Abstract
Objectives: To investigate the expression of matrix protein metalloenzymes (MMPs) during cerebral aneurysm (CA) formation and assess the effects of resveratrol (RES) on MMP expression and CA prevention. Methods: Male Sprague-Dawley rats were randomly divided into three groups: sham-operated, CA, and RES groups. CA models were constructed by ligating the renal and carotid arteries of SD rats. The RES group received a diet mixed with RES (50 mg/kg), while the CA group was given normal feed; the sham-operated group underwent simulated surgery without ligation and received normal feed. HE staining was used to observe the pathological changes in the cerebral artery aneurysm wall. Immunofluorescence (IF) staining and RT-PCR were used to detect the expression of MMP-2 and MMP-9, as well as oxidative stress markers in the cerebral artery wall tissues of rats at 1-, 2-, and 3-months post-surgery. Results: HE staining reveled that after ligation, the cerebral artery walls of SD rats exhibited irregular thickness, twisted morphology, abnormal nuclear morphology of the cells, and infiltration of inflammatory cells, confirming the successful establishment of CA model. Meanwhile, the infrared spectrogram of the RES purified from Tiger Balm closely matched that of the standard, confirming successful purification. IF staining indicated that MMP-2 and MMP-9 levels dynamically increased over time in the vessel wall of the CA rats. Subsequently, antioxidant assays showed that RES treatment enhanced antioxidant capacity, with increased levels of superoxide dismutase, glutathione peroxidase, and catalase in the vascular wall tissue. Moreover, after 3 months of RES treatment, IF staining showed a marked reduction in MMP-2 and MMP-9 levels in the vessel walls of CA rats. Meanwhile, HE staining also showed improvements in the wall structure, with a more intact wall and an increased vascular endothelial cell density. Conclusions: RES effectively inhibits the expression of MMP-2 and MMP-9, thereby preventing and delaying the development of CA.
Keywords: MMP, CA, cerebral aneurysm, resveratrol
Introduction
Cerebral aneurysm (CA) is characterized by abnormal bulge in the wall of an arterial blood vessel, influenced by a combination of genetics, environment, and lifestyle factors [1]. The global prevalence of CA is rising annually, with an increased incidence among younger populations [2]. When a CA ruptures, it can lead to acute neurological symptoms such as severe headaches and loss of consciousness, posing an immediate threat to the patient’s life [3]. Even in the absence of rupture, CA can lead to cerebral ischemia, presenting as headaches, facial paralysis, and visual or speech problems. Additionally, direct compression by CA on neural structures may result in neurological damage such as hearing loss and facial paralysis [4]. This has heightened clinical concern regarding the pathogenesis and risks associated with CA, thus driving the pursuit of novel prevention and treatment strategies.
Among the various factors contributing to CA development, matrix metalloproteinases (MMPs) have gained significant attention [5]. These enzymes are crucial for degradation and remodeling of the extracellular matrix and are involved in processes such as cell migration, tissue repair, development, and inflammation [6]. Research has increasingly focused on MMPs, particularly MMP-2 and MMP-9, due to their involvement in arterial wall remodeling and inflammatory responses, which may accelerate CA formation [7]. Tao et al. [8] demonstrated that elevated MMP levels in an atherosclerosis rat model contributed to aneurysm formation and rupture. Therefore, targeting MMPs could be a viable strategy to prevent and inhibit CA development.
Recent studies have explored the potential of natural products to inhibit MMP-2 and MMP-9 activity. For example, flavonoids in quercetin, kaempferol, and hawthorn demonstrate inhibitory activity against MMP-2 and -9. Hilliard et al. [9] showed that they exhibit specific heterogeneous activity at micromolar concentrations. Similarly, green tea polyphenols also exhibit MMP inhibitory activity, demonstrating potential therapeutic effects on CA [10,11]. However, challenges such as low bioavailability, poor stability, significant side effects, and complex structure limit the application of these natural products in experimental and therapeutic applications. Resveratrol (RES), a natural polyphenol present in red grapes, blueberries, peanuts, and red wine, is known for its antioxidant, anti-inflammatory, and anti-cancer properties [12]. Relevant studies have found that RES may reduce the damage of inflammatory factors on blood vessel walls through its powerful antioxidant and anti-inflammatory effects [13]. Moreover, RES has been reported to inhibit MMP-2 and -9 activities, thereby maintaining blood vessel wall stability [14]. In addition, it has also been shown to inhibit platelet aggregation, promote vascular endothelial cell function, and enhance blood vessel elasticity, offering further protective benefits [15]. Therefore, RES may be a promising candidate for inhibiting CA development.
Given the limited research on RES’s role in CA development, this study aims to explore the involvement of MMPs in CA formation and assess whether RES can intervene in CA development by inhibiting MMP-2 and -9 expressions. A deeper understanding of these molecular mechanisms is expected to provide new directions for the early prevention and treatment of CA, potentially improving patient outcomes and quality of life.
