Abstract
Objective: To identify risk factors for postoperative recurrence in patients with eosinophilic chronic rhinosinusitis with nasal polyps (ECRSwNP) and develop a nomogram prediction model for identifying patients at high risk of recurrence. Methods: A cohort study involving 200 ECRSwNP patients analyzed clinical data for recurrence predictors using univariate and multivariate logistic regression analyses. A nomogram model was developed and validated using Receiver Operating Characteristic (ROC) curves. Mean absolute error (MAE) calculations evaluated the model’s predictive accuracy. Results: At six-month follow-up, 39 patients (19.5%) experienced recurrence. Factors such as preoperative tissue eosinophil percentage, eosinophil cationic protein (ECP), serum-specific immunoglobulin E (IgE), interleukin-5 (IL-5), and postoperative nasal environment were identified as risk factors for recurrence. The nomogram incorporating these factors demonstrated high predictive accuracy (AUC = 0.989, MAE = 0.026). Conclusion: This study underscores the significance of individualized risk assessment in managing ECRSwNP recurrence. The developed nomogram provides a robust tool for clinical prognostication, aiding personalized treatment strategies and improving patient outcomes.
Keywords: Eosinophilic chronic sinusitis with nasal polyps, postoperative recurrence, risk factors, nomo diagram model
Introduction
Chronic rhinosinusitis (CRS) is a common upper respiratory tract disease characterized by chronic inflammation of the nasal mucosa. It is a highly heterogeneous condition resulting from interactions between environmental factors and host immune dysfunction, accompanied by multifactorial tissue remodeling, with a prevalence rate of approximately 5% to 12% [1,2]. CRS can lead to airway remodeling, marked by epithelial lesions, thickening of the basement membrane, fibrosis, and goblet cell hyperplasia. These changes significantly impact the physical and mental well-being and overall quality of life of affected individuals [3].
Nasal endoscopy categorizes CRS into two main phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps [4]. Nasal polyps usually present bilaterally and originate from the ethmoid sinuses. Currently, no specific genetic or environmental factors are strongly associated with CRSwNP development. However, based on genetic characteristics, CRSwNP is broadly classified into eosinophilic (ECRSwNP) and non-eosinophilic (NonECRSwNP) subtypes [5]. In China, the prevalence of ECRSwNP is increasing. Clinically, patients often experience nasal polyps, sinus orifice obstruction, and impaired drainage of nasal secretions, leading to nasal congestion, facial discomfort, and diminished olfaction [6].
To address these issues, functional nasal endoscopic surgery combined with pharmacologic therapy is typically employed to alleviate symptoms and improve quality of life in ECRSwNP patients. However, postoperative recurrence is common [7]. Previous studies have shown a gradual increase in recurrence rates over time, with rates exceeding 90% after ten years of follow-up [8]. Controlling ECRSwNP recurrence post-surgery remains a significant challenge for both surgeons and patients. Identifying consistent predictors of postoperative recurrence across different patient populations is crucial for improving treatment outcomes and patient prognosis.
This study addresses the urgent need to identify reliable predictors of postoperative recurrence in ECRSwNP. By retrospectively analyzing data from 200 ECRSwNP patients treated at the Department of Otolaryngology, People’s Hospital of Yubei District, Chongqing, between January 2016 and December 2022, we developed a postoperative recurrence risk model. This model facilitates the early identification of patients at high risk of recurrence, thus extending remission periods and improving long-term prognosis. The study aims to offer valuable insights into the management of ECRSwNP, reducing the risk of postoperative recurrence and enhancing the quality of life for affected patients.
Materials and methods
General information
This retrospective study analyzed clinical data from 200 patients diagnosed with ECRSwNP who were admitted to the People’s Hospital of Yubei District, Chongqing, between January 2016 and December 2022.
Inclusion criteria: (1) Met the diagnostic criteria for ECRSwNP [9]. (2) Indicated for surgery and underwent functional nasal endoscopy to remove nasal polyps and diseased tissues. (3) Received no treatment one month before surgery.
Exclusion criteria: (1) History of previous nasal endoscopic surgery. (2) Presence of fungal sinusitis, nasal or sinus deformity, or concomitant sinus malignancy. (3) Primary ciliary dysfunction or cystic fibrosis. (4) Women who were pregnant or breastfeeding. (5) Patients with significant missing clinical data (missing data rate >20%).
This study was approved by the Ethics Committee of the People’s Hospital of Yubei District, Chongqing.
Diagnostic criteria
The primary symptoms of CRSwNP included nasal congestion and the presence of thick or sticky nasal mucus. Secondary symptoms included facial pressure or pain, and a reduced or lost sense of smell. Diagnosis was confirmed if one primary symptom and at least two secondary symptoms were present, along with evidence of sinus inflammation and nasal polyps on CT scan or nasal endoscopy.
During surgery, nasal polyp samples were collected, fixed in a 4% formaldehyde solution for 24 hours, and then embedded in paraffin. The samples were sectioned into 5 μm slices and underwent hematoxylin and eosin (HE) staining. Initially, 1-2 sections with satisfactory staining and intact tissue structure were identified at low magnification (X50). Next, five fields with prominent eosinophilic infiltration were identified under low magnification (X100). Eosinophil counts were conducted in each of these five fields at high magnification (X400), and the diagnostic outcome was based on the average eosinophil count across these fields. A diagnosis of ECRSwNP was confirmed if the average eosinophil percentage was ≥10%.
