Abstract
Objective: To conduct a systematic review (SR) to find evidence for a connection between Crohn’s disease (CD) and the gut-brain axis (GBA). Methods: This study conducted a systematic review (SR) employing a search strategy and strict inclusion criteria. It was conducted by searching for studies published between 2017 and 2024 in the following databases: PUBMED, PUBMED PMC, BVS-BIREME, SCOPUS, WEB OF SCIENCE, EMBASE, and COCHRANE. Results: Fifty original research articles were included. Among these, 20 studies addressed neuroimaging methods to evaluate CD patients’ functional or structural brain changes. Neurodegenerative diseases were the second most addressed topic in the studies, with 18 articles related to different diseases such as Parkinson’s disease, Alzheimer’s disease, dementia, Amyotrophic Lateral Sclerosis, Multiple Sclerosis, and Multiple System Atrophy. Eight articles addressed sleep disorders related to CD; two explored Electroencephalography changes; one investigated Brain-Derived Neurotrophic Factor serum levels and one correlated vagotomy with CD. Conclusion: Interest in the link between CD and GBA is increasing, but studies remain varied and inconclusive, spanning from epidemiology to brain imaging and neglecting to investigate a mechanistic relationship. This SR underscores the need for further research to better understand the potential role of GBA in the prognosis and etiology of CD, highlighting its complexity.
Keywords: Brain-gut axis, gut-brain axis, Crohn’s disease
Introduction
Inflammatory Bowel Disease (IBD) is a term that comprises a group of chronic, idiopathic inflammatory diseases affecting the gastrointestinal tract (GIT), primarily represented by Crohn’s Disease (CD), Ulcerative Colitis (UC), and Indeterminate Colitis (IC). In CD, we observe transmural inflammation, which can affect the entire gastrointestinal tract, from the mouth to the anus. This multifaceted condition is believed to arise from genetic predisposition, environmental factors, and changes in the intestinal microbiota. This combination leads to dysregulation of innate and adaptive immune responses, resulting in mucosal damage and impairment of epithelial barrier function [1].
Recent findings indicate that the Gut-Brain Axis (GBA) could substantially influence the pathophysiology and presentation of CD. The GBA represents a sophisticated bidirectional communication network that enables interaction between intestinal stimuli-like immune responses and metabolites from the gut microbiota. It also regulates intestinal motor and secretory functions in conjunction with the central nervous system (CNS) [2,3].
The GBA’s functioning is not yet fully understood, particularly regarding its interaction with the intestinal microbiota and immune responses. Nonetheless, the interest in the relationship between the GBA and CD is reflected in the wide variety of topics addressed in the literature. These studies encompass a range of neurological alterations or diseases, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), sleep disorders, and the evaluation of neuroanatomical changes in CD patients [4-7].
The GBA, or Microbiota-Gut-Brain Axis, includes the CNS - comprising the brain and spinal cord, the autonomic nervous system (ANS), the enteric nervous system (ENS), and the hypothalamic-pituitary-adrenal (HPA) axis [2]. It plays a crucial role in regulating various intestinal functions, such as motility, hormone secretion, and control of intestinal permeability, as well as in regulating brain functions, such as behavior, sleep regulation, and stress response [8]. The ENS regulates intestinal functions such as muscle contraction to propel food, fluid secretion, and nutrient absorption. It also sends signals to the brain about the state of the gut, including the presence of food, bacteria, or inflammatory signals. The vagus nerve is a significant communication pathway between the gut and the brain. It transmits sensory signals from the gut to the brain and motor signals from the brain to the gut. These signals can influence intestinal motility, hormone and neurotransmitter secretion, and even pain perception [2,8,9].
The intestinal microbiota communicates with the CNS through their products, such as short-chain fatty acids, secondary bile acids, and tryptophan metabolites [10,11]. While some of these metabolites interact directly with enteroendocrine cells, enterochromaffin cells, and the immune system present in the intestinal mucosa and mucus to transmit ascending signals, others can cross the intestinal barrier and enter the systemic circulation, possibly reaching the blood-brain barrier (BBB) [11,12]. Another possibility is that microbial signals communicate through neural pathways involving vagal and spinal afferents [13]. However, whether these metabolites directly reach specific brain regions in concentrations sufficient to affect particular brain circuits remains unclear.
The HPA axis plays an important role in human cognitive function. It is one of the main neuroendocrine systems that respond to stress by producing glucocorticoids such as cortisol. Adequate cortisol concentrations are essential for neurodevelopment and cognitive processes such as learning and memory. Evidence suggests that the stress response can impact the GBA throughj the HPA axis [14].
There is a growing interest in the literature in intestinal events’ influence on the CNS’s functioning. The GBA, in particular, plays a vital role in the link between gastrointestinal and neurological diseases. Evidence suggests the influence of CD on neurological alterations or diseases, just as various neurological events are associated with alterations in the gastrointestinal ecosystem [15]. The ENS, a key player in intestinal immunity and inflammatory response, can have significant bidirectional consequences for the intestine and the CNS. Its dysfunction can lead to visceral hypersensitivity and chronic pain, which are common symptoms in CD [16,17]. Furthermore, neuroimmune inter- actions within the intestine during disease activity can mediate heightened intestinal permeability, resulting in elevated systemic levels of inflammatory factors. The dysbiosis, increased intestinal permeability, and translocation of bacteria and their metabolites in CD are being studied and recognized as important factors contributing to structural and functional alterations in the CNS [15]. This underscores the urgency and importance of our research, as chronic intestinal inflammation is associated with peripheral changes that disrupt CNS homeostasis, making it a focus of research on neurological disorders such as sleep disorders and neurodegenerative diseases. The focus of this SR was to gather evidence of the interaction between the GBA and CD.
Materials and methods
Study design
This is a systematic literature review. It was conducted by gathering all literature data that meet the eligibility criteria and by answering the question about the “Evidence of interaction between Crohn’s Disease and the Gut-Brain Axis”.
Strategies for method development
The study was constructed based on the recommendations in the “Preferred Reporting Items for SRs and Meta-Analyses (PRISMA)” of 2015 [18]. The PICO methodology was used to frame the research question to be answered, which stands for: P: Population; I: Interventions; C: Comparators; O: Outcome (Table 1).
