Abstract
Objective: To investigate the risk factors for premature rupture of membranes (PROM) and preterm birth in pregnant women following cervical conization. A nomogram model was developed and validated to predict the occurrence of PROM and preterm birth in this population. Methods: A total of 100 pregnant women who had undergone cervical conization between January 2014 and December 2023 were included. The participants were divided into two groups: 52 in the PROM group and 48 in the non-PROM group. Additionally, 43 cases were in the preterm birth group, and 57 were in the full-term group. Maternal age, body mass index (BMI) during pregnancy, and the conization method were recorded. A nomogram model was constructed to predict PROM and preterm birth, with the predictive performance evaluated using the area under the ROC curve (AUC), C-index, and decision curve analysis (DCA). Results: Univariate and multivariate regression analyses identified pre-pregnancy obesity, advanced maternal age, time after conization, and second-trimester cervical length as significant risk factors for PROM and preterm birth. These factors were incorporated into a clinical nomogram. Calibration curves demonstrated excellent internal and external accuracy for the model. The AUC for the nomogram was 0.8746. DCA showed the clinical utility of the model when the threshold probability ranged from 20% to 60%. Conclusion: Pre-pregnancy obesity, advanced maternal age, time since conization (<12 months), and second-trimester cervical length (<25 mm) were identified as independent risk factors for predicting PROM and preterm birth in pregnant women after cervical conization.
Keywords: Preterm birth, premature rupture of membranes, cervical conization, risk factors, nomogram model
Introduction
Cervical intraepithelial neoplasia (CIN) is a precancerous lesion of the cervix, confined to the cervical epithelium, and characterized by histologic changes [1]. According to recent epidemiological data, women aged 30-39 are at higher risk for CIN [2], and many are planning pregnancies when diagnosed. The standard surgical treatment for CIN is cervical conization [3], which minimally affects the patient. However, studies have demonstrated a significant increase in adverse pregnancy outcomes following cervical conization [4-6].
After cervical conization, several potential issues may arise. First, patients may experience pain and discomfort at the incision site, which can impact daily activities and quality of life in the short term. Second, the risk of premature rupture of membranes (PROM) increases due to cervical damage, weakening cervical support and closure function [5]. Additionally, there is a higher risk of preterm delivery, since structural and functional changes to the cervix may compromise its ability to sustain pregnancy. As for the optimal time to conceive after surgery, waiting 6 months to 1 year is generally considered safe [6].
Cervical conization is a significant risk factor for both preterm delivery and PROM [7]. Retrospective studies have shown an increased risk of these adverse outcomes in women who have undergone cervical conization [8,9]. A systematic review analyzing the impact of surgical treatment on pregnancy outcomes in CIN patients reported that cervical conization increases the risk of preterm delivery and PROM in subsequent pregnancies. Specifically, the risk of preterm delivery before 37 weeks increased by 1.78 times, before 32-34 weeks by 2.4 times, and before 28-30 weeks by 2.54 times. The risk of PROM before 37 weeks increased by 2.36 times [10]. Moreover, the greater the height of the cone removed during surgery, the higher the risks of preterm delivery and PROM.
Effective prediction and early prevention of PROM and preterm birth in pregnant women after cervical conization are crucial for reducing their incidence and improving neonatal outcomes. This study aimed to investigate the risk factors for PROM and preterm birth in pregnant women following cervical conization and to establish and validate a nomogram for predicting these outcomes.
Materials and methods
Study design and ethics
This retrospective study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital. A total of 100 pregnant women who underwent cervical conization between January 2014 and December 2023 were included. Among them, 52 were in the PROM group, and 48 were in the non-PROM group. Additionally, 43 cases were in the preterm birth group, and 57 were in the full-term group.
Inclusion criteria
(1) Age ≥18 years; (2) Singleton pregnancy; (3) Cervical conization due to CIN; (4) Exclusion of invasive carcinoma by post-conization pathology; (5) Complete clinical data.
Exclusion criteria
(1) History of mental illness; (2) Pregnancy complications such as gestational diabetes or hypertensive disorders; (3) History of PROM, uterine malformations, repeated vaginal bleeding, or other PROM risk factors; (4) Termination of pregnancy before 28 weeks; (5) History of smoking, alcohol use, or drug abuse; (6) History of cervical cerclage; (7) Early miscarriage; (8) Incomplete clinical data.
Data collection and measurement
Demographic and clinical characteristics were obtained from the hospital’s electronic case system, including age, pre-pregnancy BMI, multiparity, assisted reproduction, cervical length (CL), conization method, and time since conization.
Sample size estimation
Sample size was determined through power analysis, adjusted for an estimated attrition rate: corrected sample size = sample size/(1 - [% attrition/100]). The final sample size was set at approximately 100.
Statistical analysis
Data were analyzed using SPSS 20.0 software. Continuous variables were expressed as mean ± standard deviation (SD). Independent t-test or rank-sum test were used for group comparisons. Categorical variables were expressed as percentages (%), and comparisons were made using the chi-square (X2) test. Multivariate logistic regression was used to identify risk factors for PROM and preterm birth in women after cervical conization. Statistical significance was set at P<0.05. Identified risk factors were entered into R software (R 3.6.3) to construct a nomogram model predicting the risk of PROM and preterm birth. The variables and regression coefficients from the model were used to generate an ROC curve for validation.
