Abstract
Objective: To analyze the risk factors for bloodstream infection after immunosuppressive therapy in patients with aplastic anemia using logistic regression. Methods: A retrospective analysis was conducted on the clinical data from 70 patients with aplastic anemia admitted to the People’s Hospital of Zitong County and the Infectious Disease Hospital in Jiangyou City from March 2011 to March 2023. Patients were divided into two groups based on whether they developed an infection after treatment: the infection group (n = 18) and the non-infection group (n = 52). Risk factors for bloodstream infection following immunosuppressive therapy were analyzed, and the predictive value of independent risk factors was assessed. Results: Univariate analysis identified age, diabetes, disease severity, albumin levels, neutrophil count, and concurrent infections before treatment as significant risk factors for bloodstream infection following immunosuppressive therapy (all P<0.05). Multivariate analysis further confirmed that age, diabetes, disease severity, albumin levels, and neutrophil count were independent risk factors for bloodstream infection (all P<0.05). ROC curve analysis revealed that age, diabetes, disease severity, albumin levels, and neutrophil count had area under the curve (AUC) values of 0.678, 0.728, 0.698, 0.740, and 0.739, respectively, in predicting bloodstream infection after immunosuppressive therapy. The sensitivity values were 65.39%, 78.85%, 67.31%, 67.31%, and 76.92%, respectively, while the specificity values were 72.22%, 66.67%, 72.22%, 77.78%, and 61.11%, respectively. Conclusion: Age, diabetes, disease severity, albumin levels, and neutrophil count are key factors influencing bloodstream infection after immunosuppressive therapy in patients with aplastic anemia. These findings highlight the need for careful monitoring of these factors during immunosuppressive therapy to reduce the risk of bloodstream infection.
Keywords: Aplastic anemia, immunosuppressive therapy, bloodstream infection
Introduction
Aplastic anemia is a rare hematologic disorder characterized by impaired bone marrow hematopoiesis, leading to decreased numbers of red blood cells, white blood cells, and platelets [1]. This condition can result from abnormal immune attack on hematopoietic stem cells or from damage to these cells due to genetic or environmental factors [2]. Although aplastic anemia can occur in any age group, it is more common in young people and children, with a relatively lower incidence in the elderly [3]. The global incidence of aplastic anemia is low, though regional differences exist [4]. Patients typically present with symptoms such as anemia, bleeding, and infection, which significantly impact their quality of life and increase the risk of complications [5]. Early diagnosis and treatment of aplastic anemia are therefore crucial for patient outcomes.
Immunosuppressive therapy is a common treatment for aplastic anemia [6]. This therapy can reduce the immune system’s attack on hematopoietic stem cells, thereby promoting bone marrow regeneration and the restoration of normal hematopoietic function [7]. However, immunosuppressive therapy can also suppress the immune system, increasing the risk of infections and bleeding. Bloodstream infection is a common and serious side effect of immunosuppressive therapy [8]. The weakened immune response in these patients makes them more susceptible to various pathogens, leading to bloodstream infections [9]. Symptoms may include fever, chills, low blood pressure, rapid heart rate, and shortness of breath. If not promptly treated, bloodstream infections can lead to severe complications and even be life-threatening [10].
These infections pose significant risks to patients due to their high incidence and potential for severe outcomes. The reported incidence of bloodstream infections among patients undergoing immunosuppressive therapy ranges from 5% to 20%, depending on the patient population and specific immunosuppressive agents used [11]. Factors influencing the development of bloodstream infections in these patients include the type and intensity of the immunosuppressive regimen, the underlying condition being treated, the presence of indwelling medical devices such as central venous catheters, and the patient’s overall health status, including comorbidities such as diabetes or renal failure [12].
This study aims to identify the risk factors influencing bloodstream infection after immunosuppressive therapy in patients with aplastic anemia, providing valuable insights for clinical intervention and treatment strategies.
Materials and methods
Ethics statement
This research was approved by the Medical Ethics Committee of the People’s Hospital of Zitong County.
Screening process
Inclusion criteria: a. Patients who meet the diagnostic criteria for aplastic anemia [13]; b. Patients who have undergone immunosuppressive therapy; c. Patients without cognitive dysfunction; d. Patients with detailed clinical data.