Table 1.
Development of the systematic review’s guiding question using the PICO methodology
| Population | Patients with Crohn’s disease |
|---|---|
| Interventions | Studies illustrating cohorts of patients with Crohn’s disease exhibiting signs or neurological disorders |
| Comparators | Studies involving patients without Crohn’s disease presenting signs or neurological disorders |
| Outcomes | Association or lack of association between Crohn’s disease and signs or neurological disorders |
Inclusion criteria
The following criteria were adopted for the inclusion of studies in this Systematic Review: 1. Studies published in the literature from January 2017 to March 2024. 2. Studies on humans: (1) Population with IBD; (2) Adults (over 18 years old). 3. Studies analyzing signs, disorders, and neurological diseases in the presence of a CD diagnosis. 4. Observational studies (case-control, cohort, and cross-sectional). 5. Intervention studies (randomized clinical trials).
Systematic review strategy
This study was conducted as a SR, examining all relevant empirical evidence that met specific inclusion criteria. The primary aim was to investigate a specific question: “Evidence of interaction between Crohn’s Disease and the Gut-Brain Axis”. The process included the following key steps: formulating the research question, selecting appropriate databases for comprehensive exploration, defining the search timeframe, developing detailed search terms, systematically conducting searches across the selected databases, applying predefined inclusion and exclusion criteria, extracting pertinent data, selecting relevant studies, evaluating the quality and relevance of each study, and applying exclusion criteria as necessary.
On November 22, 2022, a search was conducted in the PROSPERO database to identify existing SRs on the topic of interest. PROSPERO is an international public database of SR protocols maintained by the Centre of Reviews and Dissemination at the University of York and funded by the National Institute for Health Research (NIHR). No records of SRs with the same focus as the current study were found. Consequently, the present study was registered in the PROSPERO system.
Selection of studies for comprehensive analysis
The free software Rayyan (Qatar Computing Research Institute - QCRI) was used for archiving, organizing, and selecting articles [19]. The selection of published articles was conducted in two stages. In the first stage, reviewers JFS and WMS independently assessed the titles and abstracts of the articles for inclusion or exclusion, with a third reviewer (JDCM) resolving conflicts. In the second stage, the same two reviewers independently assessed the full text of selected articles, with the third reviewer resolving conflicts as before.
Results
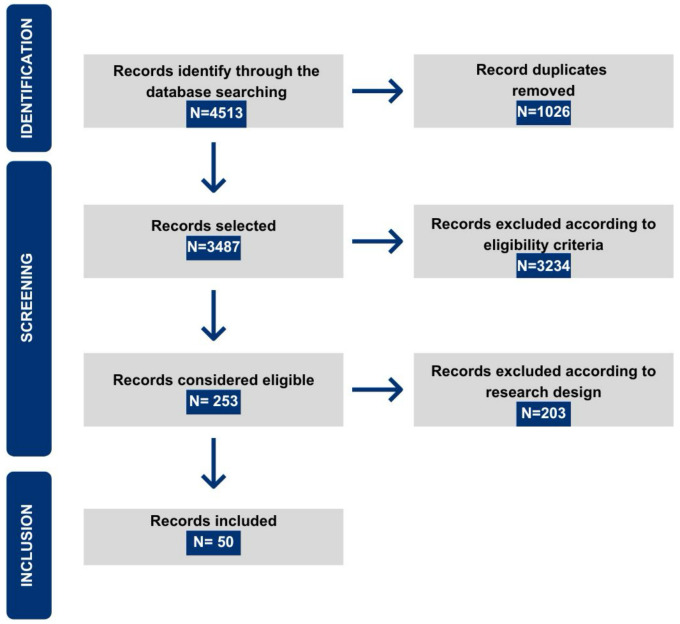
The search was conducted across seven databases for articles published between 2017 and 2024, with the final search conducted on March 8, 2024. A total of 4,513 studies were identified, and after excluding duplicates, 3,487 articles remained for analysis. The first phase of article analysis began with reading abstracts, selecting 253 studies for the second stage, which involved full-text reading. Following eligibility criteria, 50 articles were included in the SR (Figure 1).
Figure 1.
Diagram illustrating the inclusion of articles in the systematic review.
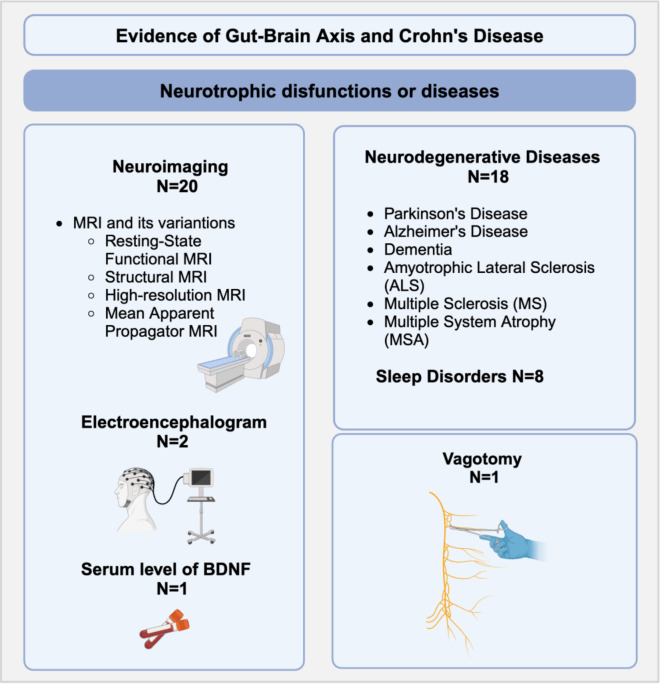
Of the 50 reviewed articles, 26 investigated various neurological disorders such as sleep disorders or neurodegenerative diseases like PD, AD, Dementia, Amyotrophic Lateral Sclerosis (ALS), Multiple Sclerosis (MS), and Multiple System Atrophy (MSA). Twenty-three articles examined the gut-brain interaction in CD through different methods, including neuroimaging analysis (20 articles), electroencephalogram (EEG) studies (2 articles), and one study on the serum level of Brain-derived neurotrophic factor (BDNF) in CD patients. Additionally, one study on vagotomy in CD patients was included. The studies exhibited considerable heterogeneity, even when addressing the same topic, resulting in occasionally conflicting findings. Tables 2, 3 and 4 show the studies and topics covered. Detailed results of the included studies can be found in Supplementary Tables 1, 2, 3. Figure 2 illustrates the methods used in the literature for functional or structural brain analysis in CD patients and neurological diseases.