Results
Comparison of clinical characteristics
Table 1 presents the characteristics of all subjects. The study included 100 pregnant women who had undergone cervical conization, comprising 52 in the PROM group and 48 in the non-PROM group. Significant differences were observed between the two groups in age, pre-pregnancy BMI, cervical length (CL), and time since conization (all P<0.05). However, there were no significant differences in cervical conization method, surgical approach, use of assisted reproduction, or multiparity between the groups (all P>0.05).
Table 1.
Comparison of clinical data between the two groups
| Clinical data | PROM group (n=52) | Non-PROM group (n=48) | t/χ2 | P |
|---|---|---|---|---|
| Age | 36.8±6.1 | 30.4±3.9 | 4.847 | 0.011 |
| BMI before pregnancy | 25.3±9.8 | 20.3±5.7 | 2.078 | 0.022 |
| Multipara | 6 (11.5%) | 5 (10.7%) | 0.026 | 0.071 |
| Assisted reproduction and pregnancy | 5 (9.6%) | 3 (6.4%) | 0.570 | 0.550 |
| CL | 23.2±5.6 | 28.3±4.6 | 4.855 | 0.023 |
| Operation mode | 2.255 | 0.063 | ||
| LEEP | 19 (36.5%) | 21 (45.6%) | ||
| CKC | 33 (63.5%) | 26 (54.3%) | ||
| Cervical canceration | 0.333 | 0.579 | ||
| CIN1 | 9 (17.3%) | 8 (17.1%) | ||
| CIN2 | 23 (44.2%) | 24 (50.7%) | ||
| CIN3 | 20 (38.4%) | 15 (32.1%) | ||
| Time after conization | 20.54 | <0.001 | ||
| 6-12 months | 10 (19.2%) | 0 (1.43%) | ||
| 12-24 months | 20 (38.5%) | 23 (48.6%) | ||
| >24 months | 22 (42.3%) | 24 (50.0%) |
Note: CL: cervical length; LEEP: Loop Electrosurgical Excision Procedure; CKC: Cold Knife Conization.
Similarly, we compared preterm birth outcomes in the cohort. The results indicated significant differences between the preterm birth and full-term groups in terms of age, CL, and time since conization (all P<0.05). However, no significant differences were found regarding cervical conization method, surgical approach, pre-pregnancy BMI, use of assisted reproduction, or multiparity (all P>0.05) (Table 2).
Table 2.
Comparison of clinical data between Preterm birth and full-term group in PROM population
| Clinical data | Preterm birth group (n=43) | Full-term group (n=57) | t/χ2 | P |
|---|---|---|---|---|
| Age | 36.2±6.1 | 32.9±4.3 | 4.847 | 0.021 |
| BMI before pregnancy | 25.2±5.8 | 23.8±7.2 | 2.078 | 0.232 |
| Multipara | 3 (7.0%) | 7 (12.3%) | 0.026 | 0.081 |
| Assisted reproduction and pregnancy | 7 (16.3%) | 4 (7.0%) | 0.570 | 0.650 |
| CL | 22.2±3.2 | 29.0±4.2 | 4.855 | 0.033 |
| Operation mode | 6.22 | 0.023 | ||
| LEEP | 4 (9.3%) | 26 (45.6%) | ||
| CKC | 40 (93.0%) | 29 (50.9%) | ||
| Cervical canceration | 4.036 | 0.079 | ||
| CIN1 | 4 (9.3%) | 11 (19.3%) | ||
| CIN2 | 13 (30.2%) | 27 (47.4%) | ||
| CIN3 | 26 (60.5%) | 19 (33.3%) | ||
| Time after conization | 8.315 | 0.002 | ||
| 6-12 months | 20 (46.5%) | 6 (10.5%) | ||
| 12-24 months | 13 (30.2%) | 24 (42.1%) | ||
| >24 months | 10 (23.3%) | 27 (47.4%) |
Note: CL: cervical length; LEEP: Loop Electrosurgical Excision Procedure; CKC: Cold Knife Conization; PROM: premature rupture of membrane.
Univariate and multivariate regression analysis
As shown in Table 3, univariate regression analysis revealed that age, pre-pregnancy BMI, CL, use of assisted reproduction, cervical conization method, and time since significantly associated with PROM in pregnant women after cervical conization (all P<0.05).
Table 3.