Exclusion criteria: a. Patients with concurrent diseases such as leukemia that could affect this study; b. Patients with coagulation disorders; c. Patients with poor compliance or cooperation; d. Pregnant and lactating women; e. Patients with concurrent autoimmune diseases or other malignancies; f. Patients with heart, liver, or kidney dysfunction.
Study design
A retrospective analysis was conducted on the clinical data from 100 patients with aplastic anemia admitted to the People’s Hospital of Zitong County and the Infectious Disease Hospital in Jiangyou City from March 2011 to March 2023.
Data extraction and outcome measures
Clinical data were meticulously collected from electronic medical records and outpatient follow-up records. The dataset included factors such as disease course, BMI, age, gender, diabetes, hypertension, smoking history, drinking history, disease severity, albumin levels, neutrophil counts, and concurrent infections prior to treatment. The primary objective was to identify risk factors associated with bloodstream infections in patients with aplastic anemia following immunosuppressive therapy. Through rigorous analysis, the study aimed to determine independent risk factors with predictive value for bloodstream infections. By comprehensively evaluating these risk factors, the study seeks to enhance understanding and improve risk assessment and management strategies in clinical practice.
Statistical analysis
Data were statistically analyzed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and visualized with GraphPad Prism 8. Measurement data were expressed as means ± standard deviation (Means ± SD), while categorical data were represented as percentages (%). Chi-square tests (χ2) were used to analyze differences in categorical data between groups. Additional sensitivity and stratified analyses by subgroups (e.g., age, gender) were conducted to evaluate the consistency of observed associations. For continuous variables, independent sample t-tests were used for comparisons between groups, and paired t-tests were applied for within-group comparisons. Logistic regression analysis identified risk factors influencing bloodstream infections following immunosuppressive therapy in patients with aplastic anemia. The predictive value of these independent risk factors was further assessed using receiver operating characteristic (ROC) curves, with areas under the curve (AUC) and sensitivity and specificity determined. Statistical significance was set at P<0.05 for all analyses, ensuring the reliability and validity of the results.
Results
Sample screening
Based on the inclusion and exclusion criteria, a total of 70 patients who met the requirements were selected, while 30 patients were excluded from the study. The clinical data of the 70 selected patients are detailed in Table 1. Patients were divided into two groups based on whether they developed infection after treatment: the infection group (n = 18) and the non-infection group (n = 52) (Figure 1).
Table 1.
Baseline data
| Factors | Cases (percentage) |
|---|---|
| Course of disease (d) (≤20/>20) | 44 (62.86%)/26 (37.14%) |
| Body mass index (BMI) (kg/m2) (≤24/>24) | 44 (62.86%)/26 (37.14%) |
| Age (≤40/>40) | 39 (55.71%)/31 (44.29%) |
| Gender (Male/Female) | 40 (57.14%)/30 (42.86%) |
| Diabetes (Yes/No) | 23 (32.86%)/47 (67.14%) |
| Hypertension (Yes/No) | 21 (30.00%)/49 (70.00%) |
| Smoking history (Yes/No) | 27 (38.57%)/43 (61.43%) |
| Drinking history (Yes/No) | 27 (38.57%)/43 (61.43%) |
| Disease severity (Severe/Extremely severe) | 30 (42.86%)/40 (57.14%) |
| Concurrent other infections before treatment (Yes/No) | 16 (22.86%)/54 (77.14%) |
Figure 1.