Table 2.
Studies about neurological disorders (neurodegenerative diseases and sleep disorders)
| Neurological disorder | Author/Year |
|---|---|
| Multiple System Atrophy | Shadrin et al. 2021 [20] |
| Dementias | Sand et al. 2022 [21], Zingel et al. 2021 [22] |
| Sleep disorders | Bar-Gil Shitrit et al. 2018 [23], Chen et al. 2021 [24], Chrobak et al. 2018 [25], Georgiana-Emmanuela et al. 2020 [26], Hastaliklari et al. 2019 [27], Iskandar et al. 2020 [28], Kyle Hoffman et al. 2022 [29], Sofia et al. 2020 [30] |
| Alzheimer’s Disease | Aggarwal et al. 2020 [31] |
| Parkinson’s Disease | Camacho-Soto et al. 2018 [32], Freuer et al. 2022 [33], Hui et al. 2018 [34], Kang et al. 2022 [35], Loosen et al. 2023 [36], Park et al. 2019 [37], Wang et al. 2024 [38], Weimers et al. 2019 [39], Witoelar et al. 2017 [40], Zheng et al. 2022 [41] |
| Neurodegenerative Diseases | Li et al. 2022 [42] |
| Amyotrophic Lateral Sclerosis | Li et al. 2021 [43] |
| Multiple Sclerosis | Sonnenberg et al. 2023 [44] |
Table 3.
Studies employing neuroimaging, electroencephalography, or serum BDNF analysis in Crohn’s disease
| Examination techniques | Author/Year |
|---|---|
| MRI and variations | Agostini et al. 2023 [46], Bao et al. 2018 [47], Chen et al. 2023 [48], Fan et al. 2020 [49], Hou et al. 2019 [51], Hou et al. 2020 [52], Kong et al. 2022 [54], Kornelsen et al. 2020 [55], Li et al. 2021 [56], Liu et al. 2018 [57], Nair et al. 2019 [58], Qiu et al. 2022 [59], Thapaliya et al. 2023 [61], Thapaliya et al. 2023 [62], Thomann et al. 2017 [6], Thomann et al. 2021 [63], Thommann et al. 2017 [64], Yeske et al. 2024 [65], Zhang et al. 2021 [66] |
| EEG | Hall et al. 2023 [50], Kelleci et al. 2019 [53] |
| Serum evaluation of BDNF | Sochai et al. 2021 [60] |
Table 4.
Study about vagotomy and Crohn’s disease
| Neurological disorder | Author/Year |
|---|---|
| Vagotomy | Liu et al. [67] |
Figure 2.
Summary of methods used in the literature for functional or structural brain analysis in patients with CD and neurological diseases, along with the number (N) of related papers: MRI = Magnetic Resonance Imaging; BDNF = Brain-Derived Neurotrophic Factor. Created using Biorender software.
Discussion
Studies that deal with neurological disorders
Neurodegenerative diseases
While the association between PD and CD was particularly emphasized, the methods and outcomes varied considerably across studies. Among the 50 selected articles, 18 investigated the association between CD and neurodegenerative diseases. Of these, ten focused on PD, two on multiple types of dementia, one on AD, one on ALS, two on MS, and one on MSA.
Parkinson’s disease and Crohn’s disease
PD is recognized as the most prevalent neurodegenerative motor disorder globally. Emerging evidence suggests a potential link between PD and chronic low-level intestinal inflammation, which may trigger the aggregation of abnormal alpha-synuclein, an essential protein for PD pathogenesis. This protein could spread to the brain through the vagus nerve or breach the blood-brain barrier, whose permeability can be influenced by chronic intestinal inflammation [68,69]. This association has sparked interest in further exploring the potential link between these two diseases.
Five articles in the SR on PD explored data from electronic medical record analysis. Camacho-Soto et al. [32] showed that PD was inversely associated with CD. On the other hand, Park et al. [37] demonstrated that CD patients have a 2.2 times higher chance of developing PD, with corticosteroid treatment being a protective factor in these cases. LIkewise, Weimers [44] observed that patients with CD had a 30% higher overall risk of PD when compared to healthy patients. However, Loosen et al. [36] and Wang et al. [38] did not find a significant correlation between the diseases.
It is important to note that in the study by Camacho-Soto and Loosen, there was control for access and frequency of medical visits, which may explain why there was a contrasting result compared to other studies. This is because greater access to healthcare services can lead to surveillance bias, as there is a higher chance of diagnosis if the patient visits healthcare services more frequently. When Weimers [39] adjusted the analysis for the number of medical visits, the increased risk of PD in patients with CD disappeared, which confirms Loosen’s findings.
Freuer et al. [33] and Witoelar et al. [40] evaluated the association between PD and CD using GWAS to assess overlapping genes related to both diseases. While the former, focusing on causality analysis, did not show a significant causal association between PD and CD, the latter found genetic overlap, albeit associated with shared susceptibility gene loci.
Other studies on PD have explored the field of genetics, such as Hui et al. [34] and Kang et al. [35]. The former study found an association between the LRRK2 gene and genetic effects similar in PD and CD through exome sequencing. In the latter, shared genetic variants were identified in both diseases, which may indicate a possible common genetic basis between them. On the other hand, Zheng et al. [41] worked with analysis of peripheral blood transcriptomic databases, finding 178 genes differentially expressed in common between the two diseases (113 genes increased and 65 genes decreased).