Materials and methods
Materials and animals
Anti-MMP2 antibody (ab92536), anti-MMP9 antibody (ab76003), anti-superoxide dismutase (SOD) antibody (ab80946), anti-glutathione peroxidase (GSH-Px) antibody (ab108427), and anti-catalase (CAT) antibody (ab209211), DAPI (ab285390), FITC (ab6717) Alexa Fluor® 568 (ab175471), Alexa Fluor® 647 (ab150079), and Alexa Fluor® 488 (ab150077) were all purchased form Abcam, USA. In addition, 45 male Sprague-Dawley (SD) rats weighing approximately 300 g were selected for the establishment of CA models. The animals were individually fed in the animal center under a controlled climate (24°C, 30 to 60% humidity) with free access to standard diet and drinking water. This study was approved by the Ethics Committee of Hebei Medical University. All experiments were conducted in accordance with the guidelines outlined in the Animal Research: Reporting of in vivo Experiments [ARRIVE] guidelines.
Preparation of RES
RES was extracted from tiger nut root in the following steps: 1) Grinding and pulverization: the roots were ground and pulverized to increase the extraction efficiency and the surface area. 2) Solvent extraction: the grounded samples were extracted with 70% ethanol in a material-to-liquid ratio of 1:20. 3) Ultrasonic extraction: the mixture was subjected to ultrasonic treatment for 1 h to accelerate the extraction process and increase the extraction efficiency. 4) Filtration and concentration: the extract was filtered through a filter or centrifuge to remove suspended matter and impurities. Then, the extract was concentrated by rotary evaporation at 50°C to obtain the crude RES extract. 5) Purification: the crude extract was further processed by adding twice the volume of petroleum ether. The petroleum ether phase was collected and the extraction repeated until the petroleum ether was colorless. 6) Drying: The petroleum ether was removed by rotary evaporation at 40°C, and the remaining was dried in a blast dryer at 50°C to obtain RES. The concentration of RES in the samples was determined using high performance liquid chromatography technique (HPLC).
Fourier transform infrared spectroscopy (FTIR) of RES
A small amount of the extracted RES was analyzed using a Fourier transform infrared spectrometer (INVENIO, Spectral Quality Analysis and Detection Technology Co., Ltd., Shanghai, China). The analysis was conducted with reference to a standard RES sample to ensure accuracy in spectral comparison.
Construction of CA models
After anesthesia by intraperitoneal injection of sodium pentobarbital (50 mg/kg), SD rats were fixed on the operating table. Subsequently, the surgical area was disinfected, and the posterior branches of the bilateral renal arteries and the left common carotid artery were exposed and ligated to induce the formation of CA [16]. In the sham operation group, the renal arteries and the left carotid artery were only exposed without ligation. Postoperatively, the rats’ temperature, pulse, and respiration were continuously monitored until extubation. After extubation, their vital signs were recorded every 5 minutes until they regained mobility. Comprehensive postoperative care, including appropriate analgesia, anti-infection, and monitoring of fecal production and food intake, was provided for 3 days postoperatively to assess the recovery of their overall body condition. Finally, the rats were divided into sham-operated and CA groups. The rats in the sham-operated group were fed in 3 cages, with 5 rats in each group, corresponding to 1-, 2-, and 3-month groups, and those in CA group were also fed in three cages, corresponding to 1-, 2-, and 3-month groups, with 10 rats in each group.
Anti-aneurysm effects of RES
RES feeding
To evaluate the anti-aneurysm effects of resveratrol (RES), 50% of the rats in the CA group were randomly assigned to the RES group. The rats were fed a diet supplemented with RES (50 mg/kg) [17] (Figure 1), and the remaining rats in CA and sham-operated groups were given normal diets.
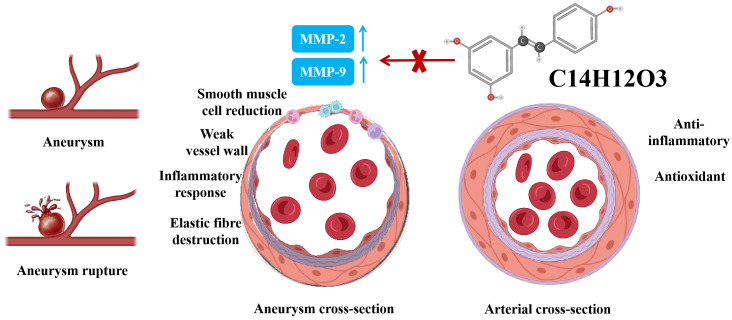
Figure 1.
Mechanism of RES in preventing progression of CA. Note: RES: resveratrol; CA: aneurysm; MMP-2: matrix protein metalloenzyme - 2; MMP-9: matrix protein metalloenzyme - 9.
HE staining of aneurysm specimens
At 1, 2, and 3 months postoperatively, rats from the sham-operated, CA, and RES groups were deeply anesthetized by tail vein injection with excess sodium pentobarbital (100 mg/kg) for euthanasia. Then, the brain of each rat was removed by craniotomy and the formation of intracranial aneurysms was carefully observed under a microscope. The vascular tissue at the site of aneurysm formation was excised, fixed, washed and dehydrated before being embedded in paraffin. Sections of 4 microns were prepared, deparaffinized, stained with hematoxylin and eosin (HE), and rehydrated for microscopic examination of the pathological changes associated with CA.