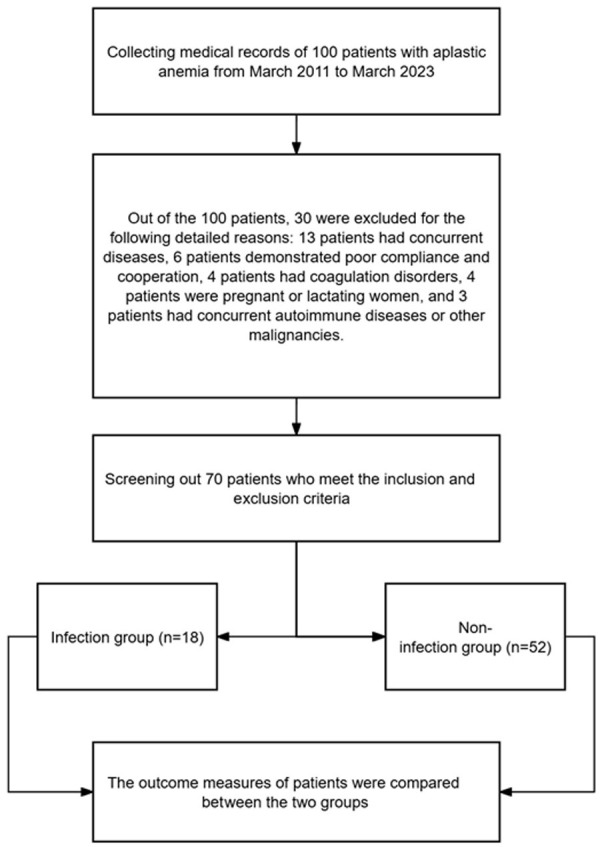
Flowchart of sample screening process based on inclusion and exclusion criteria.
Univariate analysis of factors affecting bloodstream infection after immunosuppressive therapy
A comparison of clinical data between the infection group and the non-infection group revealed significant differences in age, diabetes status, disease severity, albumin levels, neutrophil count, and the presence of other infections before treatment. In the infection group, 5 patients were aged ≤40 years, and 13 patients were aged >40 years; 12 patients had diabetes, while 6 did not; 13 patients had severe conditions, and 5 had extremely severe conditions; 14 patients had albumin levels ≤30 g/L and 4 had levels >30 g/L; 12 patients a had neutrophil count ≤0.8×109/L and 6 had a count >0.8×109/L; 8 patients had other infections before treatment, and 10 did not. In the non-infection group, 34 patients were aged ≤40 years, and 18 were aged >40 years; 11 patients had diabetes, while 41 did not; 17 patients had severe conditions, and 35 had extremely severe conditions; 17 patients had albumin levels ≤30 g/L and 35 had levels >30 g/L; 15 patients had a neutrophil count ≤0.8×109/L and 37 had a count >0.8×109/L; 8 patients had other infections before treatment and 44 did not. These factors were identified as significant risk factors influencing bloodstream infection after immunosuppressive therapy in patients with aplastic anemia (all P<0.05) (Table 2).
Table 2.
Univariate analysis
| Factors | Infection group (n = 18) | Non-infection group (n = 52) | χ2 | P |
|---|---|---|---|---|
| Course of disease (d) | 0.032 | 0.859 | ||
| ≤20 | 11 | 33 | ||
| >20 | 7 | 19 | ||
| Body mass index (BMI) (kg/m2) | 0.151 | 0.698 | ||
| ≤24 | 12 | 32 | ||
| >24 | 6 | 20 | ||
| Age | 7.664 | 0.006 | ||
| ≤40 | 5 | 34 | ||
| >40 | 13 | 18 | ||
| Gender | 0.025 | 0.875 | ||
| Male | 10 | 30 | ||
| Female | 8 | 22 | ||
| Diabetes | 12.550 | <0.001 | ||
| Yes | 12 | 11 | ||
| No | 6 | 41 | ||
| Hypertension | 0.128 | 0.720 | ||
| Yes | 6 | 15 | ||
| No | 12 | 37 | ||
| Smoking history | 0.353 | 0.553 | ||
| Yes | 8 | 19 | ||
| No | 10 | 33 | ||
| Drinking history | 0.001 | 0.975 | ||
| Yes | 7 | 20 | ||
| No | 11 | 32 | ||
| Disease severity | 8.532 | 0.004 | ||
| Severe | 13 | 17 | ||
| Extremely severe | 5 | 35 | ||
| Albumin levels (g/L) | 11.020 | 0.001 | ||
| ≤30 | 14 | 17 | ||
| >30 | 4 | 35 | ||
| Neutrophil counts (×109/L) | 8.072 | 0.005 | ||
| ≤0.8 | 12 | 15 | ||
| >0.8 | 6 | 37 | ||
| Concurrent other infections before treatment | 6.404 | 0.011 | ||
| Yes | 8 | 8 | ||
| No | 10 | 44 |
Multivariate analysis of factors affecting bloodstream infection after immunosuppressive therapy
The indicators identified as significant in Table 2 were assigned values (Table 3) and subjected to logistic regression analysis. The results indicated that age (OR: 22.262, 95% CI: 1.208-410.136), diabetes (OR: 56.675, 95% CI: 3.139-1023.185), disease severity (OR: 0.044, 95% CI: 0.003-0.680), albumin levels (OR: 52.972, 95% CI: 1.897-1478.978), and neutrophil count (OR: 29.470, 95% CI: 1.477-587.892) were independent risk factors influencing bloodstream infection after immunosuppressive therapy in patients with aplastic anemia (P<0.05) (Table 4).
