Abstract
Objective: To explore the correlation between serum interleukin-22 (IL-22) and interleukin-27 (IL-27) levels and vasculopathy in patients with diabetic nephropathy (DN). Methods: A total of 104 DN patients treated at the Shanxi University of Traditional Chinese Medicine Affiliated Hospital were selected as the observation group, with another 104 healthy individuals, serving as the control group in this retrospective study. The baseline data and the serum levels of IL-22 and IL-27 were compared between the two groups. The observation group was divided into three subgroups based on their urinary albumin excretion rate (UAER): clinical albuminuria group (microangiopathy, 29 patients), microalbuminuria group (51 patients), and normal albuminuria group (24 patients). Logistic regression was used to analyze the factors influencing the occurrence of microangiopathy. According to whether they had major adverse cardiovascular events (MACE) during 6-month follow-up, the DN patients were divided into a MACE group (n = 39) and a non-MACE group (n = 65). The serum levels of IL-22 and IL-27 were then compared between the two groups. The clinical utility of IL-22 and IL-27 in the assessment of microangiopathy and prognosis was evaluated through receiver operating characteristic (ROC) curve analysis. Results: Compared to the control group, the observation group exhibited significantly higher serum levels of fasting blood glucose, glycated hemoglobin, total cholesterol, triglycerides, low-density lipoprotein, uric acid, blood creatinine, cystatin C, IL-22 and IL-27, but lower glomerular filtration rate (all P<0.05). There were significant differences among different albuminuria groups in terms of duration of disease, serum levels of fasting blood glucose, low-density lipoprotein, cystatin C, IL-22, IL-27, and glomerular filtration rate (all P<0.05). Correlation analysis showed that the serum levels of IL-22 and IL-27 were positively correlated with the duration of disease and serum levels of fasting blood glucose, low-density lipoprotein, uric acid, blood creatinine and cystatin C. However, they were negatively correlated with glomerular filtration rate (P<0.05). The logistic regression analysis indicated that the glomerular filtration rate and serum levels of cystatin C, IL-22, and IL-27 were independent risk factors for the occurrence of microangiopathy. Compared to non-MACE group, the MACE group presented with higher serum IL-22 and IL-27 levels. ROC curve analysis showed that the AUC (Area Under the Curve) for combined detection (>0.9) of serum IL-22 and IL-27 levels was higher than that (>0.8) for each alone in assessing microangiopathy in DN patients. Additionally, the AUC for using serum IL-22 and IL-27 levels, whether individually or in combination, exceeded 0.7 when evaluating patient prognosis. Conclusion: Elevated serum IL-22 and IL-27 levels are closely associated with the severity of the DN and can serve as auxiliary indicators for assessing microangiopathy and prognosis in DN patients.
Keywords: Diabetic nephropathy, interleukin 22, interleukin 27, vasculopathy
Introduction
Diabetic nephropathy (DN) is the primary cause of end-stage renal disease, and epidemiological data show that 30%-40% of diabetic patients in China develop DN [1-5]. Clinically, early screening for DN is commonly performed through microalbuminuria detection [6,7]. However, the accuracy of urine microalbumin detection can be easily affected by various factors, resulting in reduced sensitivity and specificity. Therefore, exploring effective biomarkers for assessing microangiopathy in DN patients holds significant clinical importance [8,9]. Interleukin-27 (IL-27) is an inflammatory factor belonging to the Interleukin-12 (IL-12) family, known for its dual pro- and anti-inflammatory effects. On the one hand, IL-27 participates in inflammatory responses by inducing the differentiation of T cells into Th1 cells, which promote the secretion of interferon-γ; on the other hand, IL-27 can inhibit the expression of IL-17 and enhance the production of IL-10, thus exerting anti-inflammatory effects [10]. Interleukin-22 (IL-22), secreted by Th22 cells, plays an important role in inflammation and immune regulation [11]. Currently, there is limited research on the roles of IL-22 and IL-27 in the microangiopathy associated with DN. This study explores the correlation between serum IL-22 and IL-27 levels and microangiopathy in DN patients, aiming to provide a basis for clinical diagnosis and treatment.
Patients and methods
General information
From January 2022 to December 2023, 104 DN patients treated at Shanxi University of Traditional Chinese Medicine Affiliated Hospital were selected as the observation group in this retrospective study. The inclusion criteria for the observation group were as follows: (1) All patients diagnosed with DN either by renal biopsy or based on clinical characteristics, including a glomerular filtration rate of <60 mL·min-1·1.73 m-2, continuous proteinuria of ≥30 mg over 24 hours, or both [12]; (2) Type 2 diabetes; (3) Age ≥18 years.