Postoperative recurrence was diagnosed if patients’ symptoms did not significantly improve, nasal polyps and thick mucus were visible on nasal endoscopy, the mucosa of the sinus cavity was congested and edematous, and the nasal orifice was re-narrowed or atretic. Patients were categorized into recurrence and non-recurrence groups based on findings at the 6-month postoperative follow-up.
Observation indicators
Primary indicators
These included the collection of patients’ general information (gender, age, weight, BMI, disease duration, smoking history, and alcohol abuse history) and preoperative blood indicators, such as peripheral blood eosinophil percentage, tissue eosinophil percentage, serum specific IgE (sIgE), eosinophil cationic protein (ECP), interleukin-5 (IL-5), interleukin-6 (IL-6), and interleukin-1β (IL-1β).
Secondary indicators
These included intraoperative factors, such as the duration of nasal endoscopic surgery, and postoperative factors, such as the presence of infection in the nasal environment (including sinus and nasal tract complex obstruction) and the pH value of the rinsing fluid in the operative cavity. Postoperative rehabilitation compliance was categorized as follows: 1. Complete compliance: Patients fully adhered to medical staff instructions during the perioperative period without showing resistance. 2. Partial compliance: Patients mostly adhered to instructions, with occasional psychological resistance. 3. Non-compliance: Patients did not follow medical staff instructions, exhibiting psychological conflict and non-compliant behavior. 4. Postoperative follow-up and recurrence: Patients were followed up for six months post-surgery through a combination of telephone calls and outpatient reviews. The first follow-up occurred two weeks post-surgery to remove old blood and secretions via nasal endoscopy. The second follow-up was one month post-surgery to remove nasal scabs and visible vesicles. Subsequent follow-ups were conducted every two months, and postoperative recurrence was recorded during the six-month follow-up period.
Detection methods
Within 24 hours of admission, each patient provided two 4 mL samples of peripheral venous blood. Blood samples were processed using a centrifuge (Shaanxi Zhengyuan Science and Technology Development Co., Ltd., model: PCM20). One sample was centrifuged at 3500 rpm for 10 minutes (centrifugation radius: 11.5 cm), and the serum layer was preserved at -20°C for analysis. The other sample of whole blood was stored for future testing. Serum sIgE levels were measured using an immunoturbidimetric assay with an immunoglobulin E (IgE) kit (Hunan Yonghe Sunshine Bio-technology Co., Ltd., specification: 100 mL × 8/100 mL × 4). This assay tested for both inhalant allergens (e.g., cat or dog dander, dust, dust mites) and ingestible allergens (e.g., shrimp, crab, pineapple, milk, mango, cashews). An enzyme marker (Beijing Municipal Government of China) was used for result analysis. Serum levels of IL-5, IL-6, IL-1β, and ECP were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) with an enzyme labeling instrument (Beijing Hongrunda Science and Technology Development Co., Ltd., model: ZS-2).
Before surgery, eosinophils in peripheral blood were counted using an automatic blood cell analyzer (Shenzhen Myriad Biomedical Electronics Co., Ltd., model: BC-5310), and their percentage was calculated. For histopathological examination, nasal polyp specimens collected during surgery were fixed in a 4% formaldehyde solution, embedded in paraffin, sectioned into 5 μm slices, and stained with HE.
Statistical method
Data analysis was performed using SPSS 27.0 statistical software. Categorical data were presented as [cases (%)], and comparisons were made using the χ2 test. Continuous data, confirmed to follow a normal distribution via the Shapiro-Wilk (S-W) test, were expressed as mean ± standard deviation (x̅ ± sd), and the independent t-test was used for comparisons between groups. For non-normally distributed data, the Mann-Whitney U test was applied, with results represented by the median (M) and interquartile range (P25, P75). Risk factors for postoperative recurrence of ECRSwNP were analyzed using a multivariate logistic regression model. A nomogram prediction model was then constructed using R version 4.2.1, and its accuracy was validated. A p-value of <0.05 was considered statistically significant.
Results
General data comparison
This study included 200 patients diagnosed with ECRSwNP. Based on the six-month postoperative follow-up, patients were divided into two groups: the recurrence group (n = 39) and the non-recurrence group (n = 161), resulting in a recurrence rate of 19.50%. Univariate analysis revealed statistically significant differences (P<0.05) between the two groups in several clinical parameters. These parameters included preoperative serum sIgE levels, percentage of peripheral blood eosinophils, tissue eosinophil percentage, levels of IL-5, IL-6, IL-1β, postoperative nasal cavity environmental conditions, pH value of the rinsing fluid used during surgery, and adherence to postoperative care, as summarized in Table 1.
Table 1.