Table 3.
Assignment table
| Factors | Assignment |
|---|---|
| Age | ≤40 = 0, >40 = 1 |
| Diabetes | No = 0, Yes = 1 |
| Disease severity | Severe = 0, Extremely severe = 1 |
| Albumin levels (g/L) | ≤30 = 1, >30 = 0 |
| Neutrophil counts (×109/L) | ≤0.8 = 1, >0.8 = 0 |
| Concurrent other infections before treatment | Yes = 1, No = 0 |
| Bloodstream infection | Yes = 1, No = 0 |
Table 4.
Multivariate analysis
| B | S.E. | Wals | Sig. | Exp (B) (95% C.I.) | |
|---|---|---|---|---|---|
| Age | 3.103 | 1.487 | 4.357 | 0.037 | 22.262 (1.208-410.136) |
| Diabetes | 4.037 | 1.476 | 7.480 | 0.006 | 56.675 (3.139-1023.185) |
| Disease severity | -3.126 | 1.399 | 4.996 | 0.025 | 0.044 (0.003-0.680) |
| Albumin levels | 3.970 | 1.699 | 5.461 | 0.019 | 52.972 (1.897-1478.978) |
| Neutrophil counts | 3.383 | 1.527 | 4.908 | 0.027 | 29.470 (1.477-587.892) |
| Concurrent other infections before treatment | 2.832 | 1.484 | 3.644 | 0.056 | 16.980 (0.927-311.027) |
Predictive value of independent risk factors
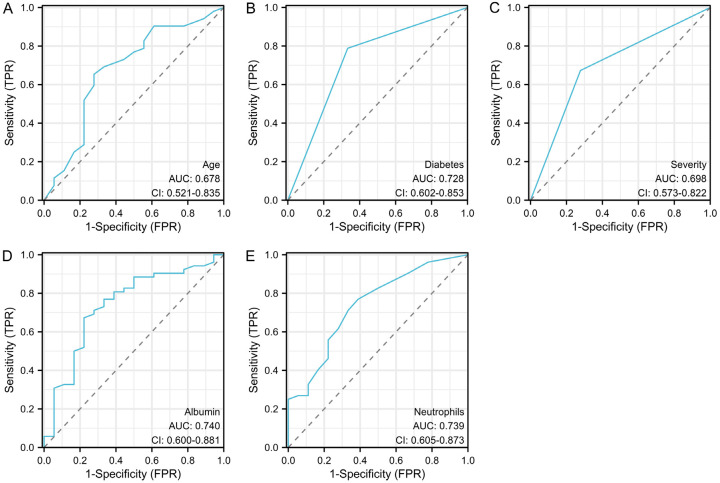
The ROC curve analysis revealed that age, diabetes status, disease severity, albumin levels, and neutrophil count had area under the curve (AUC) values of 0.678, 0.728, 0.698, 0.740, and 0.739, respectively, in predicting bloodstream infection after immunosuppressive therapy. The sensitivity values were 65.39%, 78.85%, 67.31%, 67.31%, and 76.92%, respectively, while the specificity values were 72.22%, 66.67%, 72.22%, 77.78%, and 61.11%, respectively (Table 5; Figure 2).
Table 5.