Immunofluorescence (IF) staining
Sections of rats in each group were dewaxed, dehydrated, and labelled with anti-MMP-9 antibody to detect MMP-9 in the vessel wall. After incubation overnight at 4°C, fluorescein isothiocyanate (FITC) was added as the secondary antibody to form a fluorescently labelled complex. Simultaneously, DAPI (ab285390) was used to stain the cell nuclei. Finally, the sections were observed using a confocal microscope and fluorescent images were captured. The levels of MMP-2 in the vessel wall were measured by the same method, in which anti-MMP2 and FITC were used as primary and secondary antibodies, respectively. Finally, the levels of MMP-9 and -2 in the vessel wall of rats in each group were measured by PCR for quantitative analysis (Table 1).
Table 1.
PCR primer information
| MMP-2 | 5’ forward 3’ | CTGATAACCTGGATGCAGTCGT |
| 3’ reverse 5’ | CCAGCCAGTCCGATTTGA | |
| MMP-9 | 5’ forward 3’ | TTCAAGGACGGTCGGTATT |
| 3’ reverse 5’ | CTCGAGCCTAGACCCAACTTA | |
| GAPDH | 5’ forward 3’ | AAGAAGGTGGTGAAGCAGGC |
| 3’ reverse 5’ | TCCACCACCCTGTTGCTGTA |
Note: MMP-2: matrix protein metalloenzyme - 2; MMP-9: matrix protein metalloenzyme - 9; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Antioxidant function of RES
Sections from each group were de-paraffinized, rehydrated, and subsequently labeled with anti-SOD, anti-GSH-Px, and anti-CAT antibodies to assess the level of oxidative stress within the vessel wall. Meanwhile, Alexa Fluor® 568, Alexa Fluor® 647, and Alexa Fluor® 488 conjugated fluorescent secondary antibodies were added. Confocal microscopy was used to observe the sections and capture fluorescent images. Finally, SOD, GSH-Px, and CAT levels of rats in each group were quantified using PCR (Table 1).
Statistical analysis
Data were collected from at least three independent experiments and analyzed using GraphPad Prism7 (GraphPad Prism software, San Diego, CA, USA). All data were presented as the mean ± standard deviation (SD). The student’s t-test was applied to assess significant differences between groups. Statistical significance was determined at P < 0.05.
Results
FTIR of RES
Comparison with standard RES spectrograms confirmed that our extracted RES closely matched the reference. As shown in Figure 2, both samples had similar absorption peaks near 3250 cm-1, characteristic of the alcohol phenolic hydroxyl group in RES. In addition, absorption peaks near 1630, 1604, 1510, and 1458 cm-1, corresponding to the benzene ring vibrations in RES, were similarly observed. A distinctive absorption peak at 962 cm-1, indicative of trans -C=C- bonds, was also noted. While the extraction of RES from Tiger Balm was successful, the relatively low concentration of RES in the samples likely resulted in weaker absorption peaks in their infrared spectra.
Figure 2.
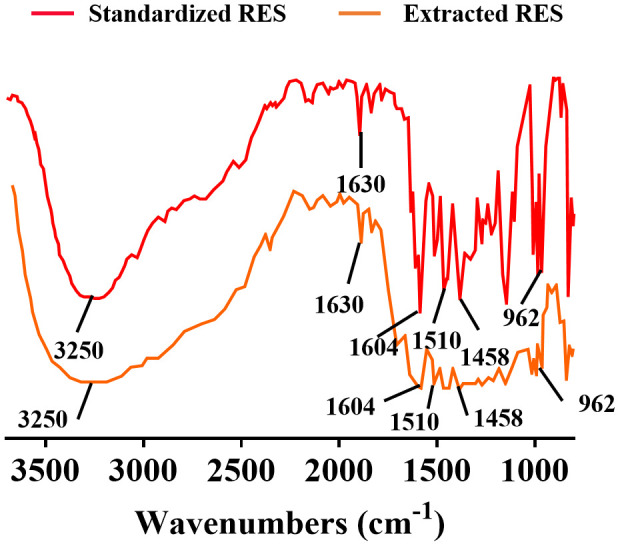
FTIR of RES extracted from tiger nut root. Note: FTIR: Fourier transform infrared spectroscopy; RES: resveratrol.
HE staining of rat arterial rings in each group
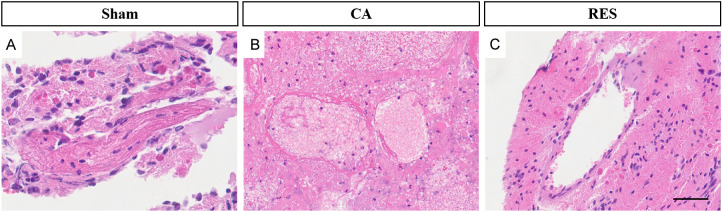
Compared with the pathological sections of the rats in the sham-operated group (Figure 3A), the vascular walls of the rats in CA group showed irregular thickness and twisted morphology, and the nuclei of the cells were abnormally shaped, showing enlargement, anisotropy, or hyper-staining. Meanwhile, damaged and degenerated elastic fibers were noted within the vascular walls, along with the presence of inflammatory cell infiltration in the local tissues (Figure 3B). These observations confirm that the successful establishment of the CA model.
