodynamic analysis was conducted to determine key parameters of blood flow. The Reynolds number (Re) was calculated using the formula Re = ρvd/μ, where ρ represents blood density (kg/m3), V is the average blood flow velocity (m/s), D stands for the inner diameter of the blood vessel (m), and μ indicates hemodynamic viscosity (Pa.s/m2). These parameters were then utilized to assess the status of blood flow, as demonstrated in Table 2. Subsequently, the hemodynamic parameters were applied to a femoral vein model with adjusted assignment conditions from a previous study on the IPCH (40°C) group. Material properties such as Young’s modulus of the venous wall (1 MPa), Young’s modulus of the venous valve (0.2 MPa), Poisson’s ratio (0.45), and density (1150 kg/m3) were employed for the linear elastic model of the blood vessel. Blood properties were considered to be incompressible viscous Newtonian fluid, with a hemodynamic viscosity of 0.0035 Pa•s and density of 1060 kg/m3. Based on ultrasonic findings and Re calculations, a laminar flow model was adopted for the blood flow in rabbit veins; the distal femoral vein and great saphenous vein served as inlet veins, and the proximal femoral vein as the outlet vein. Numerical simulations were performed using a bidirectional fluid-structure coupling method meshed by a dynamic grid approach for both the blood vessel and blood models. The simulation utilized a time step of 0.06 s with a total duration of 3 s. Both the initial and boundary conditions were maintained in alignment with the experimental parameters established in prior studies.
Table 2.
Femoral venous hemodynamic values of the two groups (n=10)
| Hemodynamic value | IPCH (42°C) | IPCH (45°C) |
|---|---|---|
| Mean blood velocity (cm/s) | 7.7±0.44 | 8.07±0.39 |
| Peak blood velocity (cm/s) | 15.18±1.54 | 18.95±1.17 |
| Blood flow per minute (ml/min) | 11.45±0.94 | 13.2±0.99 |
| Vessel diameter (cm) | 0.19±0.01 | 0.19±0.01 |
| Heart rate (Times/min) | 154.6±7.57 | 162.4±6.45 |
| Reynolds number (Re) | 38.40 | 40.25 |
| Blood flow state | Laminar flow | Laminar flow |
IPCH: intermittent pneumatic compression combined with hyperthermia.
Outcome indicators
Utilizing finite element hemodynamic analysis, simulations were conducted to examine hemodynamic behavior under various conditions, resulting in the derivation of pertinent observation indices within the framework of predefined control equations. The entire finite element analysis process was executed computationally, with boundary conditions determined based on previously acquired hemodynamic parameters. The software application was employed to model blood flow velocity in the femoral vein across different states, enabling the generation of a distribution vector depicting femoral vein blood flow velocity. As blood traverses the femoral vein, it imparts stress on both the vein itself and its venous valves. Through the use of a computer algorithm, the total deformation and equivalent stress distribution of the femoral vein and venous valve were determined under varying blood flow conditions. Additionally, the total deformation and equivalent force were computed at intervals of 0.06 s to track changes in these parameters over time, offering insights into the dynamic behavior of the femoral vein and venous valve.
Statistical methods
Data analysis was performed using SPSS version 26.0 software. Measurements conforming to a normal distribution were presented as mean ± standard deviation (x̅±S). Paired-sample t-tests were conducted to evaluate the hemodynamic parameters pre- and post-modeling, with statistical significance denoted by P < 0.05.
Results
Blood flow velocity distribution vector
The blood flow velocity distribution exhibited a pattern across the three groups, being higher near the axial flow, lower near the lateral flow, and peaking at the central open region of the venous valve. As the temperature rose incrementally, a corresponding increase in the blood flow velocity distribution was observed in all groups, with the IPCH (45°C) group demonstrating the most substantial enhancement, surpassing the improvements seen in the IPCH (40°C) and IPCH (42°C) groups (Figure 1).
Figure 1.
