Abstract
Objectives: To investigate the role of coagulation indices in assessing the severity of hypertensive disorders in pregnancy (HDP) and their predictive value for delivery outcomes. Methods: A retrospective analysis was conducted on clinical data from 54 pregnant women with HDP who delivered at Zhoushan Hospital between June 2013 and June 2023. A control group of 42 healthy pregnant women from the same period was also included. Results: In the HDP group, activated partial thromboplastin time (APTT) and prothrombin time (PT) were significantly lower, while D-Dimer (D-D) levels were significantly higher compared to the control group (all P < 0.01). Receiver operating characteristic (ROC) curve analysis showed that the areas under the curve (AUC) for APTT, D-D, PT, and the combined assay were 0.886, 0.813, 0.830, and 0.960, respectively (all P < 0.001). The combined assay demonstrated higher predictive efficacy for HDP than that of any single assay (all P < 0.05). Furthermore, APTT, D-D, and PT showed strong correlations with systolic blood pressure and 24-hour urinary protein levels in HDP patients (all P < 0.001), indicating the corresponding HDP severity. For prediction of adverse birth outcomes (ABO), APTT, D-D, PT, and the combined test all had high predictive value (all P < 0.01). The incidence of ABO, especially neonatal ABO, increased significantly when these coagulation indices (except APTT) were at their optimal cut-off points (P < 0.05). Conclusions: The combined testing of APTT, D-D, and PT provides high predictive efficacy for both HDP and ABO and is closely associated with the severity of HDP.
Keywords: Coagulation indices, hypertensive disorders in pregnancy, adverse birth outcomes, prediction
Introduction
Hypertensive disorders in pregnancy (HDP) are common and serious conditions during pregnancy. According to incomplete statistics, the incidence of HDP in China varies regionally, typically ranging from 5% to 10% [1]. The progression of HDP can lead to a range of severe complications that pose significant risks to both mother and child. For example, HDP may advance to pre-eclampsia and eclampsia, characterized by hypertension, proteinuria, and multi-organ damage, which can threaten maternal health and life [2]. In addition, HDP may lead to fetal growth restriction, preterm delivery, and even stillbirth, posing threats to fetal survival and development [3]. Therefore, targeted management and intervention of HDP, along with an in-depth study of its pathological mechanisms, are crucial to reducing HDP-related complications and ensuring the safety of both mother and infant.
Recent studies have increasingly focused on the role of coagulation changes in the pathogenesis of HDP and its impact on maternal and infant outcomes. HDP, a condition closely linked to vascular endothelial dysfunction and immune-inflammatory response, often involves abnormal coagulation changes [4]. These changes include alterations in platelet activity, variations in plasma concentrations of coagulation factors, inhibition of the fibrinolytic system, and decreased levels of anticoagulant factors [5]. These coagulation alterations can lead to a hypercoagulable state, promoting blood clot formation and deposition. Furthermore, abnormal coagulation may increase the risk of thromboembolism in the mother, raising the likelihood of postpartum hemorrhage and negatively affecting maternal fertility and postpartum recovery [6]. Abnormal coagulation can also impair placental blood supply, leading to fetal nutrient and oxygen deficiency, thereby impacting fetal growth and development [7]. Understanding the changes in coagulation function in HDP and their impact on maternal and infant outcomes is essential for clinical practice.
Currently, while numerous studies focus on coagulation changes in HDP patients, less research has been done to address the significance of these changes in predicting HDP severity and delivery outcomes. This study aims to determine whether changes in coagulation function in HDP patients can provide a strong basis for early diagnosis and risk assessment of HDP, thereby guiding effective treatment and delivery decisions.
Materials and methods
Selection and grouping of research subject
A retrospective analysis was conducted on the clinical data of pregnant women with HDP who delivered at Zhoushan Hospital between June 2013 and June 2023. This study was approved by the Ethics Committee of Zhoushan Hospital.
Inclusion criteria: (1) Meeting the diagnostic criteria outlined in the Diagnosis and Management of Hypertension in Pregnancy: Summary of Updated NICE Guidance [8]. (2) Gestational age > 20 weeks and singleton pregnancy. (3) Age between 18 and 35 years old. (4) Primigravida without prior treatment interference.
Exclusion criteria: (1) Presence of polycystic ovary syndrome. (2) Fetal congenital malformations or chromosomal abnormalities. (3) History of hypertension, diabetes mellitus, or other cardiovascular and renal disorders. (4) Presence of malignant tumors.