One-way analysis of general information
| Recurrence group (n = 39) | Non-recurrence group (n = 161) | χ2/t | P | |
|---|---|---|---|---|
| Gender [n (%)] | 0.129 | 0.432 | ||
| Male | 24 (61.54) | 94 (58.39) | ||
| Female | 15 (38.46) | 67 (41.61) | ||
| Age (years, x̅ ± sd) | 47.13±10.22 | 46.19±10.59 | 0.677 | 0.499 |
| BMI (kg/m2, x̅ ± sd) | 23.54±3.49 | 23.79±3.25 | 0.570 | 0.569 |
| Duration of disease [years, M (P25, P75)] | 5.37±1.59 | 5.46±1.71 | 0.330 | 0.742 |
| History of alcohol abuse [n (%)] | 1.161 | 0.314 | ||
| Yes | 13 (33.33) | 40 (24.84) | ||
| No | 26 (66.67) | 121 (75.16) | ||
| History of smoking [n (%)] | 0.782 | 0.240 | ||
| Yes | 21 (53.85) | 74 (45.96) | ||
| No | 18 (46.15) | 87 (54.04) | ||
| Length of surgery (h, x̅ ± sd) | 2.32±0.30 | 2.26±0.24 | 0.973 | 0.332 |
| Serum sIgE (IU/ml) | 3.77±1.21 | 2.69±0.90 | 6.472 | <0.001 |
| Peripheral blood eosinophil percentage EOS (%, x̅ ± sd) | 7.31±1.84 | 6.13±1.41 | 6.229 | <0.001 |
| Tissue eosinophil percentage (%, x̅ ± sd) | 16.13±4.79 | 12.54±4.76 | 3.959 | <0.001 |
| Preoperative IL-5 (ng/L, x̅ ± sd) | 73.56±8.77 | 56.88±7.69 | 12.047 | <0.001 |
| Preoperative ECP (μg/L, x̅ ± sd) | 60.67±9.37 | 54.38±8.67 | 4.334 | <0.001 |
| Pre-operative IL-6 (pg/mL, x̅ ± sd) | 19.31±3.46 | 15.32±2.78 | 7.821 | <0.001 |
| Preoperative IL-1β (pg/mL, x̅ ± sd) | 23.58±2.98 | 19.82±2.58 | 8.391 | <0.001 |
| Postoperative intranasal environment [n (%)] | ||||
| Good condition | 27 (69.23) | 35 (21.74) | 33.104 | <0.001 |
| Infection occurred | 12 (30.77) | 126 (78.26) | ||
| PH value of the operative cavity rinsing fluid | 17.920 | <0.001 | ||
| PH = 6 | 27 (69.23) | 62 (38.51) | ||
| PH = 8 | 12 (30.77) | 99 (61.49) | ||
| Adherence to postoperative rehabilitation [n (%)] | 4.896 | 0.027 | ||
| Yes | 26 (66.67) | 133 (82.61) | ||
| No | 13 (33.33) | 28 (17.39) |
EOS, eosinophils; ECP, eosinophil cationic protein.
Multifactorial logistic regression analysis
A multifactorial logistic regression analysis was performed, with postoperative recurrence in ECRSwNP patients as the dependent variable (coded as no = 0, yes = 1). The independent variables included those with significant results from the univariate analysis. Dichotomous variables were postoperative intranasal environment, pH value of the surgical rinse solution, and adherence to postoperative care. Continuous variables included preoperative serum sIgE levels, percentage of eosinophils in peripheral blood, tissue eosinophil percentage, IL-5, IL-6, and IL-1β levels. The analysis indicated that higher preoperative tissue eosinophil percentage, ECP, serum sIgE, and IL-5 levels, as well as postoperative infection of the nasal environment, were risk factors for postoperative recurrence of ECRSwNP. In contrast, using a rinsing solution with a pH of 8 in the operative cavity was identified as a protective factor against recurrence. These findings are detailed in Table 2. The joint predictive capability of tissue eosinophil percentage, serum sIgE, IL-5, ECP, postoperative intranasal environment, and pH of the operative lavage solution for postoperative recurrence of ECRSwNP yielded AUCs of 0.654, 0.773, 0.927, 0.680, 0.737, and 0.685, respectively (all P<0.05), as illustrated in Figure 1 and Table 3.
Table 2.
Results of logistic regression analysis of risk factors for postoperative recurrence in patients with ECRSwNP
| Variable | β | S,E | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Percentage of peripheral blood EOS | 0.316 | 1.413 | 0.050 | 0.469 | 1.074 | 0.886-1.302 |
| Percentage of EOS in tissue | 0.635 | 0.307 | 4.261 | 0.005 | 3.430 | 1.453-8.100 |
| IgE | 1.903 | 0.701 | 7.372 | 0.001 | 3.040 | 1.680-5.340 |
| IL-5 | 1.641 | 0.659 | 6.199 | 0.001 | 1.422 | 1.151-1.807 |
| L6 | 0.113 | 0.061 | 3.375 | 0.061 | 1.932 | 1.026-3.637 |
| L-1β | 0.894 | 1.094 | 0.667 | 0.564 | 1.088 | 0.817-1.450 |
| Postoperative nasal environment | 2.362 | 1.194 | 3.914 | 0.033 | 1.032 | 0.845-2.952 |
| ECP | 0.349 | 0.151 | 5.351 | 0.018 | 1.283 | 1.044-1.576 |
| PH of intraoperative print fluid | 0.580 | 0.319 | 4.217 | 0.035 | 0.754 | 0.062-6.650 |
| Postoperative rehabilitation compliance | 0.582 | 0.466 | 1.563 | 0.156 | 1.823 | 0.574-4.691 |
ECRSwNP, eosinophilic chronic rhinosinusitis with nasal polyps; ECP, eosinophil cationic protein.
Figure 1.