Predictive value of independent risk factors
| Area under the curve (AUC) | Confidence interval (CI) | Cut-off | Sensitivity | Specificity | Youden index | |
|---|---|---|---|---|---|---|
| Age | 0.678 | 0.521-0.835 | 40.000 | 65.39% | 72.22% | 37.61% |
| Diabetes | 0.728 | 0.602-0.853 | 0.500 | 78.85% | 66.67% | 45.51% |
| Disease severity | 0.698 | 0.573-0.822 | 0.500 | 67.31% | 72.22% | 39.53% |
| Albumin levels | 0.740 | 0.600-0.881 | 30.05 | 67.31% | 77.78% | 45.09% |
| Neutrophil counts | 0.739 | 0.605-0.873 | 0.750 | 76.92% | 61.11% | 38.03% |
Figure 2.
ROC curve of independent risk factors in predicting bloodstream infections after immunotherapy. A. ROC curve of age in predicting bloodstream infection; B. ROC curve of diabetes in predicting bloodstream infection; C. ROC curve of severity in predicting bloodstream infection; D. ROC curve of albumin in predicting bloodstream infection; E. ROC curve of neutrophils in predicting bloodstream infection.
Discussion
The development of aplastic anemia is a complex process, influenced by multiple interacting factors [14,15]. The disease is primarily characterized by an insufficient number of hematopoietic stem cells in the bone marrow, leading to anemia, thrombocytopenia, and leukopenia [16,17]. These symptoms can result in persistent fatigue, weakness, and increased susceptibility to bleeding and infections, significantly impacting patients’ daily lives and work capacity. Aplastic anemia can also lead to severe complications such as serious infections, bleeding, anemia-induced heart and lung dysfunction, and even life-threatening conditions [18,19].
Treatment options for aplastic anemia include immunosuppressive therapy, hematopoietic stem cell transplantation, and supportive therapy. Immunosuppressive therapy is commonly used to reduce the immune system’s attack on bone marrow hematopoietic cells, thereby promoting the restoration of hematopoietic function. While this approach can help some patients recover bone marrow function, it does not guarantee effective treatment outcomes for all patients [20,21]. The advantages of immunosuppressive therapy include its non-invasive nature and the avoidance of hematopoietic stem cell transplantation. However, a significant drawback is the increased risk of infection [22]. As the immune system is suppressed during therapy, the patient’s immune function decreases, making them more vulnerable to infections, particularly bloodstream infections. These infections can manifest as fever, chills, swelling, and pain at the infection site, and in severe cases, it may progress to sepsis and organ failure [23]. Therefore, analyzing the risk factors influencing bloodstream infections after immunosuppressive therapy are of great clinical importance.
The study by Bhargawa et al. [24] revealed that IL-6 and IL-8 levels in patients with aplastic anemia were significantly elevated and correlated with disease severity, suggesting that these patients’ immune systems are severely compromised, increasing their infection risk. This study also identified disease severity as an independent risk factor for bloodstream infections after immunosuppressive therapy, with a higher incidence of bloodstream infections in patients with extremely severe aplastic anemia. Aplastic anemia is an immune-mediated disease characterized by impaired hematopoietic function in the bone marrow, leading to reductions in red blood cells, white blood cells, and platelets. Due to an abnormal immune system, patients with aplastic anemia are more susceptible to infections and have a diminished immune response to such infections [25].
Li et al. [26] found that patients with acquired aplastic anemia exhibited bone marrow hypoplasia and pancytopenia, conditions that can lead to immune abnormalities. This study also identified lower levels of albumin and neutrophils as being associated with a higher incidence of bloodstream infections. Albumin, an important plasma protein, plays a crucial role in maintaining immune function [27]. Reduced albumin levels can impair the normal functioning of the immune system, lowering the body’s ability to combat infections and increasing the risk of bloodstream infections. Neutrophils, a key type of white blood cell, are essential for combating bacterial and other pathogenic infections [28]. Low neutrophil levels compromise the body’s immune defense, making it more susceptible to infections. Factors such as bone marrow hypoplasia, drug treatments, or other underlying medical conditions can cause low neutrophil levels. Given the close link between the immune and hematopoietic systems, bone marrow hypoplasia and pancytopenia may lead to immune abnormalities in patients with aplastic anemia, reducing their immune response and increasing their risk of infection.