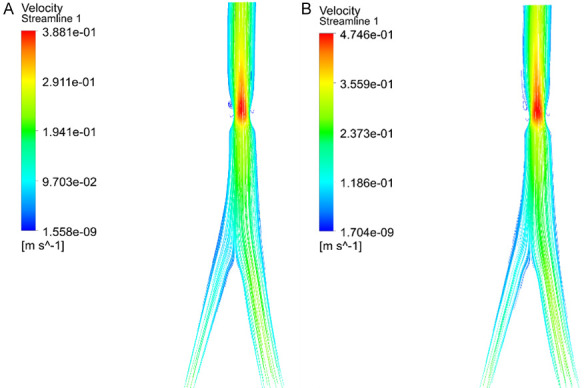
Blood velocity distribution vector of femoral vein. A. IPCH (42°C); B. IPCH (45°C). IPCH: intermittent pneumatic compression combined with hyperthermia.
Total deformation of the femoral vein and venous valve
Femoral vein
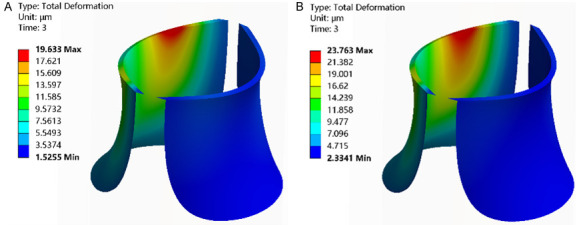
In all three groups, the region with the maximum total deformation was identified at the point where the saphenous vein intersects the femoral vein (saphenous femoral junction), displaying a decrement in deformation values from the central to the peripheral areas. Notably, while the overall deformation consistently rose, the high deformation region remained consistent. A comparison revealed higher total deformation in the IPCH (42°C) and IPCH (45°C) groups than in the IPCH (40°C) group, with the IPCH (45°C) group showing the most significant increase (Figure 2).
Figure 2.
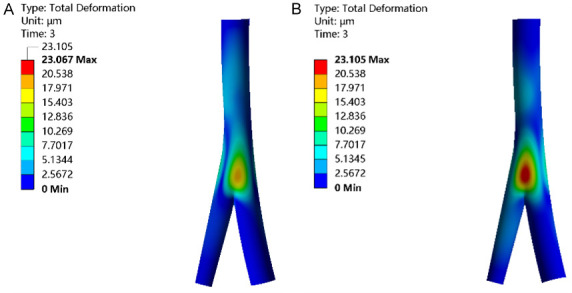
Total deformation of venous valve. A. IPCH (42°C); B. IPCH (45°C). IPCH: intermittent pneumatic compression combined with hyperthermia.
Venous valve
The most notable total deformation in the venous valve persisted predominantly within the middle and upper sections of the left valve, whereas the total deformation in the right venous valve was comparatively minor. In comparison to the IPCH (40°C) group, both the IPCH (42°C) and IPCH (45°C) groups exhibited a considerable rise in total deformation of the venous valve, with the IPCH (45°C) group demonstrating the most substantial increase (Figure 3).
Figure 3.
Equivalent stress of femoral vein. A. IPCH (42°C); B. IPCH (45°C). IPCH: intermittent pneumatic compression combined with hyperthermia.