A total of 54 pregnant women with HDP were included in the HDP group, while 42 healthy pregnant women scheduled to deliver at Zhoushan Hospital during the same period served as the control group (Table 1). Based on the severity of the conditions, the HDP group was further divided into the HDP group only (n=31), the pre-eclampsia group (PE, n=17), and the severe pre-eclampsia group (sPE, n=6). Meanwhile, the participants were classified into the good birth outcome (GBO, n=42) and the adverse birth outcome group (ABO, n=12) accordingly.
Table 1.
Comparison of general clinical information
| Factors | HDP group (n=54) | Normal group (n=42) | χ2/t | P |
|---|---|---|---|---|
| Age (years old) | 27.84 ± 4.67 | 28.44 ± 4.74 | -0.620 | 0.536 |
| Gestation period (W) | 38.92 ± 1.13 | 39.21 ± 1.22 | -1.205 | 0.231 |
| Frequency of pregnancies | 2.62 ± 0.43 | 2.51 ± 0.32 | 1.386 | 0.169 |
| Frequency of births | 1.32 ± 0.23 | 1.39 ± 0.31 | -1.270 | 0.207 |
Note: HDP, hypertensive disorders in pregnancy.
Coagulation function test
Fasting venous blood was collected from patients, centrifuged to separate the plasma, and stored at -80°C. Prothrombin time (PT) and activated partial thromboplastin time (APTT) levels were measured using a fully automatic blood coagulation analyzer [9].
Systolic blood pressure (SBP) measurement
SBP levels were measured using a mercury sphygmomanometer [10]. Three measurements were taken per patient, and the average was calculated.
24-hour Urine Protein (UPro) detection
All urine voided over a 24-hour period was collected in a clean container, beginning at a set time in the morning. After thorough mixing, a sample was taken to measure UPro concentration using the diuretic method [11].
ABO assessment
Adverse maternal, fetal, and neonatal outcomes were documented. Maternal ABO included eclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count), placental abruption, microangiopathic hemolysis, maternal death, and therapeutic induction of labor [12]. Fetal ABO included fetal growth restriction, oligohydramnios, and stillbirth. Neonatal ABO included neonatal asphyxia and death.
Statistical analysis
All experimental data were analyzed using SPSS 23.0. Sample size determination adhered to the inclusion and exclusion criteria, ensuring the minimum sample size requirement was met. Continuous data were expressed as mean ± standard deviation; differences between groups were analyzed using independent samples t-tests, while comparisons among multiple groups were conducted using one-way ANOVA. Categorical data were expressed as ratios (percentages) and compared using Chi-square (X2) tests. The predictive efficacy of each index was assessed using receiver operating characteristic (ROC) curves, and correlations between indices were analyzed using Pearson correlation coefficients.
The sample size was calculated using the following formula (n: the required sample size; Zα/2: the Z-score at the confidence interval level 1-α; ρ: the proportion of the studied subject in the population; δ: the error limits):
The ideal sample size estimated for this study was 15. The sample sizes used in this study exceeded this minimum requirement. A P-value of < 0.05 was considered statistically significant.
Results
HDP markedly enhanced the coagulation function of pregnant women
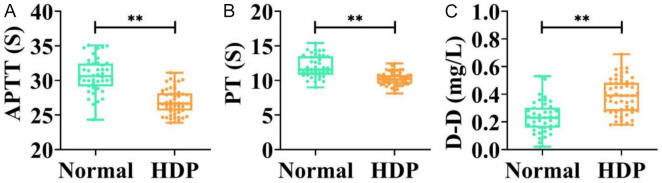
The APTT and PT levels in pregnant women with HDP were significantly lower than those in the normal group, whereas D-D levels were markedly higher (all P < 0.01, Figure 1).
Figure 1.
Comparison of APTT (A), PT (B), and D-D levels (C) in normal and HDP groups. **P < 0.01. APTT, activated partial thromboplastin time; D-D, D-Dimer; PT, prothrombin time; HDP, hypertensive disorders in pregnancy.