Univariate and Multivariate Risk analysis for PROM in pregnant women after cervical conization
| Index | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| OR [95% CI] | P value | OR [95% CI] | P value | |
| Age | ||||
| <35 years | Reference | Reference | ||
| ≥35 years | 1.68 [0.5989-0.973] | 0.006 | 2.754 [1.251-2.749] | 0.011 |
| BMI before pregnancy | ||||
| <24 | Reference | Reference | ||
| 24-28 | 1.82 [0.9206-1.982] | 0.047 | 1.24 [0.85-1.85] | 0.281 |
| ≥28 | 1.42 [0.9378-1.515] | 0.1297 | 2.97 [1.75-2.05] | 0.005 |
| Multipara | ||||
| Yes | Reference | |||
| No | 0.46 [0.133-1.636] | 0.234 | ||
| Assisted reproduction and pregnancy | ||||
| Yes | Reference | Reference | ||
| No | 0.43 [0.133-0.636] | 0.004 | 1.28 [0.77-1.38] | 0.261 |
| CL | ||||
| ≥25 | Reference | Reference | ||
| <25 | 1.25 [1.1615-1.189] | 0.028 | 1.754 [1.241-2.479] | 0.001 |
| Operation mode | ||||
| LEEP | Reference | |||
| CKC | 1.18 [1.09-2.23] | 0.294 | ||
| Cervical canceration | ||||
| CIN1 | Reference | Reference | ||
| CIN2 | 1.95 [0.6390-0.6522] | 0.022 | 1.58 [0.77-0.38] | 0.056 |
| CIN3 | 2.52 [0.9206-0.982] | 0.051 | 2.42 [0.85-0.95] | 0.058 |
| Time after conization | ||||
| 6-12 months | Reference | Reference | ||
| 12-24 months | 1.62 [0.639-0.928] | 0.014 | 1.55 [1.014-1.09] | 0.032 |
| >24 months | 5.95 [0.639-0.652] | 0.012 | 2.58 [0.57-0.88] | 0.006 |
Note: CL: cervical length; LEEP: Loop Electrosurgical Excision Procedure; CKC: Cold Knife Conization; CIN: Cervical Intraepithelial Neoplasia; PROM: premature rupture of membrane.
Multivariate regression analysis further identified pre-pregnancy obesity, advanced maternal age, time since conization, and second-trimester CL as independent predictors of PROM in this population.
We also analyzed risk factors for preterm birth in pregnant women after cervical conization. As shown in Table 4, univariate regression analysis revealed significant associations between age, pre-pregnancy BMI, CL, and time since conization with preterm birth (all P<0.05). Multivariate regression analysis confirmed that pre-pregnancy obesity, advanced age, time since cervical conization (<12 months), and second-trimester CL (<25 mm) were independent predictors of both PROM and preterm birth.
Table 4.
Univariate and multivariate risk analysis for preterm birth in pregnant women after cervical conization
| Index | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| OR [95% CI] | P value | OR [95% CI] | P value | |
| Age | ||||
| <35 years | Reference | |||
| ≥35 years | 1.69 [1.04-1.81] | 0.01 | 1.24 [0.64-1.74] | 0.072 |
| BMI before pregnancy | ||||
| <24 | Reference | Reference | ||
| 24-28 | 1.25 [1.1615-1.189] | 0.028 | 1.754 [1.241-2.479] | 0.001 |
| ≥28 | 1.36 [0.414-0.892] | 0.0086 | 2.36 [1.29-4.30] | 0.005 |
| Multipara | ||||
| Yes | Reference | |||
| No | 1.19 [1.47-1.88] | 0.42 | ||
| Assisted reproduction and pregnancy | ||||
| Yes | Reference | |||
| No | 1.25 [1.822-1.96] | 0.49 | ||
| CL | ||||
| ≥25 | Reference | Reference | ||
| <25 | 5.35 [0.039-0.052] | 0.032 | 4.58 [0.37-0.88] | 0.016 |
| Operation mode | ||||
| LEEP | Reference | |||
| CKC | 1.18 [1.09-2.23] | 0.294 | ||
| Cervical canceration | 2.52 [0.9206-0.982] | 0.051 | ||
| CIN1 | Reference | Reference | ||
| CIN2 | 1.24 [0.64-1.74] | 0.77 | ||
| CIN3 | 1.19 [0.61-1.94] | 0.87 | ||
| Time after conization | ||||
| 6-12 months | Reference | Reference | ||
| 12-24 months | 2.36 [0.414-0.892] | 0.018 | 1.66 [1.29-4.30] | 0.036 |
| >24 months | 3.45 [0.237-0.656] | 0.06 | 1.59 [1.12-2.26] | 0.021 |
Note: CL: cervical length; LEEP: Loop Electrosurgical Excision Procedure; CKC: Cold Knife Conization; CIN: Cervical Intraepithelial Neoplasia.