Figure 3.
Rat vascular wall morphology in the sham-operated (A) and CA groups (B) examined by HE staining (40 μm). Note: CA: cerebral aneurysm.
Markedly elevated MMP-2 and -9 levels were found in CA models
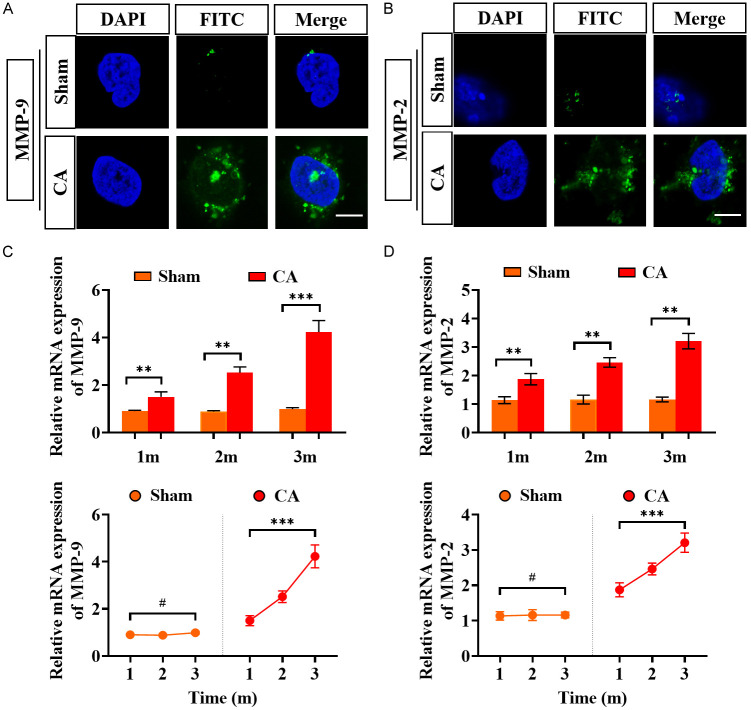
IF staining revealed that MMP-2 and -9 levels in the cytoplasm of endothelial cells of CA rats were markedly elevated compared with those of the sham-operated group (Figure 4A, 4B). In addition, PCR quantification revealed the same results, showing a time-dependent increase in MMP-2 and -9 levels, with a significantly higher level at the third month after modeling than that at the first month (Figure 4C, 4D). This indicates that MMP-2 and -9 levels dynamically increase during CA formation.
Figure 4.
Detection of MMP-2 and MMP-9 levels in CA rats. A: Fluorescent staining of nuclei, MMP-9, and both combined in the endothelial cells of rats in the sham-operated and CA groups. B: Fluorescent staining of nuclei, MMP-2, and both combined in the endothelial cells of rats in the sham-operated and CA groups. C, D: Comparison of PCR quantification results for MMP-2 and -9. #P > 0.05, **P < 0.01, ***P < 0.001. Scale bar: 5 μm. n=5. Note: MMP-2: matrix protein metalloenzyme - 2; MMP-9: matrix protein metalloenzyme - 9; CA: cerebral aneurysm.
RES exhibited notable antioxidant effects
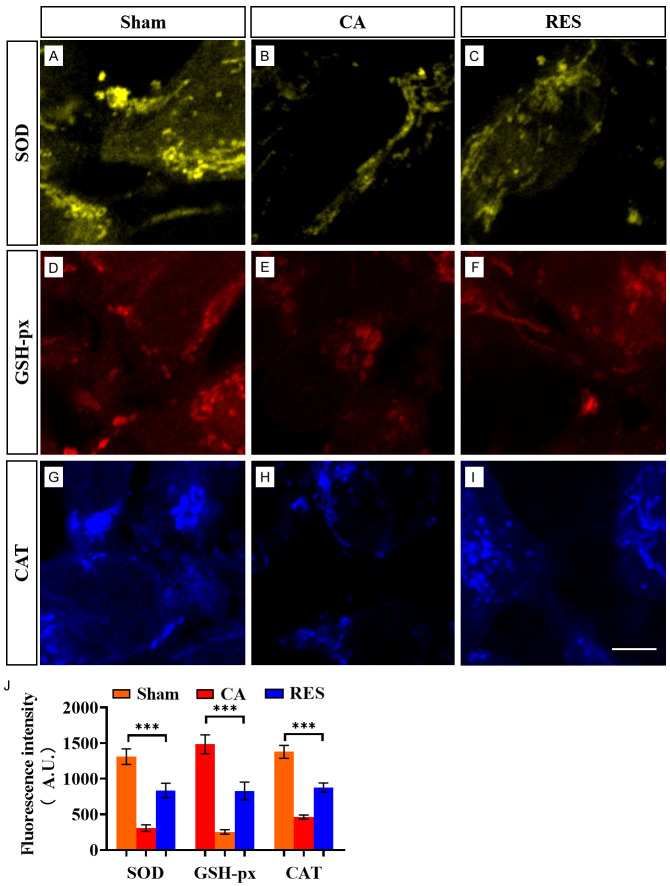
The generation of oxygen free radicals has a promotional effect on MMP levels and activity; therefore, monitoring the degree of oxidative stress can be effective in assessing the level of MMP activity. IF staining demonstrated that the levels of SOD, GSH, and CAT in the vessel wall tissue of CA rats were markedly lower than those in the sham-operated group, indicating decreased antioxidant capacity. In contrast, RES effectively improved the antioxidant capacity of CA rats, as evidenced by markedly improved levels of these enzymes (Figure 5A-I). Quantitative analysis of the fluorescence intensity confirmed these findings (Figure 5J). Therefore, RES can effectively improve the antioxidant capacity of CA rats.