Changes in the femoral vein and venous valve total deformation with time
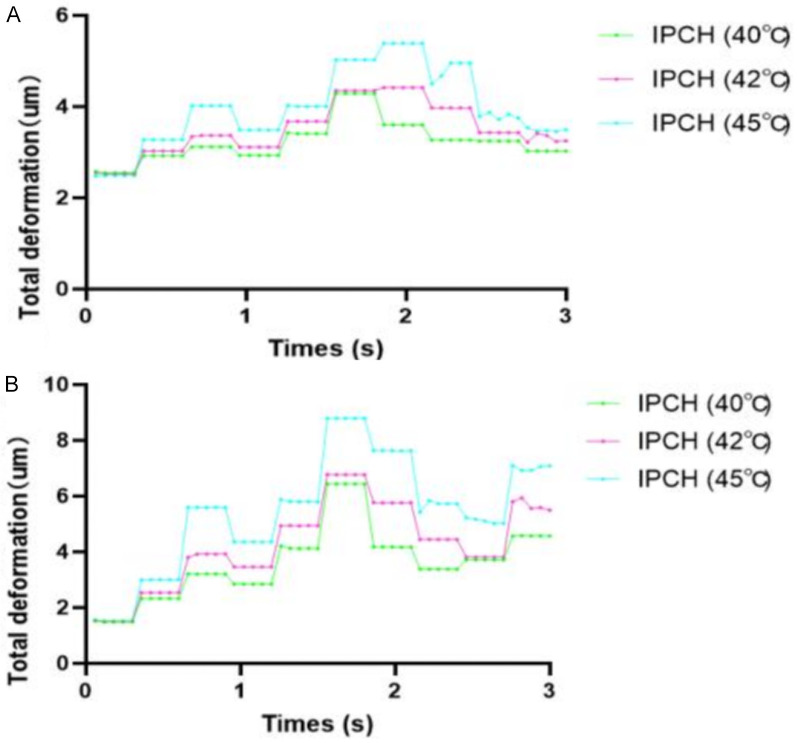
The total deformation of both the femoral vein and venous valve exhibited a pattern characterized by an initial increase followed by a subsequent decrease. Notably, the femoral vein deformation across the three groups displayed a gradual but significant rise post 0.30 seconds, peaking at 1.56 seconds before declining. The IPCH (45°C) group demonstrated the most pronounced elevation, with its peak significantly surpassing that of the other groups and maintaining superiority even when the venous valve neared closure. Similarly, the general deformation trend of the venous valve mirrored that of the femoral vein in the three groups, with the IPCH (45°C) group showcasing the most substantial increase. However, the durability of this higher deformation was comparatively shorter in the venous valve, rapidly decreasing upon valve opening. Nevertheless, the total deformation in the IPCH (45°C) group outstripped that in the IPCH (40°C) and IPCH (42°C) groups (Figure 4).
Figure 4.
Relationship between total deformation and time. A. Femoral vein; B. Venous valve. IPCH: intermittent pneumatic compression combined with hyperthermia.
Equivalent stress distribution of femoral vein and venous valve
Femoral vein
Across all three groups, the highest equivalent stress levels were observed at the saphenous femoral junction, with stress values peaking closer to the central region and diminishing towards the periphery. Comparatively, the IPCH (42°C) and IPCH (45°C) groups exhibited slightly elevated equivalent stress levels in contrast to the IPCH (40°C) group, with the most substantial increase noted in the IPCH (42°C) group (Figure 5).
Figure 5.
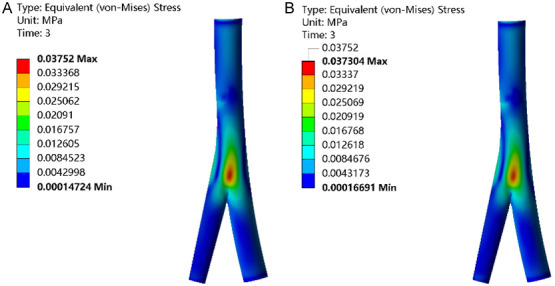
Equivalent stress of femoral vein. A. IPCH (42°C); B. IPCH (45°C). IPCH: intermittent pneumatic compression combined with hyperthermia.
Venous valve
Consistently, the distribution of equivalent stress values across the three groups revealed a predominant concentration in the left valve, notably clustered in the upper posterior section, while the right valve exhibited lower stress levels. Notably, the equivalent stress in the venous valve was higher within the IPCH (42°C) and IPCH (45°C) groups compared to the IPCH (40°C) group, with the IPCH (45°C) group showing the most notable increase (Figure 6).
Figure 6.
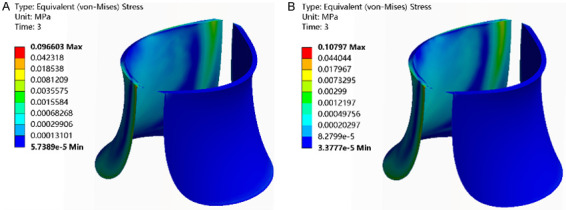
Equivalent stress of venous valve. A. IPCH (42°C); B. IPCH (45°C). IPCH: intermittent pneumatic compression combined with hyperthermia.