Abnormal coagulation indicators effectively predict HDP
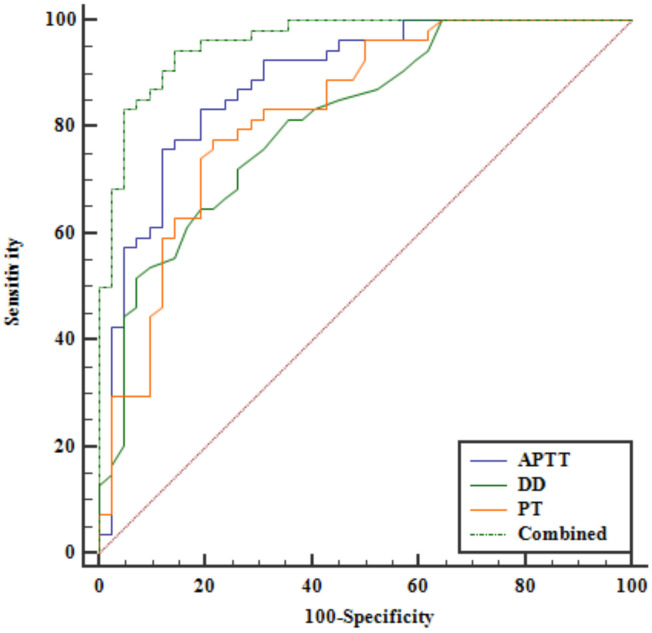
Receiver operating characteristic (ROC) curve analysis indicated that APTT, D-D, and PT levels had high predictive efficacy for HDP. The combined diagnostic approach showed even higher predictive efficacy. Youden’s index identified optimal cut-off points for predicting HDP: APTT ≤ 28.82 s, D-D ≥ 0.28 mg/L, PT ≤ 10.76 s, and combined diagnosis ≥ 0.4494 (all P < 0.001, Figure 2 and Table 2).
Figure 2.
ROC curves for APTT, D-D, PT and combined diagnostics to predict HDP. ROC, receiver operating characteristic; APTT, activated partial thromboplastin time; D-D, D-Dimer; PT, prothrombin time; HDP, hypertensive disorders in pregnancy.
Table 2.
AUC for APTT, D-D, PT and combined diagnostics to predict HDP
| Index | Cut-off value | AUC | Standard error | 95% CI |
|---|---|---|---|---|
| APTT | ≤ 28.82 s | 0.886 | 0.0352 | 0.805-0.942 |
| D-D | ≥ 0.28 mg/L | 0.813 | 0.0435 | 0.721-0.886 |
| PT | ≤ 10.76 s | 0.830 | 0.0431 | 0.739-0.899 |
| Combined | ≥ 0.4494 | 0.960 | 0.0176 | 0.899-0.990 |
| APTT vs. D-D | Z | 1.320 | ||
| P | 0.187 | |||
| APTT vs. PT | Z | 0.948 | ||
| P | 0.343 | |||
| APTT vs. Combined | Z | 2.683 | ||
| P | 0.007 | |||
| D-D vs. PT | Z | 0.289 | ||
| P | 0.773 | |||
| D-D vs. Combined | Z | 3.623 | ||
| P | 0.0003 | |||
| PT vs. Combined | Z | 3.230 | ||
| P | 0.0012 |
Note: AUC, area under the curve; APTT, activated partial thromboplastin time; D-D, D-Dimer; PT, prothrombin time; HDP, hypertensive disorders in pregnancy.
Abnormal coagulation indices correlate strongly with HDP severity
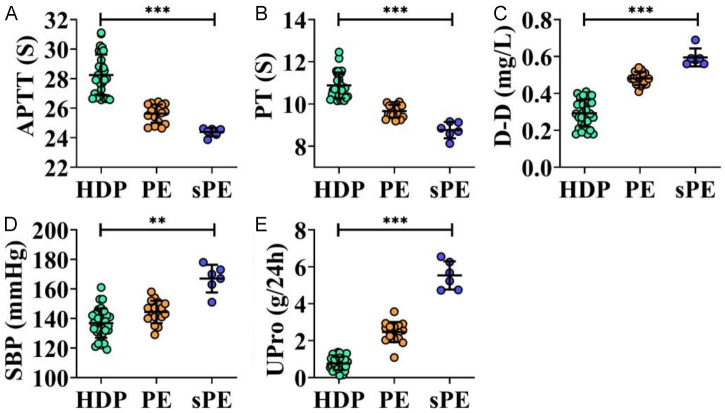
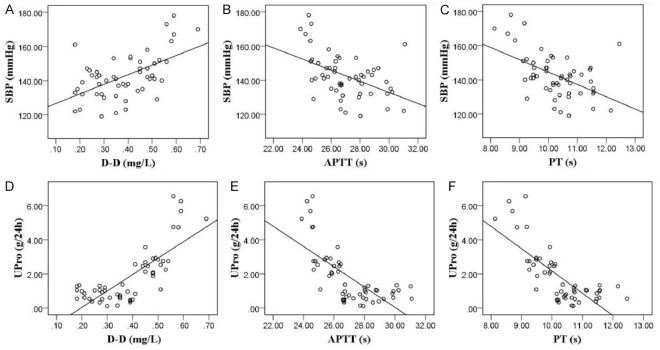
By assessing coagulation parameters, SBP, and 24-hour UPro in patients with varying HDP severity, it was observed that APTT and PT levels were significantly lower in patients with PE and sPE compared to the HDP group, particularly in the sPE group. Conversely, D-D, SBP, and UPro levels were significantly higher (all P < 0.01, Figure 3). Pearson correlation analysis showed that D-D levels were positively correlated with SBP and UPro, while APTT and PT were negatively correlated with these parameters (all P < 0.001, Figure 4).