Figure 5.
Evaluation of RES antioxidant function. A-C: Comparison of SOD levels among sham-operated, CA, or RES groups. D-F: Comparison of GSH levels among sham-operated, CA, or RES groups. G-I: Comparison of CAT levels among sham-operated, CA, or RES groups. J: Quantitative analysis of fluorescence intensity of SOD, GSH and CAT. ***P < 0.001. Scale bar: 20 μm. Note: CA: cerebral aneurysm; RES: resveratrol; SOD: superoxide dismutase; GSH-px: glutathione peroxidase; CAT: catalase.
RES effectively reduced MMP-2 and -9 levels in CA rats
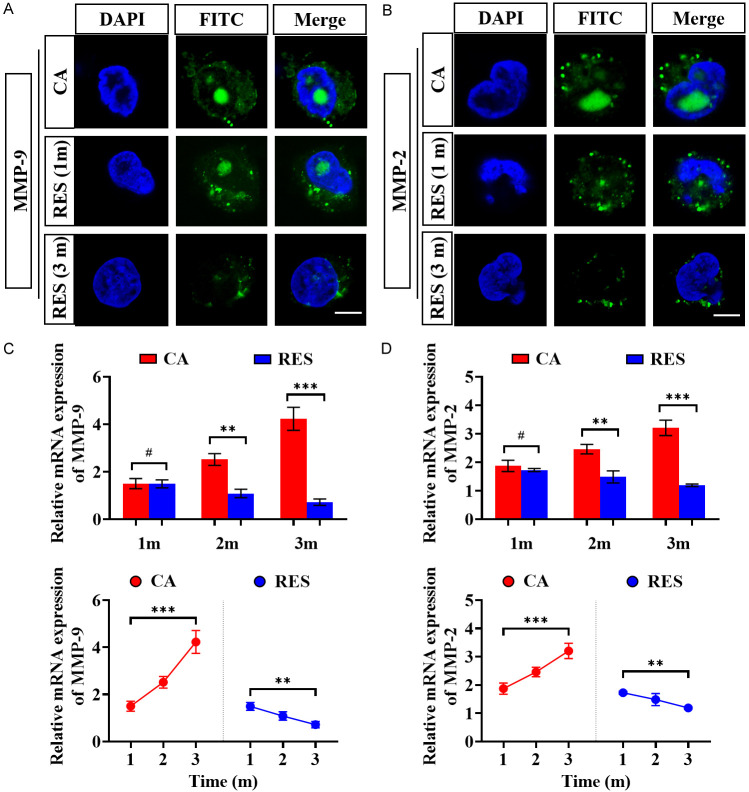
To further clarify whether RES reduces the levels of MMP-2 and -9, we examined their levels in the vessel walls of RES-fed CA rats. IF staining showed that RES feeding effectively reduced the levels of the above indexes in the vessel wall of CA rats (Figure 6A, 6B). PCR showed the same results, and a more pronounced reduction in MMP-2 and -9 levels after three months of RES feeding compared to one month (Figure 6C, 6D), suggesting that RES reduced MMP-2 and -9 levels in a time-dependent manner.
Figure 6.
Detection of MMP-2 and MMP-9 levels in RES-fed CA rats. A: Fluorescent staining of nuclei, MMP-9, and both combined in the endothelial cells of rats in the CA and RES groups (at 1st and 3rd month). B: Fluorescent staining of nuclei, MMP-2, and both combined in the endothelial cells of rats in the CA and RES groups (at 1st and 3rd month). C, D: Comparison of PCR quantification results for MMP-2 and -9. #P > 0.05, **P < 0.01, ***P < 0.001. Scale bar: 5 μm. n=5. Note: MMP-2: matrix protein metalloenzyme - 2; MMP-9: matrix protein metalloenzyme - 9; CA: cerebral aneurysm; RES: resveratrol.