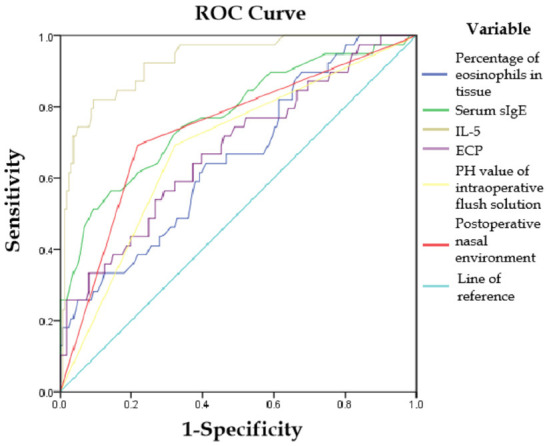
ROC curves for significant factors predicting postoperative recurrence in patients with ECRSwNP. ECRSwNP, eosinophilic chronic rhinosinusitis with nasal polyps; ECP, eosinophil cationic protein.
Table 3.
Significant factors that predict the risk of postoperative recurrence in ECRSwNP patients
| Index | Optimum cutoff value | Sensitivity | Specificity | Jordan value | AUC | P | 95% CI |
|---|---|---|---|---|---|---|---|
| Percentage of eosinophils in tissue | 13.85 | 0.641 | 0.59 | 0.231 | 0.654 | 0.003 | 0.560-0.749 |
| Serum sIGE | 3.65 IU/ml | 0.564 | 0.857 | 0.421 | 0.773 | <0.001 | 0.684-0.862 |
| IL-5 | 66.45 ng/L | 0.821 | 0.907 | 0.728 | 0.927 | <0.001 | 0.883-0.971 |
| ECP | 58.65 μg/L | 0.564 | 0.708 | 0.272 | 0.680 | <0.001 | 0.584-0.777 |
| PH value of intraoperative flush solution | - | 0.692 | 0.677 | 0.369 | 0.685 | <0.001 | 0.591-0.778 |
| Postoperative nasal environment | - | 0.692 | 0.783 | 0.475 | 0.737 | <0.001 | 0.645-0.829 |
Note: Among them, the PH value of the operative rinsing solution and the condition of the postoperative nasal internal environment were dichotomous variables. pH = 6 of the operative rinsing solution was assigned as 0 and pH = 8 as 1. The status of the postoperative nasal internal environment was assigned as 0, and the infection of the nasal internal environment as 1. ECRSwNP, eosinophilic chronic rhinosinusitis with nasal polyps; ECP, eosinophil cationic protein.
Nomogram modeling
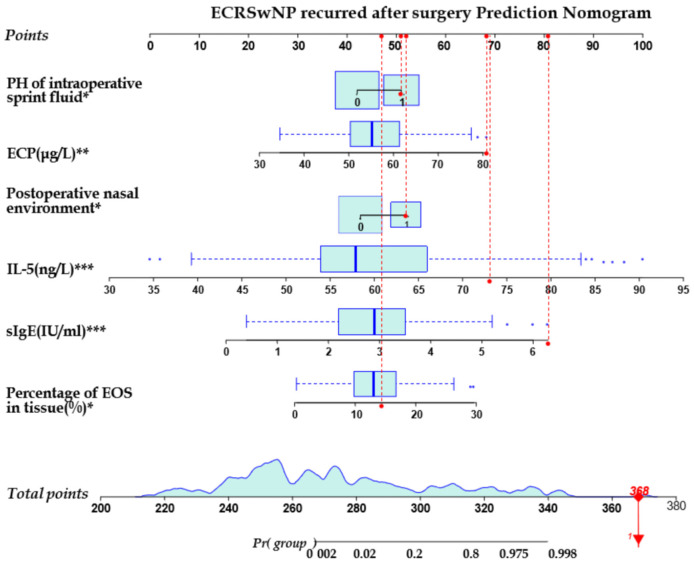
The six factors identified as significant predictors of postoperative recurrence through multifactorial logistic regression analysis were used to construct a nomogram model for predicting postoperative recurrence in ECRSwNP patients. Each predictor showed statistical significance (P<0.05), as depicted in Figure 2.
Figure 2.
Nomogram prediction model for recurrence after ECRSwNP surgery. Note: In which the pH value of the operative rinse solution and the postoperative nasal internal environment condition were dichotomous variables, pH = 6 of the operative rinse solution was assigned as 0 and pH = 8 as 1. The good status of the postoperative nasal internal environment was assigned as 0, and the infection of the nasal internal environment as 1. The prediction of postoperative recurrence at six months was statistically significant, *P<0.05, **P<0.01, ***P<0.001. ECRSwNP, eosinophilic chronic rhinosinusitis with nasal polyps; ECP, eosinophil cationic protein.
Evaluation and validation of the nomogram model
Internal validation of the nomogram model demonstrated high predictive accuracy. The calibration curve closely matched the ideal curve after 1000 iterations of resampling, with a mean absolute error (MAE) of 0.026, indicating a high level of agreement between the model’s predictions and the ideal model, as shown in Figure 3. The area under the ROC curve (AUC) was 0.989 (95% CI: 0.963-1.000), signifying excellent predictive performance, as illustrated in Figure 4. The decision curve analysis indicated that the prediction probability threshold of the model ranges from 0% to 96%, with a net benefit higher than the other two extreme curves within this range, supporting the model’s clinical validity, as shown in Figure 5.
Figure 3.