Despite epidemiological studies presenting conflicting results and limitations, genetic studies provide data that raise the possibility of interaction between PD and CD. In this sense, future studies could focus on more careful analyses to avoid the biases found in the aforementioned studies.
Alzheimer’s disease, other dementias, and Crohn’s disease
Sand et al. [21] and Zingel et al. [22] investigated the potential association between CD and the risk of developing various types of dementia using medical record data analysis. However, their findings were contradictory. Sand et al. [26] reported an elevated risk of all-cause dementia among CD patients, with a particularly heightened risk for frontotemporal dementia. In contrast, Zingel et al. [27] found that CD was not significantly associated with an increased risk of dementia.
Additionally, Aggarwal et al. [31] explored the link between AD and CD in a retrospective study utilizing medical record databases while excluding other causes of dementia. Their findings indicated that individuals with CD who were over 65 years old and of Caucasian ethnicity had significantly higher odds of developing AD (P < 0.0001).
Amyotrophic lateral sclerosis, multiple system atrophy, and multiple sclerosis
There is evidence suggesting that ALS is related to immune system dysregulation, and it has been associated with a range of autoimmune or immune-related disorders that could potentially be precursors to its development [70]. Li et al. [43] explored the genetic correlation between ALS and around ten immune-mediated diseases, including CD. The authors investigated ALS and CD through GWAS to assess genetic associations. However, they found minimal and non-significant genetic association between CD and ALS.
Another neurodegenerative disease explored was MSA, which is also associated with abnormal aggregation of alpha-synuclein found in glial cells in this condition [71]. Through genomic association study data, Shadrin et al. [20] proposed a shared genetic etiology between MSA and CD in the C7 gene, indicating that genetic variability within C7 could modulate the risks of MSA and CD.
Yang et al. [45] and Sonnenberg et al. [44] studied MS, which is characterized by an inflammatory disease of the CNS that evolves with decreased cognitive capacity, bladder control, and mobility limitations [72]. The possibility of an association between MS and CD was raised due to their common epidemiological and immunological patterns [73]. The first study identified three shared single nucleotide polymorphisms between MS and CD; however, none were significant. The second found an association between MS and simultaneous diagnosis of CD.
One of the articles [42] of this SR addressed more than one neurodegenerative disease (PD, AD, and ALS) in the same analysis, using Mendelian Randomization Analysis. This statistical approach uses known genetic variants to investigate causal relationships, in this case, between CD and other neurodegenerative diseases. This method did not suggest any causal effect of CD on PD, ALS, or AD.
Studies on sleep disorders
Eight studies included in the review explored sleep disorders in CD patients. Four [25-30] studied data from subjective analyses using the Pittsburgh Sleep Quality Index (PSQI). Three studies reported higher sleep disturbance symptoms and poorer quality in CD patients compared to healthy adults, correlating worse outcomes with active disease. However, Hastaliklaria et al. found no significant differences in PSQI scores in CD patients and showed a higher eveningpreference in these patients. Bar-Gil Shitrit et al. [23] used Ambulatory Polysomnography in studies examining objective sleep quality parameters. They found that CD patients had less REM sleep and lighter sleep than control subjects. Iskandar et al. [28] objectively analyzed sleep using an actigraph and correlated it with the Harvey Bradshaw clinical scale. The result was surprising, showing that CD patients did not exhibit a significant alteration in sleep quality compared to control groups. Chen et al. [24] conducted a genetic study exploring databases to investigate the relationship between CD patients and sleep characteristics, finding no causal effect between the studied aspects and the disease. Meanwhile, Hoffman et al. [29] evaluated the association between Obstructive Sleep Apnea (OSA) and CD by investigating medical history information from over 4 million Americans. They found an increased risk of CD patients developing OSA.
Studies that rely solely on questionnaires to analyze sleep are limited, since they are subjective regarding the perception of the disease. Additionally, Iskandar’s study [28] exposes this limitation by showing that the perception of sleep by CD patients did not confirm the data collected by actigraphy. When analyzing the use of Polysomnography for this purpose, it is important to note that it does not simulate the patient’s usual sleep situation, as they are in an ambulatory environment for the exam, unlike actigraphy, where data is collected during the patient’s usual sleep routine. Therefore, it is not possible to analyze how sleeping in a non-habitual environment would impact the sleep of CD patients compared to healthy individuals. Thus, future studies could compare objective parameters to clarify any biases.
Studies that employed radiological, blood, and other exams
Neuroimaging exams
Twenty studies were included that used Magnetic Resonance Imaging (MRI) techniques to study the brains of CD patients in different ways. The most used was Resting-state functional MRI (rs-fMRI). Structural MRI (sMRI), high-resolution MRI (hrMRI), and mean apparent propagator MRI (MAP-MRI) were also used. Many findings are purely descriptive and do not correlate with specific clinical findings or neuronal functions. The results most frequently indicate neuronal changes related to abnormal brain activity and connectivity in CD patients, suggesting abnormal functionality related to visceral sensation regulation, pain processing, and neuroimmunity. Additionally, CD patients showed activation patterns similar to those found in older healthy adults, suggesting early brain aging in CD patients compared to controls without the disease. Similar to studies on neurodegenerative diseases, studies that addressed MRI methods were also heterogeneous and showed varied results related to changes in different brain areas. However, it is essential to note that the results were unanimous in showing that there are functional or structural alterations in the brains of CD patients compared to healthy adults.
Electroencephalogram and serum BDNF levels
Kelleci et al. [53] and Hall et al. [50] studied brain alterations in CD patients using EEG analysis, demonstrating that EEG abnormalities were significantly more common in CD patients and that the diagnosis of this disease is a determining factor in the risk of developing altered brain network signatures [50].
On the other hand, Sochal [60] explored the relationship between serum BDNF levels and CD. BDNF is a protein involved in several important brain functions, such as maintaining and forming synapses and neuronal connections. The study showed that CD patients had higher serum BDNF levels than healthy adults, which positively correlated with the severity of pain.