The change of equivalent stress of femoral vein and venous valve with time
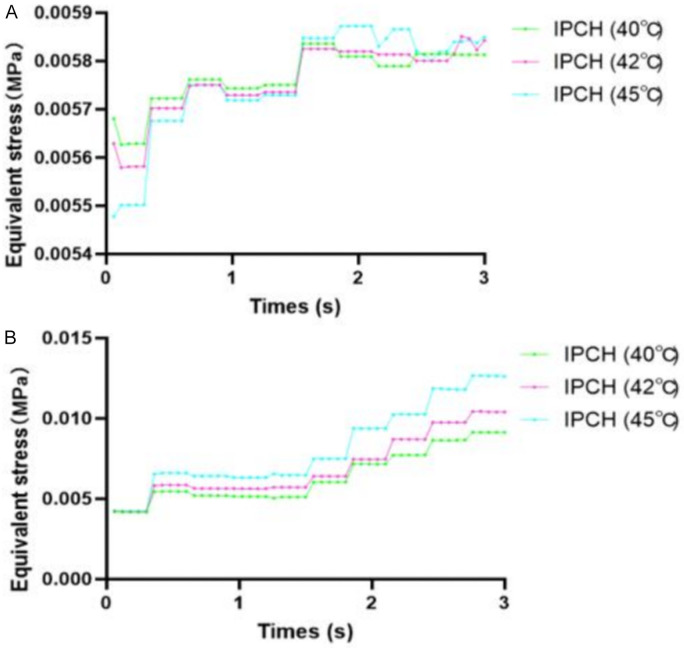
From the onset at 0 s until 1.56 s, the equivalent stress in the femoral vein of the IPCH (45°C) group initially lagged behind the other two groups, gradually catching up and slightly surpassing their levels thereafter. However, the disparities among the three groups were not statistically significant. Across the board, the equivalent stress in the venous valves experienced a slight uptick with ongoing blood impact before stabilizing. Subsequent to 1.56 s and the opening of the venous valve, there was a considerable increase in equivalent stress across all groups. While the IPCH (45°C) group exhibited the most substantial rise, the distinctions among the groups were not significant (Figure 7).
Figure 7.
Relationship between equivalent stress and time. A. Femoral vein; B. Venous valve. IPCH: intermittent pneumatic compression combined with hyperthermia.
Discussion
The main finding of this study was that the efficacy of IPCH (45°C) surpassed that of IPCH (40°C) and IPCH (42°C) interventions, despite the fact that the femoral vein exhibited increased deformation and equivalent stress levels under IPCH (45°C) conditions.
The preventive effect of IPC on DVT is mainly achieved through mechanical and biochemical effects [13]. The mechanical effect involves mimicking the “muscle pump” in the calf region, which exerts mechanical pressure on the deep veins of the lower extremities [14]. Conversely, the biochemical effect results from the extrusion-induced release of vasoactive substances by IPC, thereby reducing blood viscosity, enhancing venous return, and lowering the likelihood of DVT occurrence [15-17]. Hyperthermia, on the other hand, primarily targets local veins through thermal stimulation, triggering the activation of vasoactive substances and ATP release, culminating in improved endothelial cell viability and vasodilation [9]. Simultaneously, it augments myofibril activity and Ca2+ sensitivity, which enhances muscular contraction force and ultimately enhances blood circulation [18,19]. Our previous investigation revealed that the combined application of IPC and IPCH synergistically enhanced lower limb blood circulation compared to the individual interventions [8]. Sekins et al. [12] applied microwave diathermy therapy to induce local thigh heating, observing a substantial rise in muscle blood flow and blood vessel velocity when the local temperature exceeded 42°C. Akyurekli et al. [10] conducted a study on porcine skeletal muscle, demonstrating a fourfold surge in local blood flow and velocity within 15 to 30 min at 44.5°C hyperthermia application. Thus, it is postulated that within a safe temperature range, higher temperatures lead to more pronounced enhancement in local blood circulation and increased venous return. Specifically, temperatures ranging from 42°C to 45°C exhibit the most significant elevation in local blood flow and velocity.