The exclusion criteria for the observation group were as follows: (1) Type 1 diabetes or other types of diabetes; (2) Pregnancy or lactation; (3) Presence of acute diabetic complications; (4) Coexisting kidney diseases; (5) Coexisting diseases affecting other tissues or organs; (6) Neurological or psychiatric disorders; (7) Poor compliance.
Another 104 healthy people from the same period were selected as a control group. Inclusion criteria for the control group: (1) Healthy physical examination within the last 3 months; (2) Age ≥18 years. Exclusion criteria: (1) Patients in pregnancy or in lactation; (2) Patients with a family history of diabetes. This study was approved by the Ethics Committee of Shanxi University of Traditional Chinese Medicine Affiliated Hospital.
The observation group was further divided into three subgroups based on urinary albumin excretion rate (UAER) [13]: clinical albuminuria group (UAER ≥300 mg/24 h, also microangiopathy, 29 patients), microalbuminuria group (30 mg/24 h ≤ UAER <300 mg/24 h, 51 patients), and normal albuminuria group (UAER <30 mg/24 h, 24 patients).
Observation indicators
Clinical data were collected from all study subjects. A fasting venous blood sample of 5 ml was drawn from each patient in the early morning following enrollment. The blood was centrifuged at 3,000 r/min for 15 minutes to separate the serum. Serum levels of IL-22 and IL-27 were measured using an enzyme-linked immunosorbent assay (RT-6100 multifunctional microplate reader, Hamilton Company, Switzerland). All reagent kits were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd.
Follow up
The observation group was followed up by telephone and outpatient visit for 6 months. The endpoint event was major adverse cardiovascular events (MACE), which was defined as nonfatal myocardial infarction, nonfatal stroke, or deterioration of cardiac function. The DN patients were divided into a MACE group (n = 39) and a non-MACE group (n = 65) according to the occurrence of MACE.
Statistical methods
Data analysis was performed using SPSS 22.0. Quantitative data were presented as mean ± standard deviation (x̅ ± s). Multiple group comparisons were conducted using analysis of variance, with pairwise comparisons between groups performed using LSD-t test. Categorical data were presented as percentages (%) and compared using the chi-square test. Multifactor analysis was conducted using logistic regression. Correlation analysis was performed using Pearson’s method. Receiver Operating Characteristic (ROC) curves were plotted. A P-value of <0.05 was considered significant.
Results
Comparison of general data between the two groups
There were significant differences between the groups in terms of serum levels of fasting blood glucose, glycated hemoglobin, total cholesterol, triglycerides, low-density lipoprotein, uric acid, blood creatinine and cystatin C and glomerular filtration rate (all P<0.05), as shown in Table 1.
Table 1.
Comparison of general information between the two groups
| Index | Observation group (n = 104) | Control group (n = 104) | χ2/t | P |
|---|---|---|---|---|
| Age (years) | 54.2±4.9 | 53.0±6.1 | 1.564 | 0.119 |
| Sex | ||||
| Male | 59 | 53 | 0.696 | 0.404 |
| Female | 45 | 51 | ||
| Body mass index (kg/m2) | 22.78±2.24 | 22.34±1.51 | 1.661 | 0.098 |
| Systolic pressure (mmHg) | 119.28±9.67 | 117.12±10.15 | 1.571 | 0.118 |
| Diastolic blood pressure (mmHg) | 79.15±8.22 | 78.26±8.78 | 0.755 | 0.451 |
| Fasting blood glucose (mmol/L) | 9.53±1.59 | 5.24±0.47 | 26.387 | <0.001 |
| Glycosylated hemoglobin (%) | 7.47±0.72 | 5.28±0.39 | 27.275 | <0.001 |
| Total cholesterol (mmol/L) | 5.63±0.71 | 4.74±0.58 | 9.900 | <0.001 |
| Triglyceride (mmol/L) | 1.32±0.39 | 1.07±0.41 | 4.506 | <0.001 |
| Low density lipoprotein (mmol/L) | 3.53±0.55 | 2.79±0.52 | 9.970 | <0.001 |
| Uric acid (umol/L) | 473.16±82.31 | 224.57±35.23 | 28.315 | <0.001 |
| Serum creatinine (μmol/L) | 146.48±39.56 | 85.22±19.27 | 14.197 | <0.001 |
| Glomerular filtration rate (ml/min/1.73 m2) | 98.52±8.17 | 109.44±12.54 | 7.441 | <0.001 |
| Cystatin C (mg/L) | 1.37±0.39 | 0.65±0.21 | 16.577 | <0.001 |
Comparison of IL-22 and IL-27 levels between the two groups
The results showed that the IL-22 levels in the observation group and control group were 26.09±6.39 (pg/mL) and 5.98±2.17 (pg/mL) respectively, while the IL-27 levels were 24.64±5.69 (pg/mL) and 5.16±2.24 (pg/mL) respectively. The differences between the two groups were significant (t = 30.526, 32.487; both P<0.001), as shown in Figure 1.