Study about vagotomy
Vagotomy is a surgical procedure that partially interrupts or removes the vagus nerve and is typically used to treat peptic ulcers. Some authors suggest that this procedure might affect the intestinal inflammatory response, potentially impacting the development and progression of CD. In a study by Liu et al. [67], which analyzed medical records, a positive association was found between vagotomy and CD patients. This suggests that vagotomy could contribute to immune dysregulation and an increased risk of CD.
Limitations and challenges of the systematic review
One of the limiting factors was the prevalence of studies that addressed IBD in general, grouping CD and UC in the same analysis without specifying data pertaining solely to CD. This lack of specificity precluded the inclusion of such studies in this SR. Additionally, the absence of control groups (without CD) in some studies was another exclusion criterion, as it rendered these studies incompatible with the PICO (Patient, Intervention, Comparison, Outcome) framework requirements, thereby failing to address the primary question of this review. Moreover, most studies identified were observational, which inherently limits their ability to provide conclusions beyond correlative findings.
Another limitation was the lack of studies exploring the mechanistic pathway and molecular interaction between the GBA and CD. One reason for this could be our focus on human studies, a choice made to ensure the relevance of our findings to clinical practice. Regarding neuroimaging research on CD, the studies were quite descriptive and did not explain the findings in-depth.
These challenges underscore the complexity of conducting an SR on this topic and highlight the need for more targeted and methodologically rigorous studies to draw more definitive conclusions regarding the neurological aspects of CD and its interaction with the GBA.
Conclusion
There is a growing interest in elucidating the relationship between CD and the GBA. The extant literature encompasses diverse studies with heterogeneous methodologies, ranging from epidemiological investigations to research on functional and structural brain alterations. Despite this diversity, there is no consensus regarding the precise relationship between CD and GBA, underscoring the necessity for further research. This SR has highlighted the significant potential of this field to enhance our understanding of the prognosis and even the etiology of CD. Consequently, advancing research in this area promises to yield valuable insight that may inform more effective interventions and deepen our comprehension of the intricate mechanisms linking CD with neurological and psychiatric manifestations.
Acknowledgements
We would like to thank the librarian, Ana Paula de Morais, for her assistance in finding the articles in the database. We also thank Prof. Tristan Guillermo Torriani for reviewing the English version of the manuscript. This work was supported by the National Council for Scientific and Technological Development (CNPq) [Grant scholarship number #302557/2021-0 for R.F.L.]. J.F.S. (author) and H.D.C. (coauthor) received a Master of Science scholarship from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil), Finance Code 001. W.M.S. (coauthor) received an undergraduate scholarship from CNPq [2023].
Disclosure of conflict of interest
None.
Abbreviations
- AD
Alzheimer’s disease
- AIS
Insomnia Scale
- ALS
Amyotrophic Lateral Sclerosis
- ANS
Autonomic nervous system
- BBB
Blood-brain barrier
- BDNF
Brain-derived neurotrophic factor
- CD
Crohn’s Disease
- CNS
Central Nervous System
- EEG
Electroencephalogram
- ENS
Enteric nervous system
- FC
Functional Connectivity
- GBA
Gut-Brain Axis
- GIT
gastrointestinal tract
- HBI
Harvey-Bradshaw Index
- HPA
hypothalamic-pituitary-adrenal axis
- hrMRI
High-resolution MRI
- IC
Indeterminate Colitis
- MAP-MRI
Mean Apparent Propagator Magnetic Resonance Imaging
- MRI
Magnetic Resonance Imaging
- MS
Multiple Sclerosis
- MSA
Multiple System Atrophy
- NIHR
National Institute for Health Research
- OSA
Obstructive Sleep Apnea
- PD
Parkinson’s Disease
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSQI
Pittsburg Sleep Quality Index
- rs-fMRI
Resting-State Functional Magnetic Resonance Imaging
- sMRI
Structural MRI
- SR
Systematic Review
- UC
Ulcerative Colitis
Supporting Information
References
- 1.Dolinger M, Torres J, Vermeire S. Crohn’s disease. Lancet. 2024;403:1177–1191. doi: 10.1016/S0140-6736(23)02586-2. [DOI] [PubMed] [Google Scholar]
- 2.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 3.Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B, Wang HE, Bai YM, Tsai SJ, Su TP, Chen TJ, Wang YP, Chen MH. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2021;70:85–91. doi: 10.1136/gutjnl-2020-320789. [DOI] [PubMed] [Google Scholar]
- 5.Zhu F, Li C, Gong J, Zhu W, Gu L, Li N. The risk of Parkinson’s disease in inflammatory bowel disease: a systematic review and meta-analysis. Dig Liver Dis. 2019;51:38–42. doi: 10.1016/j.dld.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Thomann AK, Griebe M, Thomann PA, Hirjak D, Ebert MP, Szabo K, Reindl W, Wolf RC. Intrinsic neural network dysfunction in quiescent Crohn’s disease. Sci Rep. 2017;7:11579. doi: 10.1038/s41598-017-11792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Yu R, Zhu L, Hou X, Zou K. Bidirectional regulation of circadian disturbance and inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1741–1751. doi: 10.1097/MIB.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 8.Osadchiy V, Martin CR, Mayer EA. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17:322–332. doi: 10.1016/j.cgh.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer EA, Nance K, Chen S. The gut-brain axis. Annu Rev Med. 2022;73:439–453. doi: 10.1146/annurev-med-042320-014032. [DOI] [PubMed] [Google Scholar]