Development of nomogram model
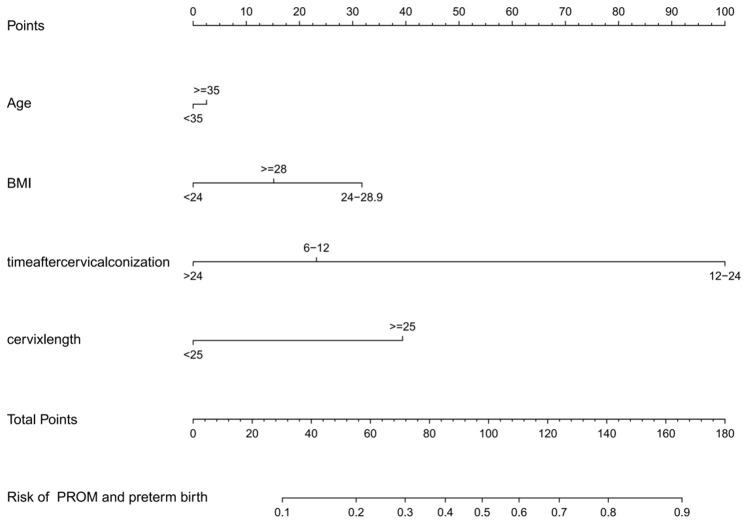
The risk factors for PROM and preterm birth in pregnant women after cervical conization were included in the prediction model using R software (R 3.6.3). The prediction probability, corresponding to the sum of the scores for each factor, represented the risk of preterm birth (Figure 1).
Figure 1.
Nomogram for predicting the premature rupture of membranes (PROM) and preterm birth in pregnant women after cervical conization.
Validation of a nomogram model
The unadjusted concordance index (C-index) for the nomogram was 0.783 [95% confidence interval (CI), 0.815-0.994]. The calibration plot of the nomogram is shown in Figure 2. The AUC for the nomogram was 0.8746 (Figure 3), indicating that the model demonstrated good discrimination and consistency in predicting the risk of PROM and preterm birth in this population.
Figure 2.
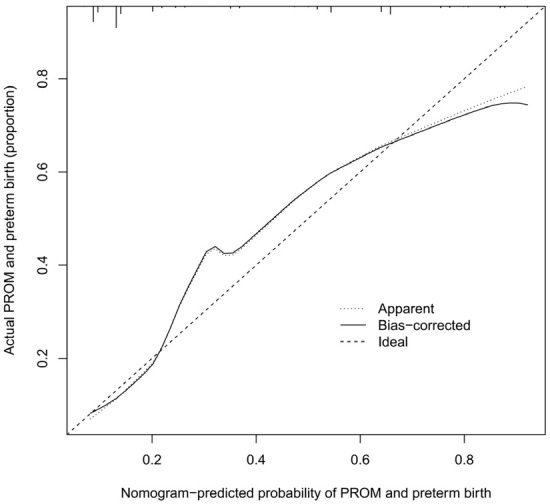
Calibration curves for predicting the premature rupture of membranes (PROM) and preterm birth in pregnant women after cervical conization.
Figure 3.
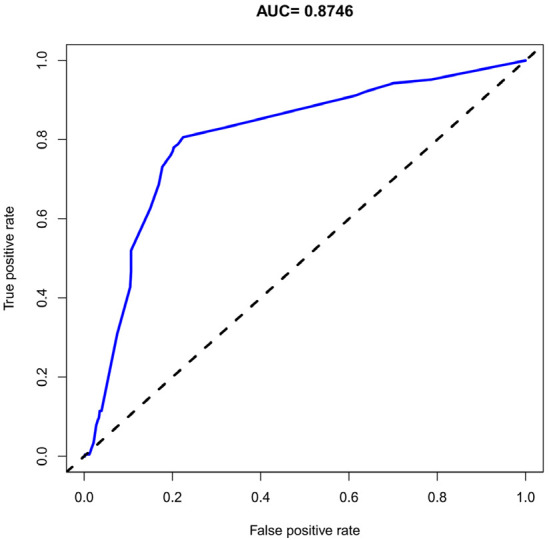
ROC curves for predicting the premature rupture of membranes (PROM) and preterm birth in pregnant women after cervical conization.
Decision curve analysis (DCA)
The DCA is shown in Figure 4. The results demonstrated that if the threshold probability for preterm birth was between 20% and 60%, the model’s validity increased. This predictive model is suitable for clinical use.
Figure 4.
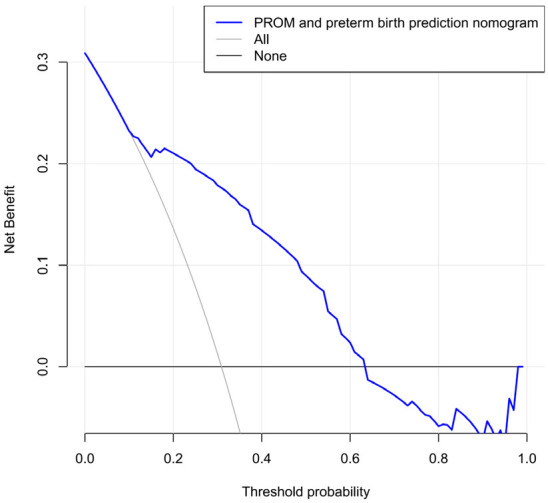
Decision curve analysis for the nomogram.