Figure 1.
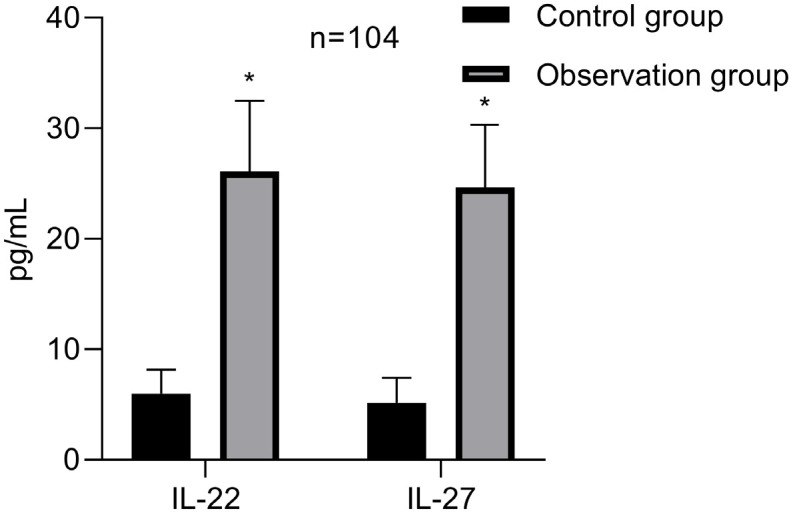
Comparison of IL-22 and IL-27 levels between the two groups. Note: *compared to the control group, P<0.05. IL-22, Interleukin-22; IL-27, interleukin-27.
Comparison of various indicators among the different albuminuria groups
There were significant differences among the different albuminuria groups in terms of duration of disease, fasting blood glucose, low-density lipoprotein, uric acid, blood creatinine, cystatin C, IL-22, IL-27 levels, and glomerular filtration rate (all P<0.05), as shown in Table 2.
Table 2.
Comparison of indicators among different urinary protein groups
| Index | Clinical proteinuria group (n = 29) | Microalbuminuria group (n = 51) | Normal proteinuria group (n = 24) | F | P |
|---|---|---|---|---|---|
| Age (years) | 54.6±3.1 | 54.1±3.0 | 53.7±3.4 | 0.557 | 0.575 |
| Sex | |||||
| Male | 17 | 27 | 15 | 0.666 | 0.717 |
| Female | 12 | 24 | 9 | ||
| Body mass index (kg/m2) | 22.95±1.31 | 22.98±1.97 | 22.13±2.28 | 1.818 | 0.168 |
| Duration of disease (years) | 12.23±2.31a,b | 10.04±2.07a | 7.55±3.20 | 24.253 | <0.001 |
| Systolic pressure (mmHg) | 120.44±7.71 | 118.29±8.75 | 119.97±7.82 | 0.735 | 0.482 |
| Diastolic blood pressure (mmHg) | 79.56±6.43 | 78.94±6.83 | 79.10±7.26 | 0.077 | 0.926 |
| Fasting blood glucose (mmol/L) | 10.42±1.01a,b | 9.36±0.75a | 8.81±0.62 | 38.820 | <0.001 |
| Glycosylated hemoglobin (%) | 7.47±0.61 | 7.50±0.52 | 7.41±0.59 | 0.209 | 0.818 |
| Total cholesterol (mmol/L) | 5.70±0.64 | 5.58±0.69 | 5.62±0.65 | 0.299 | 0.742 |
| Triglyceride (mmol/L) | 1.37±0.37 | 1.30±0.31 | 1.30±0.29 | 0.489 | 0.614 |
| Low density lipoprotein (mmol/L) | 3.68±0.27a,b | 3.51±0.20a | 3.39±0.21 | 11.420 | <0.001 |
| Uric acid (umol/L) | 496.28±48.59a,b | 472.40±64.27a | 446.86±32.28 | 5.470 | 0.006 |
| Serum creatinine (μmol/L) | 163.12±14.25a,b | 145.28±31.90a | 128.92±15.47 | 12.614 | <0.001 |
| Glomerular filtration rate (ml/min/1.73 m2) | 95.92±3.41a,b | 97.23±3.74a | 104.40±3.92 | 40.692 | <0.001 |
| Cystatin C (mg/L) | 1.62±0.24a,b | 1.34±0.21a | 1.13±0.15 | 37.750 | <0.001 |
| IL-22 (pg/mL) | 32.16±4.92 | 24.70±4.69 | 21.71±5.90 | 31.877 | <0.001 |
| IL-27 (pg/mL) | 28.58±5.01 | 24.74±4.08 | 22.54±4.21 | 13.278 | <0.001 |
compared to normal proteinuria group, P<0.05;
compared to microproteinuria group, P<0.05.