In conjunction with the beneficial impact of IPC, increasing the local hyperthermia temperature correlates with a more pronounced enhancement in promoting lower limb blood circulation [11,20]. Moreover, our investigation revealed that IPCH at varying temperatures significantly improved the blood flow velocity vector of the femoral vein in all groups, with higher temperatures of IPCH demonstrating a more effective enhancement in promoting blood return within the deep veins of the lower extremities.
IPCH induced considerable deformation of the femoral vein and venous valve by incrementally combining pressure and thermal stimulation [8]. Despite the markedly higher total deformation of the femoral vein observed in the IPCH (45°C) group compared to both the physiological state and the other groups, it is important to note that greater deformations result in more pronounced vessel deformities and increased extrusion effects on blood within the lumen, consequently facilitating more effective blood return in the vein. Notably, the total deformation of the venous valve exhibited significant alterations following IPCH treatment at varying temperatures. However, even post-treatment at 45°C, the deformation measured only 23.105 μm, considerably lower than 34.24 um under the physiological state, implying that while IPCH (45°C) effectively promotes venous blood backflow in situations of sluggish blood flow velocity, the venous valve fails to achieve optimal openness. Prolonged treatment durations may be necessary to further enhance the openness of the venous valve to its ideal physiological state.
Equivalent stress refers to the stress within the internal structures of the vessel wall. When the vessel is considered as a biological material, excessive equivalent stress prevents its elastic deformation from reaching a constant level, leading to material yielding and an elevated risk of vascular damage and rupture to some extent [21]. In the presence of both IPC and hyperthermia, the equivalent stress of the femoral vein surpasses that of the physiological state significantly. While the highest equivalent stress in the femoral vein occurred in the IPCH (42°C) group, the disparity among the three groups was not statistically significant. It is noteworthy that the equivalent stress of the femoral vein in the physiological state, where the vessel is in a relaxed state without pressure exertion, could be challenging to assess accurately. Normally, in the real physiological state, venous blood return is compressed by the “muscle pump” of the lower limbs, likely resulting in equivalent stress for the femoral vein comparable to or even higher than that under IPCH. Within 3 seconds of blood inflow, the equivalent stress of the venous valve demonstrated a gradual increase post-treatment across all groups, with the IPCH (45°C) group consistently exhibiting the most significant escalation, reaching 0.108 MPa, approximately equivalent to 0.117 MPa under physiological conditions. Hence, among the available treatment modalities, IPCH at 45°C stood out as not only the most effective in prompting deep vein return in the lower extremities but also closely mimicked the physiological conditions of the femoral vein and the venous valve, without causing harm to the vein or valve structure.
Research has indicated that integrating physical therapy with other preventive measures can significantly enhance lower limb blood circulation and prevent DVT [22]. Nevertheless, there is a paucity of studies comparing IPC in conjunction with different physical therapy modalities against IPC alone. Elbuluk et al. [23] demonstrated that IPC synchronized with respiration exhibited superior efficacy in reducing DVT and pulmonary embolism incidence compared to unsynchronized IPC. Similarly, Sakai et al. [24] observed that combining IPC with active ankle exercises yielded a more substantial improvement in blood circulation and flow rate than ankle exercises alone for DVT prevention. To date, limited research has explored the efficacy of IPCH for DVT prevention. Our previous study highlighted the superiority of IPCH over standalone IPC applications. Consequently, this study aimed to investigate the hemodynamic effects of IPCH at varying temperatures. It revealed that higher temperatures within a safe range correspond to enhanced IPCH efficacy. Although current research is animal-based and optimal adaptation temperatures differ markedly from human temperatures, the theoretical reliability of their efficacy remains intact.