- 10.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Rusch JA, Layden BT, Dugas LR. Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis. Front Endocrinol (Lausanne) 2023;14:1130689. doi: 10.3389/fendo.2023.1130689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Günther C, Rothhammer V, Karow M, Neurath M, Winner B. The gut-brain axis in inflammatory bowel disease-current and future perspectives. Int J Mol Sci. 2021;22:8870. doi: 10.3390/ijms22168870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, Regev A. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182:1606–1622. e23. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess A, Roesch J, Saake M, Sergeeva M, Hirschmann S, Neumann H, Dörfler A, Neurath MF, Atreya R. Functional brain imaging reveals rapid blockade of abdominal pain response upon anti-TNF therapy in Crohn’s disease. Gastroenterology. 2015;149:864–866. doi: 10.1053/j.gastro.2015.05.063. [DOI] [PubMed] [Google Scholar]
- 18.Galvão T, Pansani T, Harrad D. Principais itens para relatar Revisões sistemáticas e Meta-análises: a recomendação PRISMA. Epidemiologia e Serviços de Saúde. 2015;24:335–342. [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shadrin AA, Mucha S, Ellinghaus D, Makarious MB, Blauwendraat C, Sreelatha AAK, Heras-Garvin A, Ding J, Hammer M, Foubert-Samier A, Meissner WG, Rascol O, Pavy-Le Traon A, Frei O, O’Connell KS, Bahrami S, Schreiber S, Lieb W, Müller-Nurasyid M, Schminke U, Homuth G, Schmidt CO, Nöthen MM, Hoffmann P, Gieger C, Wenning G European Multiple System Atrophy Study Group. Gibbs JR, Franke A, Hardy J, Stefanova N, Gasser T, Singleton A, Houlden H, Scholz SW, Andreassen OA, Sharma M. Shared genetics of multiple system atrophy and inflammatory bowel disease. Mov Disord. 2021;36:449–459. doi: 10.1002/mds.28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rønnow Sand J, Troelsen FS, Horváth-Puhó E, Henderson VW, Sørensen HT, Erichsen R. Risk of dementia in patients with inflammatory bowel disease: a Danish population-based study. Aliment Pharmacol Ther. 2022;56:831–843. doi: 10.1111/apt.17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zingel R, Bohlken J, Kostev K. Association between inflammatory bowel disease and dementia: a retrospective cohort study. J Alzheimers Dis. 2021;80:1471–1478. doi: 10.3233/JAD-210103. [DOI] [PubMed] [Google Scholar]
- 23.Bar-Gil Shitrit A, Chen-Shuali C, Adar T, Koslowsky B, Shteingart S, Paz K, Grisaru-Granovsky S, Goldin E, Epstein Shochet G, Shitrit D. Sleep disturbances can be prospectively observed in patients with an inactive inflammatory bowel disease. Dig Dis Sci. 2018;63:2992–2997. doi: 10.1007/s10620-018-5207-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Peng WY, Tang TC, Zheng H. Differential sleep traits have no causal effect on inflammatory bowel diseases: a mendelian randomization study. Front Pharmacol. 2021;12:763649. doi: 10.3389/fphar.2021.763649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chrobak AA, Nowakowski J, Zwolińska-Wcisło M, Cibor D, Przybylska-Feluś M, Ochyra K, Rzeźnik M, Dudek A, Arciszewska A, Siwek M, Dudek D. Associations between chronotype, sleep disturbances and seasonality with fatigue and inflammatory bowel disease symptoms. Chronobiol Int. 2018;35:1142–1152. doi: 10.1080/07420528.2018.1463236. [DOI] [PubMed] [Google Scholar]
- 26.Gîlc-Blanariu GE, Ştefnescu G, Trifan AV, Moscalu M, Dimofte MG, Ştefnescu C, Drug VL, Afrsnie VA, Ciocoiu M. Sleep impairment and psychological distress among patients with inflammatory bowel disease-beyond the obvious. J Clin Med. 2020;9:2304. doi: 10.3390/jcm9072304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahbaz C, Biberci EK. Chronotype and sleep quality in patients with inflammatory bowel disease. Med Bull Haseki. 2020;58:72–77. [Google Scholar]
- 28.Iskandar HN, Linan EE, Patel A, Moore R, Lasanajak Y, Gyawali CP, Sayuk GS, Ciorba MA. Self-reported sleep disturbance in Crohn’s disease is not confirmed by objective sleep measures. Sci Rep. 2020;10:1980. doi: 10.1038/s41598-020-58807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman K, Mansoor E, Panhwar MS, Regueiro M, Cooper G, Qazi T. Prevalence of obstructive sleep apnea is increased in patients with inflammatory bowel disease: a large, multi-network study. Crohns Colitis 360. 2022;4:otac026. doi: 10.1093/crocol/otac026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sofia MA, Lipowska AM, Zmeter N, Perez E, Kavitt R, Rubin DT. Poor sleep quality in Crohn’s disease is associated with disease activity and risk for hospitalization or surgery. Inflamm Bowel Dis. 2020;26:1251–1259. doi: 10.1093/ibd/izz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal M, Alkhayyat M, Abou Saleh M, Sarmini MT, Singh A, Garg R, Garg P, Mansoor E, Padival R, Cohen BL. Alzheimer disease occurs more frequently in patients with inflammatory bowel disease: insight from a nationwide study. J Clin Gastroenterol. 2023;57:501–507. doi: 10.1097/MCG.0000000000001714. [DOI] [PubMed] [Google Scholar]