Figure 3.
Comparison of APTT (A), PT (B), D-D (C), SBP (D), and UPro (E) in HDP, PE, and sPE groups. **P < 0.01, ***P < 0.001. APTT, Activated Partial Thromboplastin Time; D-D, D-Dimer; PT, Prothrombin Time; SBP, Systolic blood pressure; UPro, Urine Protein; PE, pre-eclampsia; sPE, severe pre-eclampsia; HDP, hypertensive disorders in pregnancy.
Figure 4.
Correlation analysis of abnormal coagulation indices and HDP severity. A-C: Pearson correlation between SBP with D-D (r=0.556, P < 0.001), APTT (r=-0.471, P < 0.001), and PT (r=-0.507, P < 0.001). D-F: Pearson correlation between UPro with D-D (r=0.597, P < 0.001), APTT (r=-0.522, P < 0.001), and PT (r=-0.597, P < 0.001). APTT, Activated Partial Thromboplastin Time; D-D, D-Dimer; PT, Prothrombin Time; SBP, Systolic blood pressure; UPro, Urine Protein; HDP, hypertensive disorders in pregnancy.
Abnormal coagulation indices effectively predict ABO
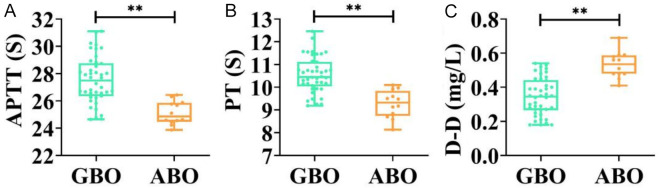
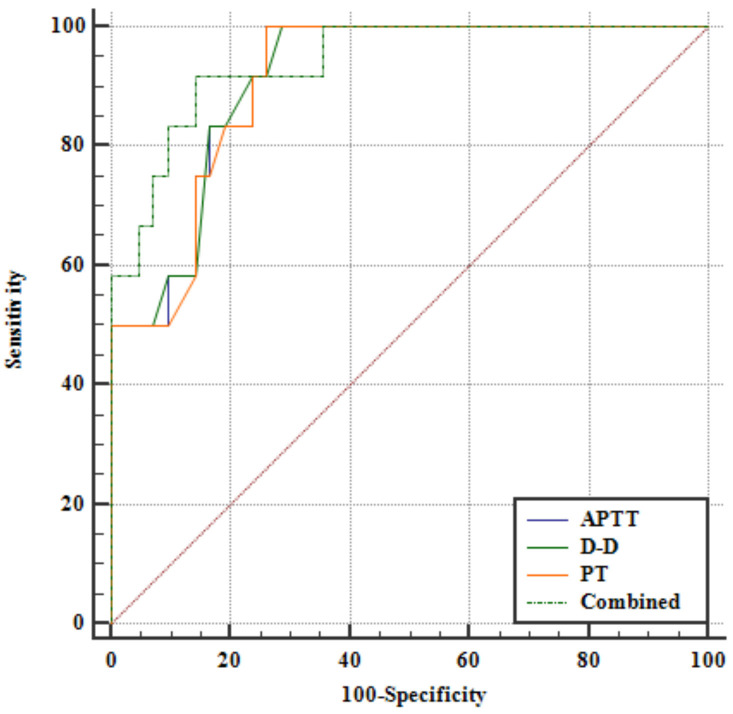
Analysis of patients with ABO revealed that APTT and PT levels were significantly lower in the ABO group compared to the GBO group, whereas D-D levels were significantly higher (all P < 0.01, Figure 5). ROC curve analysis demonstrated that APTT, D-D, and PT levels all had high predictive efficacy for ABO. Youden’s index identified optimal cut-off points for predicting ABO: APTT ≤ 26.44 s, D-D ≥ 0.4 mg/L, PT ≤ 10.1 s, and combined diagnostics ≥ 0.300 (all P < 0.001, Figure 6 and Table 3). Additionally, when D-D levels (P < 0.05) exceeded or PT levels (P < 0.05) we-re below the optimal cut-off points, the incidence of ABO, particularly neonatal ABO, was significantly increased (Table 4).