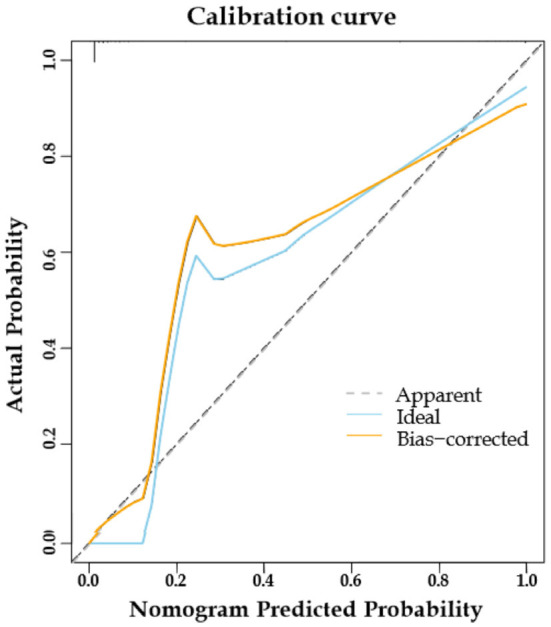
Calibration curves for predictive models.
Figure 4.
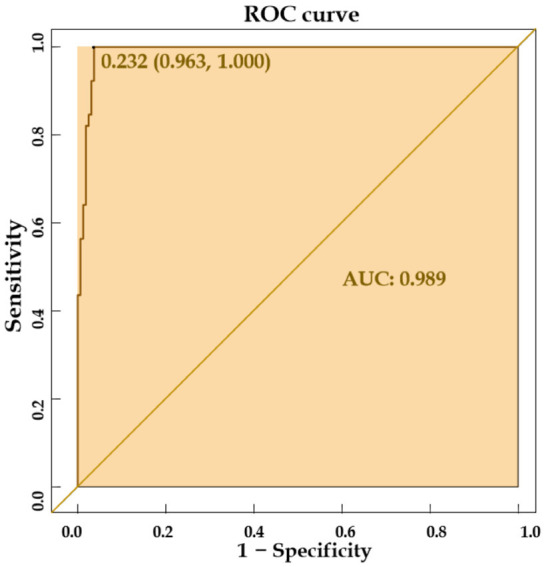
ROC curve of the predictive model.
Figure 5.
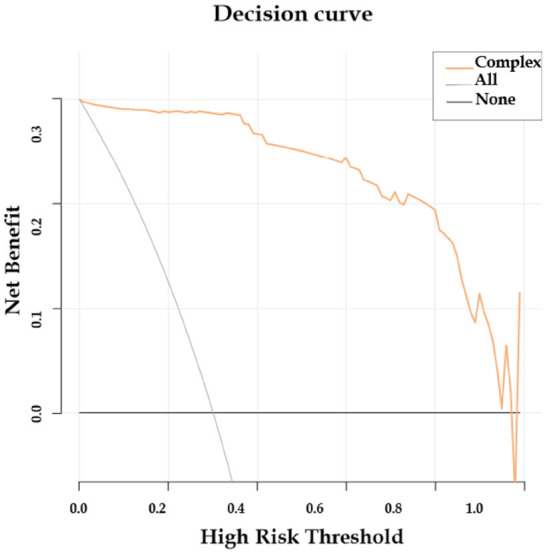
Decision curve of the prediction model.
Discussion
The global prevalence of CRSwNP is currently estimated to be around 1% to 4% [10]. Although the predominant phenotype of CRSwNP in Asian populations is not ECRSwNP, the proportion of ECRSwNP cases has been increasing annually over the past two decades, indicating that ECRSwNP may become a major subtype of CRSwNP in China in the future [11]. Eosinophils are the primary infiltrating cells in allergic and chronic inflammation, promoting the nasal mucosal inflammatory response by facilitating the release and migration of Th2 cytokines. They further exacerbate inflammation by secreting cytokines such as IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor, leading to prolonged inflammatory lesions [12]. Consequently, patients with EOS-type nasal polyps are prone to postoperative recurrence, making it crucial to identify high-risk ECRSwNP patients to provide targeted treatment. In this study, independent risk factors were identified using univariate and multifactorial logistic regression analyses.
A prediction model was then developed based on these independent factors, and its predictive performance was validated. The model’s calibration curve showed high agreement with the standard curve, and the MAE was 0.026, demonstrating superior predictive accuracy. The AUC was 0.989, indicating that the model has significant clinical utility.
The six-month postoperative recurrence rate of 19.50% observed in this study aligns with findings from previous research [13]. Multifactorial logistic regression analysis identified tissue eosinophil percentage, serum sIgE, IL-5, ECP, postoperative nasal endothelial environment, and the pH of the operative cavity rinse as factors influencing postoperative recurrence. Eosinophils, as the main infiltrating cells in allergic and chronic inflammation, promote the nasal mucosal inflammatory response through Th2 cytokine release and migration. They also worsen inflammation by secreting cytokines such as IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor, contributing to prolonged lesions [14,15].
As a crucial inflammatory indicator in CRSwNP, both peripheral blood and tissue eosinophil levels are elevated in ECRSwNP patients. In this study, eosinophil levels in treated patients gradually decreased, suggesting a strong correlation between eosinophil levels, disease severity, and postoperative recurrence [16]. The potential of peripheral blood eosinophil levels as a biomarker for predicting patient prognosis has been a topic of interest in prior studies. However, peripheral blood eosinophil levels do not necessarily reflect tissue eosinophil levels [17]. Meta-analysis results indicate that high absolute peripheral blood eosinophil values are associated with a 1.27-fold increased risk of recurrence, although substantial heterogeneity exists. This variability may be due to factors such as environmental influences, comorbid conditions, perioperative management, and differences in testing instruments [18,19].