- 32.Camacho-Soto A, Gross A, Searles Nielsen S, Dey N, Racette BA. Inflammatory bowel disease and risk of Parkinson’s disease in Medicare beneficiaries. Parkinsonism Relat Disord. 2018;50:23–28. doi: 10.1016/j.parkreldis.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freuer D, Meisinger C. Association between inflammatory bowel disease and Parkinson’s disease: a Mendelian randomization study. NPJ Parkinsons Dis. 2022;8:55. doi: 10.1038/s41531-022-00318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui KY, Fernandez-Hernandez H, Hu J, Schaffner A, Pankratz N, Hsu NY, Chuang LS, Carmi S, Villaverde N, Li X, Rivas M, Levine AP, Bao X, Labrias PR, Haritunians T, Ruane D, Gettler K, Chen E, Li D, Schiff ER, Pontikos N, Barzilai N, Brant SR, Bressman S, Cheifetz AS, Clark LN, Daly MJ, Desnick RJ, Duerr RH, Katz S, Lencz T, Myers RH, Ostrer H, Ozelius L, Payami H, Peter Y, Rioux JD, Segal AW, Scott WK, Silverberg MS, Vance JM, Ubarretxena-Belandia I, Foroud T, Atzmon G, Pe’er I, Ioannou Y, McGovern DPB, Yue Z, Schadt EE, Cho JH, Peter I. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci Transl Med. 2018;10:eaai7795. doi: 10.1126/scitranslmed.aai7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang X, Ploner A, Wang Y, Ludvigsson JF, Williams DM, Pedersen NL, Wirdefeldt K. Genetic overlap between Parkinson’s disease and inflammatory bowel disease. Brain Commun. 2023;5:fcad002. doi: 10.1093/braincomms/fcad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loosen SH, Yaqubi K, May P, Konrad M, Gollop C, Luedde T, Kostev K, Roderburg C. Association between inflammatory bowel disease and subsequent development of restless legs syndrome and Parkinson’s disease: a retrospective cohort study of 35,988 primary care patients in Germany. Life (Basel) 2023;13:897. doi: 10.3390/life13040897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S, Kim J, Chun J, Han K, Soh H, Kang EA, Lee HJ, Im JP, Kim JS. Patients with inflammatory bowel disease are at an increased risk of Parkinson’s disease: a South Korean nationwide population-based study. J Clin Med. 2019;8:1191. doi: 10.3390/jcm8081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HL, Wang ZY, Tian J, Ma DR, Shi CH. Association between inflammatory bowel disease and Parkinson’s disease: a prospective cohort study of 468,556 UK biobank participants. Front Aging Neurosci. 2024;15:1294879. doi: 10.3389/fnagi.2023.1294879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weimers P, Halfvarson J, Sachs MC, Saunders-Pullman R, Ludvigsson JF, Peter I, Burisch J, Olén O. Inflammatory bowel disease and Parkinson’s disease: a nationwide swedish cohort study. Inflamm Bowel Dis. 2019;25:111–123. doi: 10.1093/ibd/izy190. [DOI] [PubMed] [Google Scholar]
- 40.Witoelar A, Jansen IE, Wang Y, Desikan RS, Gibbs JR, Blauwendraat C, Thompson WK, Hernandez DG, Djurovic S, Schork AJ, Bettella F, Ellinghaus D, Franke A, Lie BA, McEvoy LK, Karlsen TH, Lesage S, Morris HR, Brice A, Wood NW, Heutink P, Hardy J, Singleton AB, Dale AM, Gasser T, Andreassen OA, Sharma M International Parkinson’s Disease Genomics Consortium (IPDGC), North American Brain Expression Consortium (NABEC), and United Kingdom Brain Expression Consortium (UKBEC) Investigators. Genome-wide pleiotropy between parkinson disease and autoimmune diseases. JAMA Neurol. 2017;74:780–792. doi: 10.1001/jamaneurol.2017.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng H, Qian X, Tian W, Cao L. Exploration of the common gene characteristics and molecular mechanism of Parkinson’s disease and Crohn’s disease from transcriptome data. Brain Sci. 2022;12:774. doi: 10.3390/brainsci12060774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Wen Z. Effects of ulcerative colitis and Crohn’s disease on neurodegenerative diseases: a Mendelian randomization study. Front Genet. 2022;13:846005. doi: 10.3389/fgene.2022.846005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li CY, Yang TM, Ou RW, Wei QQ, Shang HF. Genome-wide genetic links between amyotrophic lateral sclerosis and autoimmune diseases. BMC Med. 2021;19:27. doi: 10.1186/s12916-021-01903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonnenberg A, Duong HT, McCarty DJ, El-Serag HB. Concurrence of inflammatory bowel disease with multiple sclerosis or Hodgkin lymphoma. Eur J Gastroenterol Hepatol. 2023;35:1349–1353. doi: 10.1097/MEG.0000000000002657. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Musco H, Simpson-Yap S, Zhu Z, Wang Y, Lin X, Zhang J, Taylor B, Gratten J, Zhou Y. Investigating the shared genetic architecture between multiple sclerosis and inflammatory bowel diseases. Nat Commun. 2021;12:5641. doi: 10.1038/s41467-021-25768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agostini A, Benuzzi F, Ballotta D, Rizzello F, Gionchetti P, Filippini N. Differential brain structural and functional patterns in Crohn’s disease patients are associated with different disease stages. Inflamm Bowel Dis. 2023;29:1297–1305. doi: 10.1093/ibd/izad029. [DOI] [PubMed] [Google Scholar]
- 47.Bao C, Liu P, Liu H, Jin X, Shi Y, Wu L, Zeng X, Zhang J, Wang D, Calhoun VD, Tian J, Wu H. Difference in regional neural fluctuations and functional connectivity in Crohn’s disease: a resting-state functional MRI study. Brain Imaging Behav. 2018;12:1795–1803. doi: 10.1007/s11682-018-9850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen F, Zhang S, Li P, Xu K, Liu C, Geng B, Piao R, Liu P. Disruption of periaqueductal gray-default mode network functional connectivity in patients with Crohn’s disease with abdominal pain. Neuroscience. 2023;517:96–104. doi: 10.1016/j.neuroscience.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Fan Y, Bao C, Wei Y, Wu J, Zhao Y, Zeng X, Qin W, Wu H, Liu P. Altered functional connectivity of the amygdala in Crohn’s disease. Brain Imaging Behav. 2020;14:2097–2106. doi: 10.1007/s11682-019-00159-8. [DOI] [PubMed] [Google Scholar]
- 50.Hall CV, Radford-Smith G, Savage E, Robinson C, Cocchi L, Moran RJ. Brain signatures of chronic gut inflammation. Front Psychiatry. 2023;14:1250268. doi: 10.3389/fpsyt.2023.1250268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou J, Mohanty R, Nair VA, Dodd K, Beniwal-Patel P, Saha S, Prabhakaran V. Alterations in resting-state functional connectivity in patients with Crohn’s disease in remission. Sci Rep. 2019;9:7412. doi: 10.1038/s41598-019-43878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou J, Dodd K, Nair VA, Rajan S, Beniwal-Patel P, Saha S, Prabhakaran V. Alterations in brain white matter microstructural properties in patients with Crohn’s disease in remission. Sci Rep. 2020;10:2145. doi: 10.1038/s41598-020-59098-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelleci UA, Calhan T, Sahin A, Aydin-Ozemir Z, Kahraman R, Ozdil K, Sokmen HM, Yalcin AD. Electroencephalography findings in Crohn’s disease. Clin EEG Neurosci. 2019;50:129–133. doi: 10.1177/1550059418767589. [DOI] [PubMed] [Google Scholar]