Discussion
Several studies have shown that cervical conization is associated with a higher risk of preterm delivery, with the risk being five times higher compared to women with normal pregnancies [11-13]. Most preterm pregnant women initially presented with PROM, and the incidence of PROM in women after cervical conization is significantly higher than in those with normal pregnancies [14,15]. The main causes of PROM are genital tract infections and damage resulting from cervical conization. Following conization, the cervical structure changes, including alterations in cervical collagen due to the removal of glands [16]. The depth and/or width of cervical conization directly impacts the likelihood of preterm delivery [17]. However, some studies suggest that the lesion itself can damage cervical tissue, affecting its supportive function, and that CIN III is an independent risk factor for preterm delivery [18-20].
Additionally, research has found that women who experienced preterm delivery after cervical conization had shorter pregnancy intervals compared to those with full-term deliveries. The risk of preterm delivery was higher in women who became pregnant less than one year after conization [21-23]. On the other hand, one study indicated that the interval between conization and pregnancy does not significantly affect preterm delivery risk [24].
The results of this study show that pre-pregnancy obesity is a risk factor for PROM and preterm birth in pregnant women after cervical conization. This is consistent with recent international research findings [25,26] and supports the view that overweight and obesity during pregnancy increase the risk of preterm delivery. Hypertension, pre-eclampsia, hyperlipidemia, and diabetes are all associated with a higher risk of preterm delivery [27]. Pre-pregnancy BMI significantly influences pregnancy-related conditions, such as hypertension, pre-eclampsia, hyperlipidemia, and diabetes [28,29]. Additionally, overweight or obesity before pregnancy is linked to an increased risk of serious maternal conditions, including thromboembolism, sepsis, acute renal failure, and prenatal bleeding [30]. Obese women also show a significantly higher prevalence of reproductive tract infections, which have been proven to disrupt immune balance, leading to decidual chorioamnionitis, intrauterine infection, and PROM [31]. These factors suggest that pre-pregnancy overweight and obesity contribute to various complications that negatively affect pregnancy outcomes, altering the internal environment and increasing the likelihood of preterm delivery.
Advanced maternal age is another independent risk factor for PROM and preterm birth in pregnant women after cervical conization. Numerous studies, both domestic and international, have confirmed that the incidence of complications is significantly higher in older women compared to younger women. Advanced maternal age is closely associated with adverse pregnancy outcomes [32] and has become an important factor influencing the health of both mothers and babies. Advanced maternal age is strongly linked to the development of pre-eclampsia [33], with women aged 35-39 having a 1.2-fold higher risk of pre-eclampsia compared to those under 35, and women aged 40 and above showing an even higher risk [34]. This may also explain the increased incidence of PROM and preterm birth in pregnant women after cervical conization.
The time since cervical conization significantly increased the risk of perinatal preterm birth. Studies have shown that inconsistent twin development combined with growth restriction of one fetus can lead to abnormal umbilical blood flow, which increases the likelihood of cervical dilation and preterm birth [35,36]. In such cases, early pregnancy termination may be necessary, resulting in preterm delivery [37]. Therefore, adopting a healthy lifestyle, incorporating high-quality protein and an appropriate carbohydrate diet, along with regular exercise during early pregnancy, can help prevent inconsistent twin development and reduce the risk of preterm delivery.
Consistent with our findings, Regan et al. demonstrated a significantly increased risk of preterm delivery (17.9% vs. 4.6%, OR=5.6, 95% CI: 2.5-12.7) for women with a pregnancy interval of less than 12 months after conization compared to those with an interval longer than 12 months [38]. This may be related to the cervical healing process following conization, where extending the interval after surgery allows for better recovery of the cervical wound, surrounding tissue, physical structure, and local immune microenvironment, thereby reducing pregnancy-related complications. To minimize adverse outcomes such as preterm delivery, clinicians should provide individualized guidance on optimal pregnancy timing based on the patient’s recovery during and after conization.
The results of this study also indicate that a shortened CL in the second trimester is another risk factor for PROM and preterm birth in pregnant women after cervical conization. While CL slightly decreases during pregnancy, significant differences have been observed. Research shows that the shorter the CL, and the earlier the gestational week at which preterm delivery occurs, the higher the likelihood of premature birth [39]. Wang et al. [40] found that among women with a CL of less than 25 mm, the probability of spontaneous preterm delivery increased by 3% for every 1 mm decrease in CL. When CL was less than 15 mm, the risk of preterm delivery increased exponentially.
This study has certain limitations. First, it is a retrospective study, which may reduce the strength of validation. Additionally, the prediction model was validated internally, without external validation. Furthermore, the sample size may not be large enough to represent all possible variations, potentially affecting the generalizability of the model. Future research should aim to expand the sample size through multi-center collaboration, explore and control for potential confounding factors, and continuously monitor and evaluate the model’s predictive role in assessing the risk of PROM and preterm birth in pregnant women after cervical conization.
In conclusion, this study developed and validated a clinical nomogram to predict PROM and preterm birth in pregnant women after cervical conization. We confirmed that pre-pregnancy obesity, advanced age, time since conization (<12 months), and second-trimester CL (<25 mm) were independent risk factors for PROM and preterm birth.
Disclosure of conflict of interest
None.