Inhibition of local eosinophil infiltration during clinical treatment may improve symptoms of ECRSwNP [20]. Peripheral blood eosinophil levels are susceptible to various inflammatory responses in the body, limiting their clinical value in diagnosing ECRSwNP, which is consistent with the findings of this study. The tissue eosinophil percentage in this study effectively predicted postoperative recurrence. Histological analysis showed that eosinophils infiltrate the epithelium, penetrate through the thickened basement membrane, and spread into the sinus cavity [21]. Persistent eosinophilic inflammation disrupts the mucus cilia clearance system, leading to impaired immune function. This disruption results in epithelial hyperplasia and metaplasia, promoting polyp formation and increasing the risk of postoperative recurrence [22].
This study found that preoperative serum levels of IL-5, IL-6, and IL-1β were higher in the recurrence group compared to the non-recurrence group, with preoperative IL-5 levels having predictive value for postoperative recurrence. Interaction between eosinophils and commensal bacteria or fungi can trigger or exacerbate local inflammatory responses. During the development of ECRSwNP, eosinophil recruitment to nasal polyp tissues is activated, leading to the secretion of numerous factors and a type 2 inflammatory response characterized by elevated inflammatory factor expression in sinus tissues [23,24]. IL-5, in particular, enhances the activity and migration of chemokines to eosinophils, reflecting the intensity of the inflammatory response. IL-5 is a common T-lymphocyte subpopulation factor that is released in response to tissue damage or pathogen invasion. Continuous stimulation of IL-5 secretion contributes to the onset and progression of inflammation [25]. If this inflammatory response remains intense, it can persist postoperatively, increasing the likelihood of recurrence.
ECP is an inflammatory mediator secreted by activated eosinophils and serves as an indicator of inflammatory activity in the airway. Overexpression of ECP in mucosal epithelial cells exacerbates nasal mucosa inflammation, promoting nasal polyp development, which can create a vicious cycle leading to postoperative recurrence [26]. Upon first exposure to allergens, the immune system quickly recognizes them, prompting plasma cells to secrete serum-specific IgE (sIgE). This sIgE binds to immune cell receptors, initiating the sensitization reaction and activating a series of immune processes. This cascade releases a large number of inflammatory factors, promoting the formation of postoperative polyps [27].
Studies have confirmed that a rinsing solution with a pH of 8 promotes epithelialization of the nasal mucosa, improves mucosal cilia clearance, and aids in mucosal repair, consistent with the findings of the present study [28]. The use of a rinsing solution with a pH of 8 has been identified as a protective factor against postoperative recurrence. Lysozyme typically exhibits optimal antimicrobial activity within a pH range of 5.3 to 6.4, and nasal secretions with a pH lower than 6.5 generally result in negative bacterial cultures [29]. However, the pH of nasal secretions in CRS is often alkaline, which may facilitate bacterial colonization in the nasal cavity. Accumulation of purulent secretions in the sinuses postoperatively can exacerbate swelling and congestion. Multiple nasal polyps may further irritate the sinus mucosa, increasing the risk of postoperative recurrence.
A deviated nasal septum indicates anatomical abnormalities that can exert pressure on the maxillary sinus orifice and anterior ethmoid sinus, obstructing the drainage of the frontal sinus orifice, potentially inducing sinus cysts or inflammation, and leading to disease recurrence [30]. Postoperative nasal infections can trigger the nasal mucosa to release inflammatory mediators and increase mucus secretion, thereby raising the inflammatory load. This promotes inflammatory cell infiltration and stromal edema, which further elevates the risk of polyp formation.
To effectively reduce the risk of recurrence, it is essential to provide comprehensive preoperative and postoperative health education for patients. This education should emphasize self-management practices to prevent infection, maintain nasal cleanliness, and encourage the timely and appropriate use of rinsing solutions and antibiotics to treat postoperative infections. Such measures can significantly decrease the likelihood of recurrence.
Patients with ECRSwNP often experience symptoms such as nasal congestion and facial pain, significantly affecting their quality of life and sleep. The chronic and recurrent nature of the disease can lead to psychological issues, including anxiety and depression, which may negatively impact medication adherence and postoperative self-care, resulting in poor surgical outcomes and frequent disease recurrence. Studies have shown that adherence to comprehensive postoperative treatment is a protective factor against recurrence [31]. In the present study, although there was a significant difference in rehabilitation adherence between the recurrence and non-recurrence groups, this difference was not significant in the multifactorial analysis. This suggests that poor postoperative rehabilitation adherence could not predict recurrence outcomes, possibly due to the small sample size of this study.
For high-risk ECRSwNP patients, consistent use of nasal irrigation and glucocorticoids postoperatively can effectively reduce the infiltration of inflammatory factors into the nasal mucosa, preventing and alleviating nasal edema. This approach can promote nasal mucosa recovery and reduce the incidence of postoperative complications. Glucocorticoids provide anti-inflammatory, immunosuppressive, and anti-shock effects. They reduce vascular permeability, inhibit the synthesis and differentiation of immune cells and factors, stabilize mucosal epithelial and vascular endothelial barriers, and enhance receptor sensitivity. These actions help reduce edema and exudation, thereby effectively treating chronic rhinitis.