Figure 5.
Comparison of APTT (A), PT (B), and D-D levels (C) in GBO and ABO groups. **P < 0.01. APTT, Activated Partial Thromboplastin Time; D-D, D-Dimer; PT, Prothrombin Time; GBO, good birth outcome; ABO, adverse birth outcomes.
Figure 6.
ROC curves for APTT, D-D, PT and combined diagnostics to predict ABO. APTT, Activated Partial Thromboplastin Time; D-D, D-Dimer; PT, Prothrombin Time; ROC, The Receiver Operating Characteristic; ABO, adverse birth outcomes.
Table 3.
AUC for APTT, D-D, PT, and combined diagnostics to predict ABO
| Index | Cut-off value | AUC | Standard error | 95% CI |
|---|---|---|---|---|
| APTT | ≤ 26.44 s | 0.913 | 0.0391 | 0.804-0.972 |
| D-D | ≥ 0.4 mg/L | 0.914 | 0.0388 | 0.805-0.973 |
| PT | ≤ 10.1 s | 0.910 | 0.0399 | 0.800-0.971 |
| Combined | ≥ 0.300 | 0.940 | 0.0348 | 0.841-0.987 |
| APTT vs. D-D | Z | 0.260 | ||
| P | 0.795 | |||
| APTT vs. PT | Z | 1.096 | ||
| P | 0.273 | |||
| APTT vs. Combined | Z | 0.973 | ||
| P | 0.331 | |||
| D-D vs. PT | Z | 0.754 | ||
| P | 0.451 | |||
| D-D vs. Combined | Z | 0.971 | ||
| P | 0.332 | |||
| PT vs. Combined | Z | 1.071 | ||
| P | 0.284 |
Note: AUC, area under the curve; APTT, activated partial thromboplastin time; D-D, D-Dimer; PT, prothrombin time; ABO, adverse birth outcomes.
Table 4.
Correlation of APTT, D-D and PT levels with ABO
| Index | Group | n | Maternal ABO | Fetal ABO | Neonatal ABO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| N (%) | χ2 | P | N (%) | χ2 | P | N (%) | χ2 | P | |||
| D-D | ≥ 0.4 mg/L | 24 | 1 (4.17) | 0.013 | 0.910 | 3 (12.5) | 0.570 | 0.450 | 7 (29.17) | 4.550 | 0.033 |
| < 0.4 mg/L | 30 | 0 | 1 (3.33) | 0 | |||||||
| APTT | ≤ 26.44 s | 23 | 1 (4.35) | 0.023 | 0.880 | 2 (8.70) | 0.046 | 0.831 | 5 (21.74) | 1.548 | 0.213 |
| > 26.44 s | 31 | 0 | 2 (6.45) | 2 (6.45) | |||||||
| PT | ≤ 10.1 s | 20 | 1 (5.00) | 0.073 | 0.786 | 3 (15.00) | 1.201 | 0.273 | 6 (30.00) | 5.949 | 0.015 |
| > 10.1 s | 34 | 0 | 1 (2.94) | 1 (2.94) | |||||||
Note: APTT, activated partial thromboplastin time; D-D, D-Dimer; PT, prothrombin time; ABO, adverse birth outcomes.