RES was effective in preventing CA
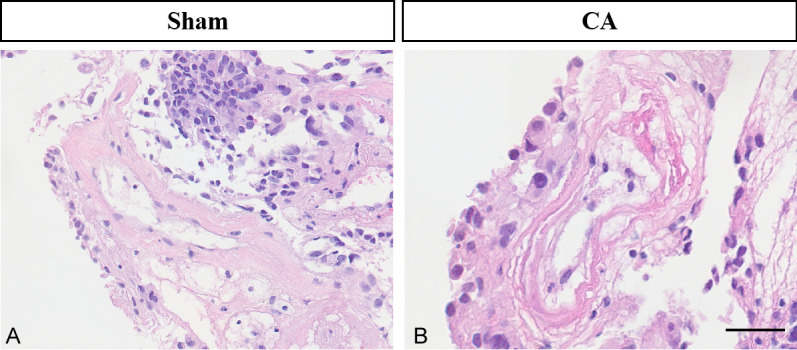
HE staining demonstrated significant improvements in the vascular wall tissues of CA rats following RES supplementation. Notable changes included increased regularity of the vascular lumen, a more intact vessel wall, and an increased number of endothelial cells (Figure 7). These results indicate that RES can effectively delay the development of CA.
Figure 7.
Rat vascular wall tissue morphology in the sham-operated (A), CA (B), and RES groups (C) examined by HE staining (60 μm). Note: CA: cerebral aneurysm; RES: resveratrol.
Discussion
Resveratrol (RES) is a naturally occurring polyphenolic compound found in various plants, renowned for its diverse biological functions including antioxidant, anti-tumor, and neuroprotective effects [18,19]. This study focuses on exploring RES’s effect on MMP-2 and -9 levels during CA formation and assess its potential effectiveness in CA prevention and treatment. We successfully extracted RES from Tiger Balm and confirmed its purity through FTIR analysis, which revealed characteristic absorption peaks at 3250, 1630, 1604, 1510, 1458, and 962 cm-1. These characteristic absorption peaks confirmed the successful extraction of RES, providing a reliable foundation for exploring its biological effects in CA treatment.
In this study, an aneurysm model was constructed by ligating the renal and carotid arteries. HE staining revealed pathological changes in the aneurysm wall, including thinning, unclear structural boundaries, and inflammatory cell infiltration, confirming the successful establishment of the CA model. Meanwhile, MMP-2 and -9, normally present at low levels and involved in extracellular matrix remodeling and repair, were significantly elevated in the aneurysm wall of the CA model. The reason may be attributed to altered intravascular hemodynamics after ligation of the renal arteries, causing the onset of stress-induced damage and structural changes in the arterial wall, while MMP-2 and -9, which degrade and remodel extracellular matrix proteins, are upregulated to facilitate matrix remodeling and aneurysm formation [20,21]. Further, elevated MMP-2 and MMP-9 activity supports endothelial and smooth muscle cell migration, adhesion, and proliferation, contributing to aneurysm development through matrix changes and neovascularization [22,23]. Therefore, a significant increase in MMP-2 and -9 within the aneurysm wall plays an important role in enabling endothelial and smooth muscle cells to cooperatively remodel the peri-aneurysm tissue, including matrix changes and neovascularization. Moreover, early aneurysm formation may also be linked to vessel wall injury or inflammation, with endothelial cells producing inflammatory mediators that amplify local inflammation and MMP activity [24-26]. Therefore, excessive MMP-2 and -9 activity is associated with vessel wall inflammation, disruption, and aneurysm formation.
RES administration significantly enhanced antioxidant capacity in CA rats and led to a marked reduction in MMP-2 and MMP-9 levels. This effect is likely due to RES’s ability to mitigate oxidative stress and inflammation. During aneurysm formation, inflammatory stimuli and metabolic abnormalities generate excessive oxygen free radicals, causing vascular endothelial cell damage, collagen degradation, and inflammatory responses. These oxygen free radicals further activate MMPs, exacerbating matrix degradation and remodeling [27,28]. RES’s antioxidant properties reduce free radicals production, thereby decreasing the level of oxidative stress and inflammation, and mitigating MMPs overactivation [19,29]. In addition, it has been suggested that RES may affect the synthesis and activity of MMP-2 and -9 by directly or indirectly regulating their gene expression [30,31]. Therefore, RES not only markedly reduced MMP-2 and -9 levels in CA rats but also enhanced the in vivo antioxidant level, positively impacting CA prevention.
However, this study has some limitations. First, while RES’s effects on MMPs during CA formation were investigated, the carrier used for RES, which may affect its stability and bioavailability in vivo, was not considered. Second, no positive control drug group, such as doxycycline, was included to further assess RES’s anti-CA effects. Third, the study did not explore the anti-inflammatory effects of RES, which are important in CA formation. Finally, the relatively short study duration limited the ability to fully evaluate the long-term effects of RES on CA treatment. Therefore, future studies should take these factors into account more comprehensively to fully assess the effects and mechanisms of RES as a potential therapeutic agent for CA.