The predictive model developed in this study aims to help clinicians identify high-risk patients preoperatively and optimize postoperative care plans, enabling targeted treatment for ECRSwNP patients and improving their quality of life. However, this study has limitations. The retrospective design may introduce selection and information biases, suggesting a need for future prospective studies with larger sample sizes and extended follow-up periods. This study relied mainly on data from a single center, so future research should include multicenter and cross-geographic studies to minimize the impact of geographical and medical differences on the results. While this study identified potential predictors of recurrence, the complex interplay of genetic, environmental, and lifestyle factors in ECRSwNP recurrence requires further in-depth research to optimize treatment strategies and improve patient outcomes.
In summary, higher preoperative tissue eosinophil percentage, ECP, serum sIgE, and IL-5 levels, along with postoperative infection in the nasal environment, are risk factors for postoperative recurrence of ECRSwNP. Targeted clinical measures can improve patient prognosis and reduce the risk of recurrence. This study provides valuable insights into the clinical management of ECRSwNP and the prevention of postoperative recurrence.
Disclosure of conflict of interest
None.
References
- 1.Liu Z, Yao Y, Xie H, Zhou A, Fan Y, Liu J, Jiao Q. Visual and bibliometric analysis of chronic rhinosinusitis and nasal polyps. J Allergy Clin Immunol Glob. 2024;3:100211. doi: 10.1016/j.jacig.2024.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milovanović J, Božić DD, Pavlović B, Jotić A, Brkić S, Ćirković I. Biofilm-producing bacteria and quality of life after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Am J Rhinol Allergy. 2024;38:159–168. doi: 10.1177/19458924241236233. [DOI] [PubMed] [Google Scholar]
- 3.Wu AW, Garcia Ruiz EA, Higgins TS, Tang DM, Illing EA, Carle TR, Vasquez M, Ting JY, Sreenath SB, Halawi A, Chen PG. Sinonasal symptom correlation with the postoperative polyp scale (POPS) Ann Otol Rhinol Laryngol. 2024;133:485–489. doi: 10.1177/00034894241232475. [DOI] [PubMed] [Google Scholar]
- 4.Le TT, Emmanuel B, Katial R, Tran TN, Kwiatek JJ, Cohen DS, Daniel SR, Cao Y, Shih VH, Melcón MG, Devouassoux G, Pelaia G RANS Study Investigators. Benralizumab in severe eosinophilic asthma and chronic rhinosinusitis with nasal polyps: the real-world, multi-country RANS observational study. J Asthma Allergy. 2024;17:313–324. doi: 10.2147/JAA.S437190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelsamie AM, Abdelazeem HM, Dawood GK, Abdelaal TM. Prognostic value of polypoid changes of the middle turbinate in relapsed nasal polypi after FESS: a prospective cohort study. Int Arch Otorhinolaryngol. 2023;28:e226–e233. doi: 10.1055/s-0043-1776730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia Y, Nie J, Wang H, Han Z, Zhang Z, Li L. Correlation study of NCF2 in chronic rhinosinusitis with nasal polyps. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024;38:303–309. doi: 10.13201/j.issn.2096-7993.2024.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann AS, Betz CS, Böscke R, Weber RK. Decision-making in the treatment of chronic rhinosinusitis with nasal polyps in the era of biologics. HNO. 2024;72:225–230. doi: 10.1007/s00106-024-01430-1. [DOI] [PubMed] [Google Scholar]
- 8.Tam B, Le J, Tang DM, Wu AW, Hopp ML, Borrelli M, Rice DH, Wrobel BB, Hur K. Gender-specific differences in preoperative concerns in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2024;133:454–457. doi: 10.1177/00034894231219129. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, Orlandi RR, Palmer JN, Patel ZM, Peters A, Walsh SA, Corrigan MD. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(Suppl):S1–S39. doi: 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- 10.Okano M, Kanai K, Oka A. Pathogenesis-based application of biologics for chronic rhinosinusitis: current and future perspectives. Auris Nasus Larynx. 2024;51:371–378. doi: 10.1016/j.anl.2023.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Kemp P, van der Lans RJL, Otten JJ, Adriaensen GFJPM, Benoist LBL, Cornet ME, Hoven DR, Rinia B, Verkest V, Fokkens WJ, Reitsma S. Hypereosinophilia during dupilumab treatment in patients with chronic rhinosinusitis with nasal polyps. Rhinology. 2024;62:202–207. doi: 10.4193/Rhin23.357. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Yang P, Zhang D. LASSO regression based risk prediction model for postoperative control in chronic sinusitis with nasal polyps. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024;38:200–206. doi: 10.13201/j.issn.2096-7993.2024.