References
- 1.Wang H, Jiang Y, Liang Y, Wei L, Zhang W, Li L. Observation of the cervical microbiome in the progression of cervical intraepithelial neoplasia. BMC Cancer. 2022;22:362. doi: 10.1186/s12885-022-09452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sykes PH, Simcock BJ, Innes CR, Harker D, Williman JA, Whitehead M, van der Griend RA, Lawton BA, Hibma M, Fitzgerald P, Dudley NM, Petrich S, Eva L, Bergzoll C, Kathuria J, McPherson G, Tristram A, Faherty J, Hardie D, Robertson A, Robertson V, Pather S, Wrede CD, Gastrell F, Fentiman G, John M, White E, Parker C, Sadler L. Predicting regression of cervical intraepithelial neoplasia grade 2 in women under 25 years. Am J Obstet Gynecol. 2022;226:222.e1–222.e13. doi: 10.1016/j.ajog.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Dempster-Rivett K, Innes CR, Simcock BJ, Harker D, Williman JA, Van Der Griend RA, Whitehead M, Hibma M, Lawton BA, Fitzgerald P, Dudley NM, Petrich S, Faherty J, Bergzoll C, Eva L, Sadler L, Pather S, Wrede CD, Sykes PH. Evaluation of guidelines for observational management of cervical intraepithelial neoplasia 2 in young women. Am J Obstet Gynecol. 2020;223:408.e1–408.e11. doi: 10.1016/j.ajog.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Vogelsang TLR, Schmoeckel E, Kuhn C, Blankenstein T, Temelkov M, Heidegger H, Kolben TM, Kolben T, Mahner S, Mayr D, Jeschke U, Vattai A. Regulation of LCoR and RIP140 expression in cervical intraepithelial neoplasia and correlation with CIN progression and dedifferentiation. J Cancer Res Clin Oncol. 2020;146:1847–1855. doi: 10.1007/s00432-020-03178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashiguchi M, Takezawa T, Nagase K, Tayama-Abe M, Matsuhisa F, Kitajima S, Morito S, Yamaji K, Futamata M, Sakata Y, Akutagawa T, Yokoyama M, Toda S, Aoki S. Collagen vitrigel membrane-coated nylon line prevents stenosis after conization of the cervix uteri. Tissue Eng Part A. 2021;27:1480–1489. doi: 10.1089/ten.TEA.2020.0374. [DOI] [PubMed] [Google Scholar]
- 6.Bhakhri R, Messner L. Case report: downbeat nystagmus due to epidural puncture during labor with undiagnosed arnold-chiari malformation. Optom Vis Sci. 2022;99:721–724. doi: 10.1097/OPX.0000000000001916. [DOI] [PubMed] [Google Scholar]
- 7.Cho GJ, Ouh YT, Kim LY, Lee TS, Park GU, Ahn KH, Hong SC, Oh MJ, Kim HJ. Cerclage is associated with the increased risk of preterm birth in women who had cervical conization. BMC Pregnancy Childbirth. 2018;18:277. doi: 10.1186/s12884-018-1765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T, Jiang R, Yao Y, Huang X. Can prophylactic transvaginal cervical cerclage improve pregnancy outcome in patients receiving cervical conization? A meta-analysis. Ginekol Pol. 2021;92:704–713. doi: 10.5603/GP.a2021.0020. [DOI] [PubMed] [Google Scholar]
- 9.Caballero A, Dudley D, Ferguson J, Pettit K, Boyle A. Maternal human papillomavirus and preterm premature rupture of membranes: a retrospective cohort study. J Womens Health (Larchmt) 2019;28:606–611. doi: 10.1089/jwh.2018.7043. [DOI] [PubMed] [Google Scholar]
- 10.Patrelli TS, Anfuso S, Vandi F, Valitutto S, Migliore M, Salvati MA, De Ioris A, Condemi V, Fadda GM, Bacchi Modena A, Nardelli GB. Preterm delivery and premature rupture of membranes after conization in 80 women. Preliminary data. Minerva Ginecol. 2008;60:295–298. [PubMed] [Google Scholar]
- 11.Gao Y, Wang H, Xiao Y. The effect of cold-knife conization on pregnancy outcomes in patients with cervical lesions. PLoS One. 2022;17:e0278505. doi: 10.1371/journal.pone.0278505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, Sun Z, Gao H, Nan Y, Pizzella S, Xu H, Lau J, Lin Y, Wang H, Woodard PK, Krigman HR, Wang Q, Wang Y. Whole cervix imaging of collagen, muscle, and cellularity in term and preterm pregnancy. Nat Commun. 2024;15:5942. doi: 10.1038/s41467-024-48680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Šimják P, Cibula D, Pařízek A, Sláma J. Management of pregnancy after fertility-sparing surgery for cervical cancer. Acta Obstet Gynecol Scand. 2020;99:830–838. doi: 10.1111/aogs.13917. [DOI] [PubMed] [Google Scholar]