Discussion
HDP are common conditions that can lead to serious complications, including preeclampsia and HELLP syndrome, posing significant threats to the health and life of both mother and child [13]. While clinical symptoms and basic biochemical markers are used for diagnosing and monitoring HDP, existing methods do not provide sufficient accuracy to determine the severity of the condition or the risk of ABO [14]. Therefore, we for the first time propose to measure coagulation parameters, including APTT, PT, and D-D levels, to improve the accuracy of diagnosing HDP severity and predicting labor outcomes. This study utilized multi-parametric data, successfully establishing a strong relationship between these coagulation indices and the clinical manifestations of HDP. This approach provides new insights for early diagnosis and individualized treatment of HDP, potentially enhancing management efficacy and reducing the risk of adverse clinical outcomes.
By comparing coagulation indices in HDP patients, we found significant enhancement of coagulation function. Similarly, Shi et al. [15] analyzed coagulation indices in HDP patients and observed significantly shorter APTT and PT, linking enhanced coagulation function to elevated coagulation factors in the plasma. This may be due to increased blood volume and progesterone levels during pregnancy, which promote the production and release of coagulation factors, leading to enhanced coagulation [16]. In addition, HDP is often associated with systemic inflammation and immune responses, which may activate the coagulation system [17]. Endothelial damage caused by hypertension can further trigger platelet activation and the coagulation cascade, enhancing coagulation function [18]. Consequently, patients with HDP exhibit significant enhancement of coagulation function.
ROC curve analysis indicated that the combined diagnostic efficacy of APTT, D-D, and PT provided a more accurate prediction of HDP development. APTT reflects the intrinsic coagulation pathway and is indicative of the activity of factors such as prothrombin, and coagulation factors VIII, IX, and XI. PT assesses the extrinsic coagulation pathway by measuring coagulation factor II activity, while D-D, a fibrinolytic product, indicates fibrinolytic activity. In pregnant women with HDP, increased blood volume, progesterone effects, and hypertension-related endothelial damage lead to increased coagulant activity, resulting in decreased APTT and PT levels and elevated D-D levels [19,20]. Therefore, monitoring APTT, PT, and D-D levels in HDP patients may effectively predict HDP development. Zhang et al. [21] found that APTT and PT were significantly negatively correlated with disease severity in HDP patients, while D-D showed a positive correlation. The combined indicators demonstrated higher predictive efficacy for pregnancy outcomes than individual markers, aligning with our findings. Another study also identified PT as a potential predictor of disease severity and adverse pregnancy outcomes in HDP patients, consistent with our results [22].
Additionally, in assessing the correlation between coagulation indices and the severity of HDP, we found that patients with sPE exhibited more pronounced coagulation enhancement. This is likely due to the progressive increase in systemic inflammation and immune response as HDP advances, which can stimulate the coagulation cascade directly or indirectly, leading to enhanced coagulation [23,24]. Furthermore, as HDP severity escalates, patients may experience increased endothelial damage, which activates platelets and coagulation cascade reactions, further enhancing coagulation to maintain the integrity of damaged vessels [25]. Severe HDP may also lead to the release of more coagulation factors, which are crucial in the coagulation cascade [21]. Moreover, HDP can increase fibrin production, a key component in blood clot formation, contributing to further coagulation enhancement [26].
We also observed that APTT, PT, and D-D levels were significantly associated with SBP and 24 h UPro. Elevated UPro levels, a critical marker of HDP severity, are linked to structural and functional alterations in the kidneys caused by HDP, such as damage to the glomerular filtration membrane and tubular dysfunction [27]. Changes in blood levels of coagulation factors and fibronectin can further affect the permeability of the glomerular filtration membrane, increasing the risk of infiltration [28]. The significant association of altered coagulation factors with SBP and UPro highlights the complexity of HDP, aiding in assessing its severity and providing insights into treatment strategies.
We also evaluated coagulation indices in patients with different delivery outcomes to explore their predictive value. Our results suggest that coagulation abnormalities significantly contribute to ABO in HDP patients. The enhanced coagulation typically seen in HDP patients may reduce the risk of bleeding but increases the risk of thrombosis. This complex coagulation state can lead to serious complications during labor, such as obstructed labor or hemorrhage, posing a threat to maternal health [5]. Thrombotic events may also lead to embolism of maternal organs, potentially causing organ dysfunction and further health risks [19]. Coagulation issues may also affect the fetus by reducing placental blood flow, thereby compromising oxygen and nutrient supply [24]. Understanding coagulation abnormalities in HDP patients is essential for preventing ABO and safeguarding maternal and fetal health.