Conclusion
In conclusion, during the formation of CA, the levels of MMP-2 and -9 are dynamically elevated and lead to the destruction and degeneration of elastic fibers in the aneurysm wall, causing remodeling of the vessel wall. RES has significant antioxidant effects and can effectively reduce the level of oxidative stress within the aneurysm wall. Meanwhile, RES can also effectively inhibit the expression of MMP-2 and -9, thus preventing and delaying the occurrence of CA.
Disclosure of conflict of interest
None.
References
- 1.Silva MA, Chen S, Starke RM. Unruptured cerebral aneurysm risk stratification: background, current research, and future directions in aneurysm assessment. Surg Neurol Int. 2022;13:182. doi: 10.25259/SNI_1112_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sukun A, Cekic B. Assessment of BNP and BDNF results in elective endovascular cerebral aneurysm treatment. Ir J Med Sci. 2022;191:1899–1903. doi: 10.1007/s11845-021-02791-0. [DOI] [PubMed] [Google Scholar]
- 3.Monsour M, Croci DM, Grüter BE, Taussky P, Marbacher S, Agazzi S. Cerebral aneurysm and interleukin-6: a key player in aneurysm generation and rupture or just one of the multiple factors. Transl Stroke Res. 2023;14:631–639. doi: 10.1007/s12975-022-01079-4. [DOI] [PubMed] [Google Scholar]
- 4.Kamide T, Misaki K, Tsutsui T, Nambu I, Yoshikawa A, Nakada M. Comparison of endovascular therapy for ruptured cerebral aneurysm during spasm and nonspasm period. Asian J Neurosurg. 2022;17:412–415. doi: 10.1055/s-0042-1750782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Guo Z, Xie D, Lin Z, Lin R. Relationship between MMP-9 gene polymorphism and intracranial aneurysm. Cell Mol Biol (Noisy-le-grand) 2022;68 doi: 10.14715/cmb/2022.68.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Amin SA, Jha T. Inhibitors of gelatinases (MMP-2 and MMP-9) for the management of hematological malignancies. Eur J Med Chem. 2021;223:113623. doi: 10.1016/j.ejmech.2021.113623. [DOI] [PubMed] [Google Scholar]
- 7.Nascimento GC, De Paula BB, Gerlach RF, Leite-Panissi C. Temporomandibular inflammation regulates the matrix metalloproteinases MMP-2 and MMP-9 in limbic structures. J Cell Physiol. 2021;236:6571–6580. doi: 10.1002/jcp.30341. [DOI] [PubMed] [Google Scholar]
- 8.Tao Y, Zhang L, Yang R, Yang Y, Jin H, Zhang X, Hu Q, He B, Shen Z, Chen P. Corilagin ameliorates atherosclerosis by regulating MMP-1, -2, and -9 expression in vitro and in vivo. Eur J Pharmacol. 2021;906:174200. doi: 10.1016/j.ejphar.2021.174200. [DOI] [PubMed] [Google Scholar]
- 9.Hilliard A, Mendonca P, Russell TD, Soliman K. The protective effects of flavonoids in cataract formation through the activation of Nrf2 and the inhibition of MMP-9. Nutrients. 2020;12:3651. doi: 10.3390/nu12123651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar J, Nandy SK, Chowdhury A, Chakraborti T, Chakraborti S. Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomed Pharmacother. 2016;84:340–347. doi: 10.1016/j.biopha.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Setozaki S, Minakata K, Masumoto H, Hirao S, Yamazaki K, Kuwahara K, Ikeda T, Sakata R. Prevention of abdominal aortic aneurysm progression by oral administration of green tea polyphenol in a rat model. J Vasc Surg. 2017;65:1803–1812. e2. doi: 10.1016/j.jvs.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Agbadua OG, Kúsz N, Berkecz R, Gáti T, Tóth G, Hunyadi A. Oxidized resveratrol metabolites as potent antioxidants and xanthine oxidase inhibitors. Antioxidants (Basel) 2022;11:1832. doi: 10.3390/antiox11091832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahjabeen W, Khan DA, Mirza SA. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: a randomized, placebo-controlled trial. Complement Ther Med. 2022;66:102819. doi: 10.1016/j.ctim.2022.102819. [DOI] [PubMed] [Google Scholar]
- 14.Chang WS, Tsai CW, Yang JS, Hsu YM, Shih LC, Chiu HY, Bau DT, Tsai FJ. Resveratrol inhibited the metastatic behaviors of cisplatin-resistant human oral cancer cells via phosphorylation of ERK/p-38 and suppression of MMP-2/9. J Food Biochem. 2021;45:e13666. doi: 10.1111/jfbc.13666. [DOI] [PubMed] [Google Scholar]