The current studies have some limitations. First, the choice of the New Zealand white rabbit as the experimental animal, with comparatively smaller calf muscles than humans, may diminish the extrusion effect of IPC. Additionally, variations between human and animal adaptation to IPCH temperatures introduce potential bias into the results. Secondly, despite employing finite element analysis based on data from ultrasound and CT perfusion imaging, disparities between simulated analyses and actual physiological hemodynamic shifts introduce some degree of bias. Lastly, this study primarily focused on simulating and examining hemodynamic alterations in the femoral vein under different IPCH temperature regimens, failing to conduct a quantitative analysis of changes in vascular bioactive substances and blood components resulting from diverse treatments. Further research is necessary to substantiate the current conclusions.
Conclusions
Among IPCH at different temperatures, IPCH at 45°C demonstrates superior efficacy in enhancing blood circulation within the deep veins of the lower extremities to prevent DVT compared to IPCH at 40°C and 42°C. Notably, operating within a safe temperature range, IPCH at 45°C not only avoids causing damage to blood vessels and local soft tissues but also contributes to the generation and activation of vasoactive substances, vasodilation, and the augmentation of blood return flow and velocity.
Acknowledgements
This study was funded by the Leading Talents Project of Xinjiang Uygur Autonomous Region (No. 2022TSYCLJ0027); and the Key Research and Development Program of Xinjiang Uygur Autonomous Region (No. 2022B03011-1).
Disclosure of conflict of interest
None.
References
- 1.Jacobs B, Henke PK. Evidence-based therapies for pharmacologic prevention and treatment of acute deep vein thrombosis and pulmonary embolism. Surg Clin North Am. 2018;98:239–253. doi: 10.1016/j.suc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 3.Schiff RL, Kahn SR, Shrier I, Strulovitch C, Hammouda W, Cohen E, Zukor D. Identifying orthopedic patients at high risk for venous thromboembolism despite thromboprophylaxis. Chest. 2005;128:3364–71. doi: 10.1378/chest.128.5.3364. [DOI] [PubMed] [Google Scholar]
- 4.Dunn N, Ramos R. Preventing venous thromboembolism: the role of nursing with intermittent pneumatic compression. Am J Crit Care. 2017;26:164–167. doi: 10.4037/ajcc2017504. [DOI] [PubMed] [Google Scholar]
- 5.Morris RJ, Roberts CH. Haematological effects of intermittent pneumatic compression for deep vein thrombosis prophylaxis. Thromb Haemost. 2020;120:912–923. doi: 10.1055/s-0040-1710016. [DOI] [PubMed] [Google Scholar]
- 6.Chiesa ST, Trangmar SJ, González-Alonso J. Temperature and blood flow distribution in the human leg during passive heat stress. J Appl Physiol (1985) 2016;120:1047–58. doi: 10.1152/japplphysiol.00965.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen C, Li N, Chen B, Wu J, Wu Z, Hua D, Wang L, Chen D, Shao Z, Ren C, Xu J. Thermotherapy for knee osteoarthritis: a protocol for systematic review. Medicine (Baltimore) 2021;100:e25873. doi: 10.1097/MD.0000000000025873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Ma P, Muheremu A, Sun R, Chai H, Jiang K. Hemodynamic analysis of intermittent pneumatic compression combined with hyperthermia after total hip arthroplasty: an experiment on male rabbits. Am J Transl Res. 2022;14:3344–3359. [PMC free article] [PubMed] [Google Scholar]
- 9.Kumaran B, Watson T. Thermophysiological responses to capacitive resistive monopolar radiofrequency electromagnetic radiation in patients with osteoarthritis of the knee joint: a randomised controlled experimental study. Electromagn Biol Med. 2021;40:210–221. doi: 10.1080/15368378.2020.1846556. [DOI] [PubMed] [Google Scholar]
- 10.Akyürekli D, Gerig LH, Raaphorst GP. Changes in muscle blood flow distribution during hyperthermia. Int J Hyperthermia. 1997;13:481–96. doi: 10.3109/02656739709023547. [DOI] [PubMed] [Google Scholar]