In addition, our study revealed that age and diabetes are independent risk factors for bloodstream infection following immunosuppressive therapy. As people age, their immune function gradually declines, which can affect the quantity and functionality of immune cells [29]. In individuals with diabetes, immune system function is often compromised, particularly in a hyperglycemic state where immune cell activity and function are suppressed, leading to immune dysfunction and increased susceptibility to infections. Diabetes can also cause blood circulation disorders and microangiopathy, further reducing the body’s resistance to infections [30].
The study by Lin et al. [31] identified chemotherapy, transplantation status, hypertension, and coronary artery disease as independent risk factors for immunosuppressed patients over 65 years old. Similarly, our study also identified age and underlying conditions such as diabetes as independent risk factors for bloodstream infections. This parallel with Lin et al.’s findings underscores the importance of vigilant monitoring and management of older patients and those with preexisting conditions to mitigate the risk of infections [31]. Furthermore, it highlights the need for additional research to explore these risk factors in greater detail and to develop targeted preventive strategies to improve outcomes in this vulnerable population.
The ROC analyses in our study provided significant insights into the predictive ability of various independent risk factors for bloodstream infection after immunosuppressive therapy. Specifically, the AUC values for age, diabetes, severity, albumin, and neutrophils were 0.678, 0.728, 0.698, 0.740, and 0.739, respectively. These values indicate that while all these factors have varying degrees of predictive power, albumin and neutrophils show particularly strong potential, with AUCs closer to 1. Additionally, the sensitivity and specificity of these indicators further elucidate their predictive capacity. Age, diabetes, severity, albumin, and neutrophils demonstrated sensitivities of 65.39%, 78.85%, 67.31%, 67.31%, and 76.92%, and specificities of 72.22%, 66.67%, 72.22%, 77.78%, and 61.11%, respectively. These metrics suggest that certain factors, notably albumin and diabetes, are effective in identifying patients at risk for bloodstream infection, while also accurately excluding those without the infection. Overall, the analysis underscores the importance of these factors in predicting bloodstream infections, which could inform future clinical strategies and patient management protocols.
In this study, logistic regression analysis was utilized to identify factors influencing bloodstream infection after immunosuppressive therapy in patients with aplastic anemia. However, there are limitations to this study. For instance, as a retrospective study, it may introduce recall bias and inaccuracies in information collection. Since data were obtained from patients’ medical records, there is a possibility of missing or erroneous data. Additionally, there may be other potential factors beyond those assessed here that could also impact patient outcomes. Future research should consider incorporating a broader range of relevant clinical and demographic indicators into the analysis to improve the accuracy and comprehensiveness of understanding the complex factors influencing the prognosis of individuals with aplastic anemia. It would also be beneficial to employ larger sample sizes and prospective study designs in future research to obtain more representative results.
To summarize, age, diabetes, disease severity, albumin levels, and neutrophil count are key factors influencing bloodstream infection after immunosuppressive therapy in patients with aplastic anemia. These findings suggest that special attention should be given to these factors during immunosuppressive therapy to reduce the risk of bloodstream infection.
Disclosure of conflict of interest
None.