- 15.Rabbani N, Xue M, Weickert MO, Thornalley PJ. Reversal of insulin resistance in overweight and obese subjects by trans-resveratrol and hesperetin combination-link to dysglycemia, blood pressure, dyslipidemia, and low-grade inflammation. Nutrients. 2021;13:2374. doi: 10.3390/nu13072374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo D, Wang YW, Ma J, Yan L, Li TF, Han XW, Shui SF. Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm. Asian Pac J Trop Med. 2016;9:499–502. doi: 10.1016/j.apjtm.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Li WF, Guo Y, Yang X, Zhang B, Zhang J. The effects of resveratrol on EPCs of peripheral blood, VEGF and TGF-β expression levels in rats with cerebral aneurysm. CSTPCD. 2020;27:618–621. [Google Scholar]
- 18.Mu Q, Najafi M. Resveratrol for targeting the tumor microenvironment and its interactions with cancer cells. Int Immunopharmacol. 2021;98:107895. doi: 10.1016/j.intimp.2021.107895. [DOI] [PubMed] [Google Scholar]
- 19.Zaparina O, Rakhmetova AS, Kolosova NG, Cheng G, Mordvinov VA, Pakharukova MY. Antioxidants resveratrol and SkQ1 attenuate praziquantel adverse effects on the liver in Opisthorchis felineus infected hamsters. Acta Trop. 2021;220:105954. doi: 10.1016/j.actatropica.2021.105954. [DOI] [PubMed] [Google Scholar]
- 20.Gajewska B, Śliwińska-Mossoń M. Association of MMP-2 and MMP-9 Polymorphisms with diabetes and pathogenesis of diabetic complications. Int J Mol Sci. 2022;23:10571. doi: 10.3390/ijms231810571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao HM, Jin L, Liu Y, Hong X. Changes in expressions of miR-22-3p and MMP-9 in rats with thoracic aortic aneurysm and their significance. Eur Rev Med Pharmacol Sci. 2020;24:6949–6954. doi: 10.26355/eurrev_202006_21686. [DOI] [PubMed] [Google Scholar]
- 22.Wang JC, Tsai SH, Tsai HY, Lin SJ, Huang PH. Hyperuricemia exacerbates abdominal aortic aneurysm formation through the URAT1/ERK/MMP-9 signaling pathway. BMC Cardiovasc Disord. 2023;23:55. doi: 10.1186/s12872-022-03012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K, Cui MZ, Zhang KW, Wang GQ, Zhai ST. Effect of miR-21 on rat thoracic aortic aneurysm model by regulating the expressions of MMP-2 and MMP-9. Eur Rev Med Pharmacol Sci. 2020;24:878–884. doi: 10.26355/eurrev_202001_20072. [DOI] [PubMed] [Google Scholar]
- 24.Qi F, Liu Y, Zhang K, Zhang Y, Xu K, Zhou M, Zhao H, Zhu S, Chen J, Li P, Du J. Artificial intelligence uncovers natural MMP inhibitor crocin as a potential treatment of thoracic aortic aneurysm and dissection. Front Cardiovasc Med. 2022;9:871486. doi: 10.3389/fcvm.2022.871486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Li R, Li Y, Li G, Zhao Y, Mou H, Chen Y, Xiao L, Gong K. Transcription factor TCF3 promotes macrophage-mediated inflammation and MMP secretion in abdominal aortic aneurysm by regulating miR-143-5p/CCL20. J Cardiovasc Pharmacol. 2023;82:458–469. doi: 10.1097/FJC.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Z, Wang J, Su Q, Luan M, Chen X, Xu X. The role of MMP-2 and MMP-9 in the metastasis and development of hypopharyngeal carcinoma. Braz J Otorhinolaryngol. 2021;87:521–528. doi: 10.1016/j.bjorl.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan X, Bian X, Wei W, Bao Q, Liu P, Jiang W. miR-34a regulates phenotypic modulation of vascular smooth muscle cells in intracranial aneurysm by targeting CXCR3 and MMP-2. Genet Mol Biol. 2021;44:e20200124. doi: 10.1590/1678-4685-GMB-2020-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji Y, Huang W, Chen Y, Zhang X, Wu F, Tang W, Lu Z, Huang C. Inhibition of MMP-2 and MMP-9 attenuates surgery-induced cognitive impairment in aged mice. Brain Res Bull. 2023;204:110810. doi: 10.1016/j.brainresbull.2023.110810. [DOI] [PubMed] [Google Scholar]
- 29.Alharris E, Mohammed A, Alghetaa H, Zhou J, Nagarkatti M, Nagarkatti P. The ability of resveratrol to attenuate ovalbumin-mediated allergic asthma is associated with changes in microbiota involving the gut-lung axis, enhanced barrier function and decreased inflammation in the lungs. Front Immunol. 2022;13:805770. doi: 10.3389/fimmu.2022.805770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amontree M, Nelson M, Stefansson L, Pak D, Maguire-Zeiss K, Turner RS, Conant K. Resveratrol differentially affects MMP-9 release from neurons and glia; implications for therapeutic efficacy. J Neurochem. 2024 doi: 10.1111/jnc.16031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Kodarahmian M, Amidi F, Moini A, Kashani L, Shabani Nashtaei M, Pazhohan A, Bahramrezai M, Berenjian S, Sobhani A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: a randomized exploratory trial. Gynecol Endocrinol. 2019;35:719–726. doi: 10.1080/09513590.2019.1576612. [DOI] [PubMed] [Google Scholar]