IL-22, Interleukin-22; IL-27, interleukin-27.
Correlation analysis
Correlation analysis showed that in DN patients, levels of IL-22 and IL-27 were positively correlated with the duration of the disease, fasting blood glucose, low-density lipoprotein, uric acid, blood creatinine, and cystatin C levels, but negatively correlated with glomerular filtration rate (all P<0.05), as shown in Table 3.
Table 3.
Results of correlation analysis
| Index | IL-22 (pg/mL) | IL-27 (pg/mL) | ||
|---|---|---|---|---|
|
|
|
|||
| r | P | r | P | |
| Duration of disease (years) | 0.429 | <0.001 | 0.378 | 0.037 |
| Fasting blood glucose (mmol/L) | 0.441 | <0.001 | 0.416 | <0.001 |
| Low density lipoprotein (mmol/L) | 0.398 | 0.011 | 0.412 | 0.015 |
| Uric acid (μmol/L) | 0.415 | 0.003 | 0.398 | <0.001 |
| Serum creatinine (μmol/L) | 0.442 | <0.001 | 0.425 | 0.002 |
| Glomerular filtration rate (ml/min/1.73 m2) | -0.395 | 0.005 | -0.376 | 0.022 |
| Cystatin C (mg/L) | 0.404 | <0.001 | 0.388 | 0.029 |
IL-22, Interleukin-22; IL-27, interleukin-27.
Logistic regression analysis
Variables that showed statistical significance in the univariate analysis were assigned values, as shown in Table 4. The results of the multivariate logistic regression analysis indicated that glomerular filtration rate, cystatin C, IL-22, and IL-27 levels were independent risk factors for the occurrence of microangiopathy, as shown in Table 5.
Table 4.
Assignment table
| Index | Assignment |
|---|---|
| Duration of disease (years) | ≥12.23 = 1; <12.23 = 0 |
| Fasting blood glucose (mmol/L) | ≥10.42 = 1; <10.42 = 0 |
| Low density lipoprotein (mmol/L) | ≥3.68 = 1; <3.68 = 0 |
| Uric acid (μmol/L) | ≥496.28 = 1; <496.28 = 0 |
| Serum creatinine (μmol/L) | ≥163.12 = 1; <163.12 = 0 |
| Glomerular filtration rate (ml/min/1.73 m2) | ≥95.92 = 1; >95.92 = 0 |
| Cystatin C (mg/L) | ≥1.62 = 1; <1.62 = 0 |
| IL-22 (pg/mL) | ≥32.16 = 1; <32.16 = 0 |
| IL-27 (pg/mL) | ≥28.58 = 1; <28.58 = 0 |
IL-22, Interleukin-22; IL-27, interleukin-27.
Table 5.
Logistic regression analysis results
| Index | β | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Glomerular filtration rate (ml/min/1.73 m2) | 1.550 | 0.492 | 9.925 | 4.71 | 3.10-6.84 | <0.001 |
| Cystatin C (mg/L) | 2.115 | 0.617 | 11.750 | 8.29 | 3.98-25.26 | <0.001 |
| IL-22 (pg/mL) | 0.820 | 0.295 | 7.727 | 2.27 | 1.87-5.09 | <0.001 |
| IL-27 (pg/mL) | 0.445 | 0.216 | 4.244 | 1.56 | 1.16-4.25 | 0.019 |
IL-22, Interleukin-22; IL-27, interleukin-27.
Clinical value of IL-22 and IL-27 in the assessment of microangiopathy
The ROC curve analysis showed that IL-22 or IL-27 for assessing patients’ microangiopathy yielded an Area Under the Curve (AUC) greater than 0.80. Notably, combined testing resulted in an AUC greater than 0.90, as shown in Table 6 and Figure 2.
Table 6.
ROC curve results
| Variable | Cut-off value | AUC | 95% CI | Sensitivity | Specificity | P |
|---|---|---|---|---|---|---|
| IL-22 (pg/mL) | 30.65 | 0.84 | 0.75, 0.92 | 0.83 | 0.85 | <0.001 |
| IL-27 (pg/mL) | 24.50 | 0.78 | 0.68, 0.88 | 0.76 | 0.75 | <0.001 |
| Joint assessment | - | 0.95 | 0.84, 0.97 | 0.90 | 0.84 | <0.001 |
IL-22, Interleukin-22; IL-27, interleukin-27.
Figure 2.