Abbreviations
- BMI
Body mass index
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- AUC
Area under the curve
References
- 1.Altay D, Yılmaz E, Ozcan A, Karakukcu M, Unal E, Arslan D. Hepatitis-associated aplastic anemia in pediatric patients: single center experience. Transfus Apher Sci. 2020;59:102900. doi: 10.1016/j.transci.2020.102900. [DOI] [PubMed] [Google Scholar]
- 2.Ghanei-Shahmirzadi A, Reihani H, Abbasi-Kashkooli A, Karbasian F, Hedayati SB, Bordbar M, Ataollahi M, Dehghani SM, Geramizadeh B. Aplastic anemia: a new complication in the recent mysterious hepatitis outbreak among children worldwide: two case reports. J Med Case Rep. 2022;16:422. doi: 10.1186/s13256-022-03542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cugno C, Cesaro S. Epidemiology, risk factors and therapy of candidemia in pediatric hematological patients. Pediatr Rep. 2012;4:e9. doi: 10.4081/pr.2012.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed P, Chaudhry QUN, Satti TM, Mahmood SK, Ghafoor T, Shahbaz N, Khan MA, Satti HS, Akram Z, Iftikhar R. Epidemiology of aplastic anemia: a study of 1324 cases. Hematology. 2020;25:48–54. doi: 10.1080/16078454.2019.1711344. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Wu Q, Zheng Y. Persistent elevated bone marrow plasma levels of thrombopoietin in patients with aplastic anemia. Cytokine. 2016;85:11–13. doi: 10.1016/j.cyto.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Cınar F, Bulbuloglu S. The effect of adherence to immunosuppressant therapy on gastrointestinal complications after liver transplantation. Transpl Immunol. 2022;71:101554. doi: 10.1016/j.trim.2022.101554. [DOI] [PubMed] [Google Scholar]
- 7.Kawabe S, Iwata N, Nishida E, Kohagura T, Abe N, Nakaseko H, Morita A. Combination immunosuppressant therapy for rapidly progressive pyoderma gangrenosum in a child. Mod Rheumatol Case Rep. 2021;5:137–140. doi: 10.1080/24725625.2020.1749359. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Zhuang JL, Duan MH, Zhang W, Li J, Zhu TN, Cai HC, Cao XX, Feng J, Yang C, Zhang Y, Zhang L, Zhou DB, Han B. Posaconazole as primary prevention of fungal infection in intensive immunosuppressive therapy for severe aplastic anemia. Zhonghua Xue Ye Xue Za Zhi. 2018;39:128–131. doi: 10.3760/cma.j.issn.0253-2727.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdolrasouli A, Schelenz S. Photo Quiz: bloodstream infection in a neutropenic patient with severe aplastic anemia. J Clin Microbiol. 2023;61:e0056623. doi: 10.1128/jcm.00566-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdolrasouli A, Schelenz S. Answer to the Photo Quiz: bloodstream infection in a neutropenic patient with severe aplastic anemia. J Clin Microbiol. 2023;61:e0057623. doi: 10.1128/jcm.00576-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camp J, Glaubitz L, Filla T, Kaasch AJ, Fuchs F, Scarborough M, Kim HB, Tilley R, Liao CH, Edgeworth J, Nsutebu E, Lopez-Cortes LE, Morata L, Llewelyn M, Fowler VG, Thwaites G, Seifert H, Kern WV, Kuss O, Rieg S. Impact of immunosuppressive agents on clinical manifestations and outcome of staphylococcus aureus bloodstream infection: a propensity score-matched analysis in 2 large, prospectively evaluated cohorts. Clin Infect Dis. 2021;73:1239–1247. doi: 10.1093/cid/ciab385. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Gao Y, Qiu Y, Zhu H, Zhang S, Summah HD, Shi G, Cheng T, Yang Z, Feng Y. The prognostic factors of bloodstream infection in immunosuppressed elderly patients: a retrospective, single-center, five-year cohort study. Clin Interv Aging. 2022;17:1647–1656. doi: 10.2147/CIA.S386922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JC British Society for Standards in Haematology. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187–207. doi: 10.1111/bjh.13853. [DOI] [PubMed] [Google Scholar]
- 14.Yu W, Yang W. Interlukin-27 rs153109 polymorphism confers the susceptibility and prognosis of aplastic anemia in Chinese population. Int J Lab Hematol. 2022;44:150–156. doi: 10.1111/ijlh.13700. [DOI] [PubMed] [Google Scholar]