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavaliere C, Masieri S, Begvarfaj E, Loperfido A, Baroncelli S, Cascone F, Ciofalo A. Long-term perspectives on chronic rhinosinusitis with nasal polyps: evaluating recurrence rates after functional endoscopic sinus surgery in the biologics Era-A 5-year follow-up study. J Pers Med. 2024;14:297. doi: 10.3390/jpm14030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvanese L, Fabbris C, Brescia G, Di Pasquale Fiasca VM, Deretti A, Finozzi F, Franz L, Frigo AC, Marioni G. Polyps’ extension and recurrence in different endotypes of chronic rhinosinusitis: a series of 449 consecutive patients. J Clin Med. 2024;13:1125. doi: 10.3390/jcm13041125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Zhu J, Zhang M, Wang Y, Cheng F, Ma W, Li M. Systemic inflammation response index predicts the postoperative recurrence of chronic rhinosinusitis with nasal polyps: a retrospective study in the Chinese population. Eur Arch Otorhinolaryngol. 2024;281:207–217. doi: 10.1007/s00405-023-08182-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Yang Y, Guo J, Yu P, Wang G, Liu X, Zhang Z, Li T, Zhang Y, Song X. The tissue lymphocyte-to-eosinophil ratio predicted long-term recurrence of eosinophilic CRSwNP. Am J Rhinol Allergy. 2023;37:563–570. doi: 10.1177/19458924231179615. [DOI] [PubMed] [Google Scholar]
- 17.Sima Y, Wang X, Zhang L. Interaction of eosinophilic and neutrophilic inflammation in patients with chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2024;24:25–31. doi: 10.1097/ACI.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 18.Du K, Zheng M, Zhao Y, Jiao C, Xu W, Hao Y, Wang Y, Zhao J, Wang X, Zhang L. A nomogram combing peripheral parameters for estimation of CRSwNP recurrence. Am J Rhinol Allergy. 2021;35:578–586. doi: 10.1177/1945892420978957. [DOI] [PubMed] [Google Scholar]
- 19.Xu WW, Zhang H, Su J, Wang S, Feng J. Study on the correlation between eosinophils and chronic rhinosinusitis with nasal polyps in Xinjiang region of China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;56:819–823. doi: 10.3760/cma.j.cn115330-202000902-00716. [DOI] [PubMed] [Google Scholar]
- 20.Xiao Y, Luo HW, Wu J, Zhang WL, Wang YX. Clinical characteristics of non-eosinophilic and eosinophilic chronic sinusitis with nasal polyps. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;33:607–610. doi: 10.13201/j.issn.1001-1781.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 21.McHugh T, Snidvongs K, Xie M, Banglawala S, Sommer D. High tissue eosinophilia as a marker to predict recurrence for eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2018;8:1421–1429. doi: 10.1002/alr.22194. [DOI] [PubMed] [Google Scholar]
- 22.Huang Z, Li X, Li Y, Huang W, Lai X, Wu H, Chen X, Zhang Y, Chang L, Zhang G. Interleukin-19 enhances eosinophil infiltration through upregulation of epithelium-derived RANTES expression via the ERK/NF-κB signalling pathway in patients with eosinophilic CRSwNP. Inflamm Res. 2024;73:499–513. doi: 10.1007/s00011-024-01851-2. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Seet JE, Yap QV, Chao SS, Thong MKT, Wang DY, Ong YK. Latent class analysis of structured histopathology in prognosticating surgical outcomes of chronic rhinosinusitis with nasal polyps in Singapore. Rhinology. 2023;61:358–367. doi: 10.4193/Rhin22.455. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Xu Z, Fu Y, Zhang N, Lu T, Li Z, Li J, Bachert C, Wen W, Wen Y. A novel inflammatory endotype diagnostic model based on cytokines in chronic rhinosinusitis with nasal polyps. World Allergy Organ J. 2023;16:100796. doi: 10.1016/j.waojou.2023.100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staricha KL, Ali HM, Stokken JK. State of the art medical management of nasal polyps. Am J Rhinol Allergy. 2023;37:153–161. doi: 10.1177/19458924221145256. [DOI] [PubMed] [Google Scholar]
- 26.Gevaert P, Han JK, Smith SG, Sousa AR, Howarth PH, Yancey SW, Chan R, Bachert C. The roles of eosinophils and interleukin-5 in the pathophysiology of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2022;12:1413–1423. doi: 10.1002/alr.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G, Zheng H, Chen X, Zheng J, Zhan J, Li R, Qi Y, Ye Y, Zeng M, Wei X. Exploration of predictive biomarkers for postoperative recurrence in chronic rhinosinusitis with nasal polyps based on serum multiple-cytokine profiling. Mediators Inflamm. 2022;2022:1061658. doi: 10.1155/2022/1061658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Kang H, Hong S, Shen Y. Effect of postoperative specific immunotherapy combined with nasal irrigation on chronic rhinosinusitis with allergic rhinitis. Iran J Allergy Asthma Immunol. 2021;20:432–440. [PubMed] [Google Scholar]
- 29.Wang L, Lv Y, Chang X, Wang M, Wang J, Yang J, Zhang C. The effectiveness of evidence-based nursing intervention for nasal irrigation after endoscopic sinus surgery in patients with chronic rhinosinusitis: a randomized controlled trial. Eur Arch Otorhinolaryngol. 2024;281:2451–2462. doi: 10.1007/s00405-023-08431-w. [DOI] [PubMed] [Google Scholar]
- 30.Ho J, Li W, Grayson JW, Alvarado R, Rimmer J, Sewell WA, Harvey RJ. Systemic medication requirement in post-surgical patients with eosinophilic chronic rhinosinusitis. Rhinology. 2021;59:59–65. doi: 10.4193/Rhin20.073. [DOI] [PubMed] [Google Scholar]
- 31.Hentati F, Kim J, Hoying D, D’Anza B, Rodriguez K. Revision rates and symptom trends following endoscopic sinus surgery: impact of race on outcomes. Laryngoscope. 2023;133:2878–2884. doi: 10.1002/lary.30647. [DOI] [PubMed] [Google Scholar]