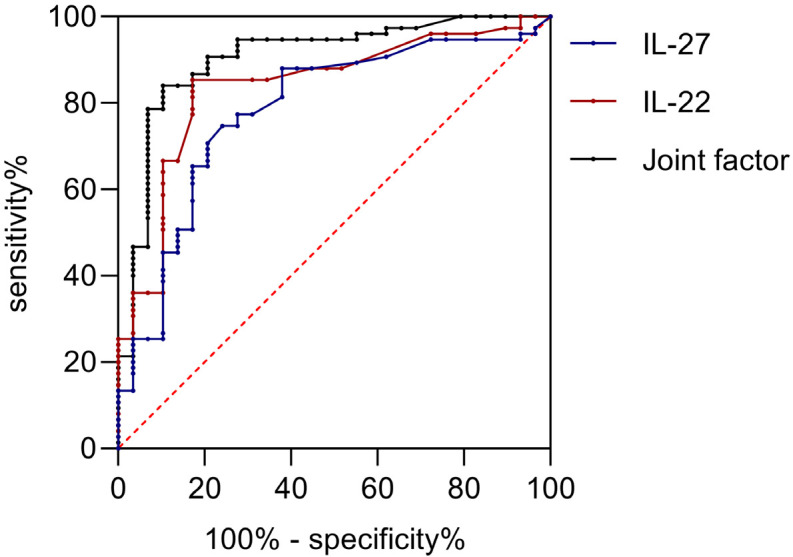
ROC curve. IL-22, Interleukin-22; IL-27, interleukin-27.
Comparison of IL-22 and IL-27 levels between MACE and non-MACE groups
The results of comparing IL-22 and IL-27 levels between MACE and non-MACE groups showed that both IL-22 and IL-27 levels were higher in the MACE group compared to the non-MACE group (all P<0.05), as shown in Table 7.
Table 7.
Comparison of IL-22 and IL-27 levels between MACE group and non-MACE group
| Index | MACE group (n = 39) | Non-MACE group (n = 65) | t | P |
|---|---|---|---|---|
| IL-22 (pg/mL) | 29.19±6.02 | 24.23±5.90 | 4.116 | <0.001 |
| IL-27 (pg/mL) | 27.11±5.49 | 23.16±5.32 | 3.623 | <0.001 |
MACE, major adverse cardiovascular events; IL-22, Interleukin-22; IL-27, interleukin-27.
Clinical value of IL-22 and IL-27 levels in assessing patient prognosis
The results showed that both IL-22 and IL-27, alone or in combination, had an AUC of >0.7 for assessing the prognosis of the patients, as shown in Table 8 and Figure 3.
Table 8.
The clinical value of IL-22 and IL-27 levels in evaluating the prognosis of patients
| Variable | Cut-off value | AUC | 95% CI | Sensitivity | Specificity | P |
|---|---|---|---|---|---|---|
| IL-22 (pg/mL) | 23.89 | 0.72 | 0.62, 0.82 | 0.74 | 0.66 | <0.001 |
| IL-27 (pg/mL) | 23.95 | 0.70 | 0.59, 0.81 | 0.69 | 0.72 | <0.001 |
| Joint factor | - | 0.77 | 0.67, 0.87 | 0.54 | 0.92 | <0.001 |
IL-22, Interleukin-22; IL-27, interleukin-27.
Figure 3.
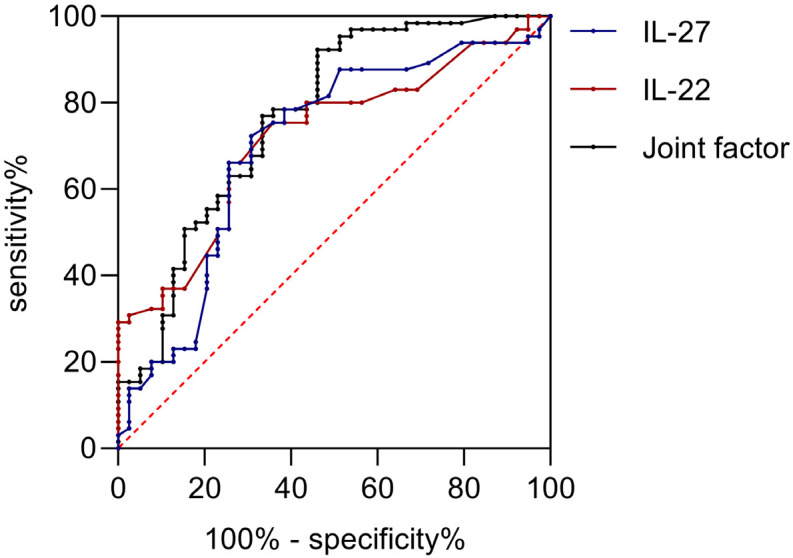
ROC curve of IL-22 and IL-27 levels in assessing patient prognosis. IL-22, Interleukin-22; IL-27, interleukin-27.