- 54.Kong N, Gao C, Zhang F, Zhang M, Yue J, Lv K, Zhang Q, Fan Y, Lv B, Zang Y, Xu M. Neurophysiological effects of the anterior cingulate cortex on the exacerbation of Crohn’s disease: a combined fMRI-MRS study. Front Neurosci. 2022;16:840149. doi: 10.3389/fnins.2022.840149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornelsen J, Wilson A, Labus JS, Witges K, Mayer EA, Bernstein CN. Brain resting-state network alterations associated with Crohn’s disease. Front Neurol. 2020;11:48. doi: 10.3389/fneur.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Ma J, Xu JG, Zheng YL, Xie Q, Rong L, Liang ZH. Brain functional changes in patients with Crohn’s disease: a resting-state fMRI study. Brain Behav. 2021;11:e2243. doi: 10.1002/brb3.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, Ma J, Hua X, Zhou Y, Qiu Y, Zhu Z, Zheng Y, Xie Q, Liang Z, Xu J. Altered intra- and inter-network functional connectivity in patients with Crohn’s disease: an independent component analysis-based resting-state functional magnetic resonance imaging study. Front Neurosci. 2022;16:855470. doi: 10.3389/fnins.2022.855470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nair VA, Dodd K, Rajan S, Santhanubosu A, Beniwal-Patel P, Saha S, Prabhakaran V. A verbal fluency task-based brain activation fMRI study in patients with Crohn’s disease in remission. J Neuroimaging. 2019;29:630–639. doi: 10.1111/jon.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu Y, Li Q, Wu D, Zhang Y, Cheng J, Cao Z, Zhou Y. Altered mean apparent propagator-based microstructure and the corresponding functional connectivity of the parahippocampus and thalamus in Crohn’s disease. Front Neurosci. 2022;16:985190. doi: 10.3389/fnins.2022.985190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sochal M, Małecka-Panas E, Gabryelska A, Fichna J, Talar-Wojnarowska R, Szmyd B, Białasiewicz P. Brain-derived neurotrophic factor is elevated in the blood serum of Crohn’s disease patients, but is not influenced by anti-TNF-α treatment-A pilot study. Neurogastroenterol Motil. 2021;33:e13978. doi: 10.1111/nmo.13978. [DOI] [PubMed] [Google Scholar]
- 61.Thapaliya G, Eldeghaidy S, Asghar M, McGing J, Radford S, Francis S, Moran GW. The relationship between Central Nervous System morphometry changes and key symptoms in Crohn’s disease. Brain Imaging Behav. 2023;17:149–160. doi: 10.1007/s11682-022-00742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thapaliya G, Eldeghaidy S, Radford SJ, Francis ST, Moran GW. An examination of resting-state functional connectivity in patients with active Crohn’s disease. Front Neurosci. 2023;17:1265815. doi: 10.3389/fnins.2023.1265815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomann AK, Schmitgen MM, Kmuche D, Ebert MP, Thomann PA, Szabo K, Gass A, Griebe M, Reindl W, Wolf RC. Exploring joint patterns of brain structure and function in inflammatory bowel diseases using multimodal data fusion. Neurogastroenterol Motil. 2021;33:e14078. doi: 10.1111/nmo.14078. [DOI] [PubMed] [Google Scholar]
- 64.Thomann AK, Reindl W, Wüstenberg T, Kmuche D, Ebert MP, Szabo K, Wolf RC, Hirjak D, Niesler B, Griebe M, Thomann PA. Aberrant brain structural large-scale connectome in Crohn’s disease. Neurogastroenterol Motil. 2019;31:e13593. doi: 10.1111/nmo.13593. [DOI] [PubMed] [Google Scholar]
- 65.Yeske B, Hou J, Chu DY, Adluru N, Nair VA, Beniwal-Patel P, Saha S, Prabhakaran V. Structural brain morphometry differences and similarities between young patients with Crohn’s disease in remission and healthy young and old controls. Front Neurosci. 2024;18:1210939. doi: 10.3389/fnins.2024.1210939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang S, Chen F, Wu J, Liu C, Yang G, Piao R, Geng B, Xu K, Liu P. Altered structural covariance and functional connectivity of the insula in patients with Crohn’s disease. Quant Imaging Med Surg. 2022;12:1020–1036. doi: 10.21037/qims-21-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu B, Wanders A, Wirdefeldt K, Sjölander A, Sachs MC, Eberhardson M, Ye W, Ekbom A, Olén O, Ludvigsson JF. Vagotomy and subsequent risk of inflammatory bowel disease: a nationwide register-based matched cohort study. Aliment Pharmacol Ther. 2020;51:1022–1030. doi: 10.1111/apt.15715. [DOI] [PubMed] [Google Scholar]
- 68.Spielman LJ, Gibson DL, Klegeris A. Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem Int. 2018;120:149–163. doi: 10.1016/j.neuint.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M, Adler CH, Derkinderen P, Beach TG. Does Parkinson’s disease start in the gut? Acta Neuropathol. 2018;135:1–12. doi: 10.1007/s00401-017-1777-8. [DOI] [PubMed] [Google Scholar]
- 70.Beers DR, Appel SH. Immune dysregulation in amyotrophic lateral sclerosis: mechanisms and emerging therapies. Lancet Neurol. 2019;18:211–220. doi: 10.1016/S1474-4422(18)30394-6. [DOI] [PubMed] [Google Scholar]
- 71.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 72.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622–1636. doi: 10.1016/S0140-6736(18)30481-1. [DOI] [PubMed] [Google Scholar]
- 73.Kosmidou M, Katsanos AH, Katsanos KH, Kyritsis AP, Tsivgoulis G, Christodoulou D, Giannopoulos S. Multiple sclerosis and inflammatory bowel diseases: a systematic review and meta-analysis. J Neurol. 2017;264:254–259. doi: 10.1007/s00415-016-8340-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.