- 14.Pennell PB, French JA, May RC, Gerard E, Kalayjian L, Penovich P, Gedzelman E, Cavitt J, Hwang S, Pack AM, Sam M, Miller JW, Wilson SH, Brown C, Birnbaum AK, Meador KJ MONEAD Study Group. Changes in seizure frequency and antiepileptic therapy during pregnancy. N Engl J Med. 2020;383:2547–2556. doi: 10.1056/NEJMoa2008663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes BL, Clifton RG, Rouse DJ, Saade GR, Dinsmoor MJ, Reddy UM, Pass R, Allard D, Mallett G, Fette LM, Gyamfi-Bannerman C, Varner MW, Goodnight WH, Tita ATN, Costantine MM, Swamy GK, Gibbs RS, Chien EK, Chauhan SP, El-Sayed YY, Casey BM, Parry S, Simhan HN, Napolitano PG, Macones GA Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436–444. doi: 10.1056/NEJMoa1913569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cong C, Xia Y, Gong S, Li T, Liu H, Zhong G, Chen D, Zhao W, Yu W, Yao Y, Liu J, Wei D, Cao H, Huang F. Infectious hepatitis E virus excreted into the vagina. FASEB J. 2024;38:e23500. doi: 10.1096/fj.202301519RR. [DOI] [PubMed] [Google Scholar]
- 17.Guo HJ, Guo RX, Liu YL. Effects of loop electrosurgical excision procedure or cold knife conization on pregnancy outcomes. Eur J Gynaecol Oncol. 2013;34:79–82. [PubMed] [Google Scholar]
- 18.Zeng Z, Chen YD, Yin MW, Chen XJ, Wang T, Xu J, Ma JH. Comparison of restrictive and liberal red blood cell suspension transfusion and analysis of influencing factors on prognosis of premature infants. Transfus Clin Biol. 2023;30:382–386. doi: 10.1016/j.tracli.2023.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Fan C, Xu C, Zhang Y, Liu J, Zhou C, Feng S, Fan Y. Serum calcium level at 32 weeks of gestation could be applied as a predictor of preterm delivery: a retrospective study. Eur J Med Res. 2024;29:400. doi: 10.1186/s40001-024-01984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao L, Yang Y, Ding L, Zhang Z, Li X, Yao H, Tang X. Profiling the clinical characteristics and surgical efficacy of laryngomalacia in children. Eur Arch Otorhinolaryngol. 2024;281:273–281. doi: 10.1007/s00405-023-08254-9. [DOI] [PubMed] [Google Scholar]
- 21.Barsosio HC, Madanitsa M, Ondieki ED, Dodd J, Onyango ED, Otieno K, Wang D, Hill J, Mwapasa V, Phiri KS, Maleta K, Taegtmeyer M, Kariuki S, Schmiegelow C, Gutman JR, Ter Kuile FO. Chemoprevention for malaria with monthly intermittent preventive treatment with dihydroartemisinin-piperaquine in pregnant women living with HIV on daily co-trimoxazole in Kenya and Malawi: a randomised, double-blind, placebo-controlled trial. Lancet. 2024;403:365–378. doi: 10.1016/S0140-6736(23)02631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bistervels IM, Buchmüller A, Wiegers HMG, Ní Áinle F, Tardy B, Donnelly J, Verhamme P, Jacobsen AF, Hansen AT, Rodger MA, DeSancho MT, Shmakov RG, van Es N, Prins MH, Chauleur C, Middeldorp S Highlow Block writing committee; Highlow Investigators. Intermediate-dose versus low-dose low-molecular-weight heparin in pregnant and post-partum women with a history of venous thromboembolism (highlow study): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;400:1777–1787. doi: 10.1016/S0140-6736(22)02128-6. [DOI] [PubMed] [Google Scholar]
- 23.González R, Nhampossa T, Mombo-Ngoma G, Mischlinger J, Esen M, Tchouatieu AM, Mendes A, Figueroa-Romero A, Zoleko-Manego R, Lell B, Lagler H, Stoeger L, Dimessa LB, El Gaaloul M, Sanz S, Méndez S, Piqueras M, Sevene E, Ramharter M, Saúte F, Menendez C MAMAH study group. Safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnant women with HIV from Gabon and Mozambique: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2024;24:476–487. doi: 10.1016/S1473-3099(23)00738-7. [DOI] [PubMed] [Google Scholar]
- 24.Park HS, Kim HS, Lee SA, Yoon J, Kim EH. Prophylactic cerclage to prevent preterm birth after conization: a cohort study using data from the National Health Insurance Service of Korea. Yonsei Med J. 2021;62:1083–1089. doi: 10.3349/ymj.2021.62.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Zhu S, Wu Y, Chen D, Liang Z. Association between maternal second-trimester stress and adverse pregnancy outcomes according to pre-pregnancy body mass index and gestational weight gain. Front Psychiatry. 2023;14:1129014. doi: 10.3389/fpsyt.2023.1129014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle TJ, Kiros GE, Schmitt-Matzen EN, Propper R, Thompson A, Phillips-Bell GS. Maternal and perinatal outcomes associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy, Florida, 2020-2021: a retrospective cohort study. Clin Infect Dis. 2022;75(Suppl 2):S308–S316. doi: 10.1093/cid/ciac441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson K, Bodnar LM, Stephansson O, Abrams B, Hutcheon JA. Safety of low weight gain or weight loss in pregnancies with class 1, 2, and 3 obesity: a population-based cohort study. Lancet. 