Discussion
Early detection of vasculopathy in patients with diabetic nephropathy (DN) and timely intervention are of great clinical importance. Urinary albumin, as one of the biomarkers of endothelial dysfunction, has been widely used for the early diagnosis of DN [14,15]. This study found that compared to the normal population, patients with DN have increased glucose and lipids. In addition, there were significant differences between DN patients and the normal population in terms of uric acid, blood creatinine, glomerular filtration rate, and cystatin C, aligning with previous research findings [16]. However, these indicators have limitations, such as low specificity and sensitivity for diagnosing DN. Therefore, identifying effective biomarkers for assessing the condition of DN and timely predicting vasculopathy is of significant clinical importance.
Current research suggests that body-related inflammatory factors can serve as potential targets for the diagnosis and treatment of DN [17]. IL-27 has both anti- and pro-inflammatory effects; it promotes the differentiation of CD4+ T cells towards Th1 and stimulates the expression of inflammatory cytokines such as IL-6 and IL-12 in monocytes [18,19]. Studies have found that Th1 is closely related to the level of urinary protein in diabetic patients, yet there is limited research on IL-27 expression levels in DN patients [20]. The results of this study show that compared to the normal population, IL-27 levels were significantly elevated in patients with DN, and they increased along with urinary protein levels. This suggests that IL-27 expression is associated with the progression of DN. The possible mechanism is that IL-27 may contribute to the progression of DN by disrupting the balance of Th1/Th2 cell ratios, promoting the secretion of inflammatory factors, leading to growth and proliferation of glomerular mesangial cells, thickening of the extracellular matrix, and expansion of the mesangial areas, thereby worsening the condition of DN.
IL-22 is a member of the IL-10 family, primarily secreted by Th22 cells, and plays a role in the development and progression of several inflammatory diseases [21,22]. On the one hand, IL-22 can protect the host by being highly expressed in response to external threats. On the other hand, excessive secretion of IL-22, influenced by the local cellular cytokine environment, can cause damage to the body. Previous research has observed increased IL-22 expression in conditions such as rheumatoid arthritis and psoriasis [23,24]. However, reports on its role in kidney diseases are still scarce.
The results of this study indicate that IL-22 levels are also elevated in patients with DN. Furthermore, this study divided DN patients into different subgroups based on their levels of proteinuria for further analysis. The results showed that as proteinuria levels increase, patients exhibit higher fasting blood glucose, low-density lipoprotein, cystatin C, IL-22, and IL-27 levels, while their glomerular filtration rates decreased. This suggests that higher proteinuria levels are indicative of more severe disease progression [25]. Additionally, the study also found that IL-22 and IL-27 levels in DN patients were closely related to disease duration, fasting blood glucose, low-density lipoprotein, cystatin C levels, and glomerular filtration rates, further confirming that IL-22 and IL-27 are involved in the process of microvascular disease in DN patients and are closely related to the severity of the condition.
The results of the logistic regression analysis showed that glomerular filtration rate, cystatin C, IL-22, and IL-27 were independent risk factors for the occurrence of microangiopathy. This indicates that in clinical practice, patients with high levels of cystatin C, high expression of IL-22 and IL-27, and low glomerular filtration rates should be closely monitored, and timely interventions should be taken to prevent further progression of the disease.
The study further investigated the clinical value of IL-22 and IL-27 in assessing microangiopathy as well as in evaluating the prognosis of patients. The findings revealed that IL-22 and IL-27 levels were significantly higher in patients with MACE compared to those without MACE. In addition, the AUCs of IL-22 and IL-27 alone in assessing microangiopathy were both greater than 0.7, and the AUC of the combine test exceeded 0.9, suggesting that the combined test of the two has higher clinical value in assessing microangiopathy. Furthermore, the AUC of IL-22 and IL-27, whether measured individually or in combination, was greater than 0.7 for assessing patient prognosis. This provides a new perspective for evaluating the prognosis of patients with DN.
However, this study has limitations, including the fact that the sample was from a single center, resulting in a relatively small sample size. Furthermore, the differences in the levels of IL-22 and IL-27 in patients with different diabetic complications were not further analyzed, making it difficult to analyze the specificity of the indexes.
In summary, the levels of IL-22 and IL-27 are elevated in patients with DN and are closely related to the severity of the disease. They can be used as auxiliary indicators for assessing microangiopathy and prognosis in DN patients.
Disclosure of conflict of interest
None.