- 11.Giombini A, Giovannini V, Di Cesare A, Pacetti P, Ichinoseki-Sekine N, Shiraishi M, Naito H, Maffulli N. Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. Br Med Bull. 2007;83:379–96. doi: 10.1093/bmb/ldm020. [DOI] [PubMed] [Google Scholar]
- 12.Sekins KM, Lehmann JF, Esselman P, Dundore D, Emery AF, deLateur BJ, Nelp WB. Local muscle blood flow and temperature responses to 915MHz diathermy as simultaneously measured and numerically predicted. Arch Phys Med Rehabil. 1984;65:1–7. [PubMed] [Google Scholar]
- 13.Chen AH, Frangos SG, Kilaru S, Sumpio BE. Intermittent pneumatic compression devices -- physiological mechanisms of action. Eur J Vasc Endovasc Surg. 2001;21:383–92. doi: 10.1053/ejvs.2001.1348. [DOI] [PubMed] [Google Scholar]
- 14.Pavon JM, Adam SS, Razouki ZA, McDuffie JR, Lachiewicz PF, Kosinski AS, Beadles CA, Ortel TL, Nagi A, Williams JW Jr. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical patients: a systematic review. J Arthroplasty. 2016;31:524–32. doi: 10.1016/j.arth.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Dai G, Tsukurov O, Chen M, Gertler JP, Kamm RD. Endothelial nitric oxide production during in vitro simulation of external limb compression. Am J Physiol Heart Circ Physiol. 2002;282:H2066–75. doi: 10.1152/ajpheart.00288.2001. [DOI] [PubMed] [Google Scholar]
- 16.Kohro S, Yamakage M, Sato K, Sato JI, Namiki A. Intermittent pneumatic foot compression can activate blood fibrinolysis without changes in blood coagulability and platelet activation. Acta Anaesthesiol Scand. 2005;49:660–4. doi: 10.1111/j.1399-6576.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 17.Rifkind JM, Nagababu E, Dobrosielski DA, Salgado MT, Lima M, Ouyang P, Silber HA. The effect of intermittent pneumatic compression of legs on the levels of nitric oxide related species in blood and on arterial function in the arm. Nitric Oxide. 2014;40:117–22. doi: 10.1016/j.niox.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Ishii S, Oyama K, Shintani SA, Kobirumaki-Shimozawa F, Ishiwata S, Fukuda N. Thermal activation of thin filaments in striated muscle. Front Physiol. 2020;11:278. doi: 10.3389/fphys.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Périard JD, Eijsvogels TMH, Daanen HAM. Exercise under heat stress: thermoregulation, hydration, performance implications, and mitigation strategies. Physiol Rev. 2021;101:1873–1979. doi: 10.1152/physrev.00038.2020. [DOI] [PubMed] [Google Scholar]
- 20.Inoue S, Takemoto M, Chishaki A, Ide T, Nishizaka M, Miyazono M, Sawatari H, Sunagawa K. Leg heating using far infra-red radiation in patients with chronic heart failure acutely improves the hemodynamics, vascular endothelial function, and oxidative stress. Intern Med. 2012;51:2263–70. doi: 10.2169/internalmedicine.51.7115. [DOI] [PubMed] [Google Scholar]
- 21.Schwiedrzik JJ, Wolfram U, Zysset PK. A generalized anisotropic quadric yield criterion and its application to bone tissue at multiple length scales. Biomech Model Mechanobiol. 2013;12:1155–68. doi: 10.1007/s10237-013-0472-5. [DOI] [PubMed] [Google Scholar]
- 22.Herring B, Lowen D, Ho P, Hodgson R. A systematic review of venous thromboembolism mechanical prophylaxis devices during surgery. Langenbecks Arch Surg. 2023;408:410. doi: 10.1007/s00423-023-03142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elbuluk AM, Kim KY, Chen KK, Anoushiravani AA, Schwarzkopf R, Iorio R. Respiratory synchronized versus intermittent pneumatic compression in prevention of venous thromboembolism after total joint arthroplasty: a systematic review and meta-analysis. Orthop Clin North Am. 2018;49:123–133. doi: 10.1016/j.ocl.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Sakai K, Takahira N, Tsuda K, Akamine A. A novel device for lower leg intermittent pneumatic compression synchronized with active ankle exercise for prevention of deep vein thrombosis. Phlebology. 2022;37:507–515. doi: 10.1177/02683555221089618. [DOI] [PubMed] [Google Scholar]