- 15.Giudice V, Selleri C. Aplastic anemia: pathophysiology. Semin Hematol. 2022;59:13–20. doi: 10.1053/j.seminhematol.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Biswajit H, Pratim PP, Kumar ST, Shilpi S, Krishna GB, Aditi A. Aplastic anemia: a common hematological abnormality among peripheral pancytopenia. N Am J Med Sci. 2012;4:384–388. doi: 10.4103/1947-2714.100980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storb R. Allogeneic bone marrow transplantation for aplastic anemia. Int J Hematol. 2024;119:220–230. doi: 10.1007/s12185-022-03506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Zhu G, Cai L, Yuan M, Wu R, Jia C, Wang B, Zheng J, Ma J, Qin M, Li S. Efficacy of hematopoietic stem cell transplantation in the treatment of children with non-severe aplastic anemia. Pediatr Transplant. 2022;26:e14340. doi: 10.1111/petr.14340. [DOI] [PubMed] [Google Scholar]
- 19.Pan P, Chen C, Hong J, Gu Y. Autoimmune pathogenesis, immunosuppressive therapy and pharmacological mechanism in aplastic anemia. Int Immunopharmacol. 2023;117:110036. doi: 10.1016/j.intimp.2023.110036. [DOI] [PubMed] [Google Scholar]
- 20.Uyar B. The analysis of immunosuppressant therapy adherence, depression, anxiety, and stress in kidney transplant recipients in the post-transplantation period. Transpl Immunol. 2022;75:101686. doi: 10.1016/j.trim.2022.101686. [DOI] [PubMed] [Google Scholar]
- 21.Urbanowicz I, Nahaczewska W, Celuch B. Narrative review of aplastic anemia-the importance of supportive treatment. Ann Palliat Med. 2021;10:694–699. doi: 10.21037/apm-20-1957. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Kim YH, Yi NJ, Kim HS, Lee HS, Lee BK, Kim H, Choi YR, Hong G, Lee KW, Suh KS. Impact of immunosuppressant therapy on early recurrence of hepatocellular carcinoma after liver transplantation. Clin Mol Hepatol. 2014;20:192–203. doi: 10.3350/cmh.2014.20.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamara JF, Righi E, Wright H, Hartel GF, Harris PNA, Paterson DL. Long-term morbidity and mortality following bloodstream infection: a systematic literature review. J Infect. 2018;77:1–8. doi: 10.1016/j.jinf.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Bhargawa SK, Singh A, Yadav G, Kushwaha R, Verma SP, Tripathi AK, Singh US. Aplastic anemia severity and IL-6 and IL-8 blood levels. Discoveries (Craiova) 2022;10:e157. doi: 10.15190/d.2022.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afolabi BE. Cancer and immunology-the homeostasis dance. J Mod Med Oncol. 2022;2:5. [Google Scholar]
- 26.Li JP, Zheng CL, Han ZC. Abnormal immunity and stem/progenitor cells in acquired aplastic anemia. Crit Rev Oncol Hematol. 2010;75:79–93. doi: 10.1016/j.critrevonc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Coley-Grant D, Herbert M, Cornes MP, Barlow IM, Ford C, Gama R. The impact of change in albumin assay on reference intervals, prevalence of ‘hypoalbuminaemia’ and albumin prescriptions. Ann Clin Biochem. 2016;53:112–116. doi: 10.1177/0004563215599560. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Sun B. Pleiotropic regulations of neutrophil receptors response to sepsis. Inflamm Res. 2017;66:197–207. doi: 10.1007/s00011-016-0993-3. [DOI] [PubMed] [Google Scholar]
- 29.Waterlow NR, Cooper BS, Robotham JV, Knight GM. Antimicrobial resistance prevalence in bloodstream infection in 29 European countries by age and sex: an observational study. PLoS Med. 2024;21:e1004301. doi: 10.1371/journal.pmed.1004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H, Yang L, Fang J, Gao Y, Zhu H, Zhang S, Summah HD, Shi G, Sun J, Ni L, Feng Y. Clinical characteristics of bloodstream infection in immunosuppressed patients: a 5-year retrospective cohort study. Front Cell Infect Microbiol. 2022;12:796656. doi: 10.3389/fcimb.2022.796656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H, Gao Y, Qiu Y, Du W, Zhu H, Li J, Wang P, Xu Y, Feng Y. Impact of age group on bloodstream infection risk evaluation in immunosuppressed patients: a retrospective, single-centre, 5-year cohort study. Aging Clin Exp Res. 2023;35:357–366. doi: 10.1007/s40520-022-02299-2. [DOI] [PubMed] [Google Scholar]