References
- 1.An J, Nichols GA, Qian L, Munis MA, Harrison TN, Li Z, Wei R, Weiss T, Rajpathak S, Reynolds K. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9:e001847. doi: 10.1136/bmjdrc-2020-001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai N, Koppisetti H, Pande S, Shukla H, Sirsat B, Ditani AS, Mallick PP, Kathar U, Kalia K, Tekade RK. Nanomedicine in the treatment of diabetic nephropathy. Future Med Chem. 2021;13:663–686. doi: 10.4155/fmc-2020-0335. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, Zhang X, Li C, Huang Z, Sun X, Wang L, Zhou M, Wu J, Wang Y. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. 2021;326:2498–2506. doi: 10.1001/jama.2021.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun B, Luo Z, Zhou J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc Diabetol. 2021;20:9. doi: 10.1186/s12933-020-01200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YH, Zhao LC. Serum levels of inflammatory factors associated with disease progression in diabetic kidney disease. South Chin J Prev Med. 2021;47:1228–1230. [Google Scholar]
- 6.Nowak N. Protective factors as biomarkers and targets for prevention and treatment of diabetic nephropathy: from current human evidence to future possibilities. J Diabetes Investig. 2020;11:1085–1096. doi: 10.1111/jdi.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueki K, Sasako T, Okazaki Y, Miyake K, Nangaku M, Ohashi Y, Noda M, Kadowaki T J-DOIT3 Study Group. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int. 2021;99:256–266. doi: 10.1016/j.kint.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Yang M, Wang X, Han Y, Li C, Wei L, Yang J, Chen W, Zhu X, Sun L. Targeting the NLRP3 inflammasome in diabetic nephropathy. Curr Med Chem. 2021;28:8810–8824. doi: 10.2174/0929867328666210705153109. [DOI] [PubMed] [Google Scholar]
- 9.Ke G, Chen X, Liao R, Xu L, Zhang L, Zhang H, Kuang S, Du Y, Hu J, Lian Z, Dou C, Zhang Q, Zhao X, Zhang F, Zhu S, Ma J, Li Z, Li S, He C, Chen X, Wen Y, Feng Z, Zheng M, Lin T, Li R, Li B, Dong W, Chen Y, Wang W, Ye Z, Deng C, Xiao H, Xiao J, Liang X, Shi W, Liu S. Receptor activator of NF-κB mediates podocyte injury in diabetic nephropathy. Kidney Int. 2021;100:377–390. doi: 10.1016/j.kint.2021.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Aparicio-Siegmund S, Garbers C. The biology of interleukin-27 reveals unique pro- and anti-inflammatory functions in immunity. Cytokine Growth Factor Rev. 2015;26:579–586. doi: 10.1016/j.cytogfr.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Imperiale BR, García A, Minotti A, González Montaner P, Moracho L, Morcillo NS, Palmero DJ, Sasiain MDC, de la Barrera S. Th22 response induced by Mycobacterium tuberculosis strains is closely related to severity of pulmonary lesions and bacillary load in patients with multi-drug-resistant tuberculosis. Clin Exp Immunol. 2021;203:267–280. doi: 10.1111/cei.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinese Diabetes Society. Chinese guidelines for the prevention and treatment of type 2 diabetes (2017 edition) Chin J Diabetes. 2018;10:4–67. [Google Scholar]
- 13.Shao Y, Lv C, Yuan Q, Wang Q. Levels of serum 25(OH)VD3, HIF-1α, VEGF, vWf, and IGF-1 and their correlation in type 2 diabetes patients with different urine albumin creatinine ratio. J Diabetes Res. 2016;2016:1925424. doi: 10.1155/2016/1925424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafez EA, Hassan SAE, Teama MAM, Badr FM. Serum uric acid as a predictor for nephritis in Egyptian patients with systemic lupus erythematosus. Lupus. 2021;30:378–384. doi: 10.1177/0961203320979042. [DOI] [PubMed] [Google Scholar]