2024;403:1472–1481. doi: 10.1016/S0140-6736(24)00255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Zhang S, Yu W, Li G, Li J, Ji J, Mi Y, Luo X. Pre-pregnancy body mass index and glycated-hemoglobin with the risk of metabolic diseases in gestational diabetes: a prospective cohort study. Front Endocrinol (Lausanne) 2023;14:1238873. doi: 10.3389/fendo.2023.1238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karimi M, Mofidi Nejad M, Tabaeifard R, Omid N, Rezaei Z, Azadbakht L. The association between dietary habits and self-care behavior of pregnant women with pregnancy complications. Sci Rep. 2024;14:19681. doi: 10.1038/s41598-024-70162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara A, Hedderson MM, Zhu Y, Avalos LA, Kuzniewicz MW, Myers LC, Ngo AL, Gunderson EP, Ritchie JL, Quesenberry CP, Greenberg M. Perinatal complications in individuals in california with or without SARS-CoV-2 infection during pregnancy. JAMA Intern Med. 2022;182:503–512. doi: 10.1001/jamainternmed.2022.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surve MV, Anil A, Kamath KG, Bhutda S, Sthanam LK, Pradhan A, Srivastava R, Basu B, Dutta S, Sen S, Modi D, Banerjee A. Membrane vesicles of group B streptococcus disrupt Feto-maternal barrier leading to preterm birth. PLoS Pathog. 2016;12:e1005816. doi: 10.1371/journal.ppat.1005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zawiejska A, Wróblewska-Seniuk K, Gutaj P, Kippen J, Gomulska A, Wender-Ozegowska E. Markers of maternal insulin resistance and lipid ratios measured in early pregnancy are related to adverse fetomaternal outcomes in women treated for hyperglycemia detected in early pregnancy-data from a retrospective cohort study. J Clin Med. 2022;11:1777. doi: 10.3390/jcm11071777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurrell A, Webster L, Sparkes J, Battersby C, Brockbank A, Clark K, Duhig KE, Gill C, Green M, Hunter RM, Seed PT, Vowles Z, Myers J, Shennan AH, Chappell LC PARROT-2 trial group. Repeat placental growth factor-based testing in women with suspected preterm pre-eclampsia (PARROT-2): a multicentre, parallel-group, superiority, randomised controlled trial. Lancet. 2024;403:619–631. doi: 10.1016/S0140-6736(23)02357-7. [DOI] [PubMed] [Google Scholar]
- 34.Islami F, Baeker Bispo J, Lee H, Wiese D, Yabroff KR, Bandi P, Sloan K, Patel AV, Daniels EC, Kamal AH, Guerra CE, Dahut WL, Jemal A. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74:136–166. doi: 10.3322/caac.21812. [DOI] [PubMed] [Google Scholar]
- 35.Lees CC, Romero R, Stampalija T, Dall’Asta A, DeVore GA, Prefumo F, Frusca T, Visser GHA, Hobbins JC, Baschat AA, Bilardo CM, Galan HL, Campbell S, Maulik D, Figueras F, Lee W, Unterscheider J, Valensise H, Da Silva Costa F, Salomon LJ, Poon LC, Ferrazzi E, Mari G, Rizzo G, Kingdom JC, Kiserud T, Hecher K. Clinical opinion: the diagnosis and management of suspected fetal growth restriction: an evidence-based approach. Am J Obstet Gynecol. 2022;226:366–378. doi: 10.1016/j.ajog.2021.11.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao H, Zeng Z, Liu H, Hu Q, Yu H. Resolution of preeclampsia after selective termination in discordant twins: a case report and literature review. Medicine (Baltimore) 2022;101:e31484. doi: 10.1097/MD.0000000000031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suresh S, Freedman A, Adams M, Hirsch E, Ernst LM. Placental histology for targeted risk assessment of recurrent spontaneous preterm birth. Am J Obstet Gynecol. 2024;230:452.e1–452.e11. doi: 10.1016/j.ajog.2023.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Regan AK, Gissler M, Magnus MC, Håberg SE, Ball S, Malacova E, Nassar N, Leonard H, Pereira G. Association between interpregnancy interval and adverse birth outcomes in women with a previous stillbirth: an international cohort study. Lancet. 2019;393:1527–1535. doi: 10.1016/S0140-6736(18)32266-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Li S, Tian C, Li M, Zhang B, Yu H. Changes of uterocervical angle and cervical length in early and mid-pregnancy and their value in predicting spontaneous preterm birth. Front Physiol. 2024;15:1304513. doi: 10.3389/fphys.2024.1304513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Ou Q, Gao L. The increased cfRNA of TNFSF4 in peripheral blood at late gestation and preterm labor: its implication as a noninvasive biomarker for premature delivery. Front Immunol. 2023;14:1154025. doi: 10.3389/fimmu.2023.1154025. [DOI] [PMC free article] [PubMed] [Google Scholar]