- 15.Gorski M, Jung B, Li Y, Matias-Garcia PR, Wuttke M, Coassin S, Thio CHL, Kleber ME, Winkler TW, Wanner V, Chai JF, Chu AY, Cocca M, Feitosa MF, Ghasemi S, Hoppmann A, Horn K, Li M, Nutile T, Scholz M, Sieber KB, Teumer A, Tin A, Wang J, Tayo BO, Ahluwalia TS, Almgren P, Bakker SJL, Banas B, Bansal N, Biggs ML, Boerwinkle E, Bottinger EP, Brenner H, Carroll RJ, Chalmers J, Chee ML, Chee ML, Cheng CY, Coresh J, de Borst MH, Degenhardt F, Eckardt KU, Endlich K, Franke A, Freitag-Wolf S, Gampawar P, Gansevoort RT, Ghanbari M, Gieger C, Hamet P, Ho K, Hofer E, Holleczek B, Xian Foo VH, Hutri-Kähönen N, Hwang SJ, Ikram MA, Josyula NS, Kähönen M, Khor CC, Koenig W, Kramer H, Krämer BK, Kühnel B, Lange LA, Lehtimäki T, Lieb W Lifelines Cohort Study; Regeneron Genetics Center. Loos RJF, Lukas MA, Lyytikäinen LP, Meisinger C, Meitinger T, Melander O, Milaneschi Y, Mishra PP, Mononen N, Mychaleckyj JC, Nadkarni GN, Nauck M, Nikus K, Ning B, Nolte IM, O’Donoghue ML, Orho-Melander M, Pendergrass SA, Penninx BWJH, Preuss MH, Psaty BM, Raffield LM, Raitakari OT, Rettig R, Rheinberger M, Rice KM, Rosenkranz AR, Rossing P, Rotter JI, Sabanayagam C, Schmidt H, Schmidt R, Schöttker B, Schulz CA, Sedaghat S, Shaffer CM, Strauch K, Szymczak S, Taylor KD, Tremblay J, Chaker L, van der Harst P, van der Most PJ, Verweij N, Völker U, Waldenberger M, Wallentin L, Waterworth DM, White HD, Wilson JG, Wong TY, Woodward M, Yang Q, Yasuda M, Yerges-Armstrong LM, Zhang Y, Snieder H, Wanner C, Böger CA, Köttgen A, Kronenberg F, Pattaro C, Heid IM. Meta-analysis uncovers genome-wide significant variants for rapid kidney function decline. Kidney Int. 2021;99:926–939. doi: 10.1016/j.kint.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cankurtaran V, Inanc M, Tekin K, Turgut F. Retinal microcirculation in predicting diabetic nephropathy in type 2 diabetic patients without retinopathy. Ophthalmologica. 2020;243:271–279. doi: 10.1159/000504943. [DOI] [PubMed] [Google Scholar]
- 17.Umapathy D, Subramanyam PV, Krishnamoorthy E, Viswanathan V, Ramkumar KM. Association of Fetuin-A with Thr256Ser exon polymorphism of α2-Heremans Schmid Glycoprotein (AHSG) gene in type 2 diabetic patients with overt nephropathy. J Diabetes Complications. 2022;36:108074. doi: 10.1016/j.jdiacomp.2021.108074. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Ma Y, Zhang M, Wang Y, Wu W. Diagnostic accuracy of interleukin-27 in tuberculous pleurisy: a systematic review and meta-analysis. QJM. 2021;114:568–576. doi: 10.1093/qjmed/hcaa215. [DOI] [PubMed] [Google Scholar]
- 19.Jahantigh D, Ghazaey Zidanloo S, Forghani F, Doroudian M. IL-27 variants might be genetic risk factors for preeclampsia: based on genetic polymorphisms, haplotypes and in silico approach. Mol Biol Rep. 2020;47:7929–7940. doi: 10.1007/s11033-020-05871-z. [DOI] [PubMed] [Google Scholar]
- 20.Houssen ME, El-Hussiny MAB, El-Kannishy A, Sabry D, El Mahdy R, Shaker ME. Serum and aqueous humor concentrations of interleukin-27 in diabetic retinopathy patients. Int Ophthalmol. 2018;38:1817–1823. doi: 10.1007/s10792-017-0655-7. [DOI] [PubMed] [Google Scholar]
- 21.Luo JW, Hu Y, Liu J, Yang H, Huang P. Interleukin-22: a potential therapeutic target in atherosclerosis. Mol Med. 2021;27:88. doi: 10.1186/s10020-021-00353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patnaude L, Mayo M, Mario R, Wu X, Knight H, Creamer K, Wilson S, Pivorunas V, Karman J, Phillips L, Dunstan R, Kamath RV, McRae B, Terrillon S. Mechanisms and regulation of IL-22-mediated intestinal epithelial homeostasis and repair. Life Sci. 2021;271:119195. doi: 10.1016/j.lfs.2021.119195. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Q, Yang G, Xiao F, Xie J, Wang S, Lu L, Cui D. Role of Th22 cells in the pathogenesis of autoimmune diseases. Front Immunol. 2021;12:688066. doi: 10.3389/fimmu.2021.688066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao Q, Gao HY, Zhou XL, Cao CH. Expression and clinical significance of interleukin-22 in patients with rheumatoid arthritis. Chin J Immunol. 2017;33:103–107. [Google Scholar]
- 25.Guo Y. The value of urinary albumin/creatinine ratio in early renal injury of type 2 diabetes mellitus. J Med Theory Pract. 2018;31:734–736. [Google Scholar]