This study has some limitations, including a small sample size, which may reduce the statistical power of the findings. Additionally, the limited number of coagulation markers studied does not fully capture the complexity of the coagulation system. Future studies should consider increasing the sample size to enhance statistical confidence and exploring multiple aspects of the coagulation system to further elucidate the relationship between coagulation abnormalities and birth outcomes in HDP patients.
In conclusion, HDP is associated with abnormal activation of coagulation function, and the combined testing of APTT, PT, and D-D demonstrates high diagnostic efficacy for HDP. Moreover, coagulation function is significantly correlated with HDP severity and effectively predicts labor outcomes.
Acknowledgements
The Startup Fund for scientific research and the Fujian Medical University (grant number: 2020QH1259).
Disclosure of conflict of interest
None.
References
- 1.Hu L, Li DH, Wang SY. A algorithm for prediction of exudative retinal detachment risk of patients with pregnancy-induced hypertension. Int J Ophthalmol. 2022;15:1310–1315. doi: 10.18240/ijo.2022.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S, Li H, Li C, He X, Wang Y. Development and validation of a nomogram for predicting the risk of pregnancy-induced hypertension: a retrospective cohort study. J Womens Health (Larchmt) 2021;30:1182–1191. doi: 10.1089/jwh.2020.8575. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Gu C, Lei Y, Wang J, Sun L, Fan J, Wang Y, Zhang X. Interrelation among one-carbon metabolic (OCM) pathway-related indicators and its impact on the occurence of pregnancy-induced hypertension disease in pregnant women supplemented with folate and vitamin B12: real-world data analysis. Front Nutr. 2022;9:950014. doi: 10.3389/fnut.2022.950014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-induced hypertension. Hormones (Athens) 2015;14:211–223. doi: 10.14310/horm.2002.1582. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wang J, Wang K, Peng T, Liu H, Zheng H, Hu Q. Correlation of component blood transfusion of hypertension patients with pregnancy and postpartum hemorrhage. Clin Lab. 2021;67 doi: 10.7754/Clin.Lab.2020.200849. [DOI] [PubMed] [Google Scholar]
- 6.Liao Y, Ding Y, Yu L, Xiang C, Yang M. Exploring the mechanism of alisma orientale for the treatment of pregnancy induced hypertension and potential hepato-nephrotoxicity by using network pharmacology, network toxicology, molecular docking and molecular dynamics simulation. Front Pharmacol. 2022;13:1027112. doi: 10.3389/fphar.2022.1027112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy Eleti M, Agrawal M, Dewani D, Goyal N. Serum LDH levels in normotensive and preeclamptic-eclamptic pregnant women and its correlation with fetomaternal outcome. Cureus. 2023;15:e37220. doi: 10.7759/cureus.37220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster K, Fishburn S, Maresh M, Findlay SC, Chappell LC Guideline Committee. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366:l5119. doi: 10.1136/bmj.l5119. [DOI] [PubMed] [Google Scholar]
- 9.Tekle E, Gelaw Y, Dagnew M, Gelaw A, Negash M, Kassa E, Bizuneh S, Wudineh D, Asrie F. Risk stratification and prognostic value of prothrombin time and activated partial thromboplastin time among COVID-19 patients. PLoS One. 2022;17:e0272216. doi: 10.1371/journal.pone.0272216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Kim YM, Kim SH, Shin J, Lee EM. Replacing mercury sphygmomanometers with mercury-free sphygmomanometers for the national health survey in children: direct comparisons applying two types of mercury-free sphygmomanometer. Korean Circ J. 2024;54:270–287. doi: 10.4070/kcj.2023.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zheng B, Li Y, Shen X, Huang L, Zhao F, Yan S. Association of high vibration perception threshold with reduced renal function in patients with type 2 diabetes. Front Endocrinol (Lausanne) 2024;15:1357294. doi: 10.3389/fendo.2024.1357294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Than NG, Romero R, Meiri H, Erez O, Xu Y, Tarquini F, Barna L, Szilagyi A, Ackerman R, Sammar M, Fule T, Karaszi K, Kovalszky I, Dong Z, Kim CJ, Zavodszky P, Papp Z, Gonen R. PP13, maternal ABO blood groups and the risk assessment of pregnancy complications. PLoS One. 2011;6:e21564. doi: 10.1371/journal.pone.0021564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Zhang J, Qin F, Chen X, Jiang X. Evaluation of the predictive value of high sensitivity C-reactive protein in pregnancy-induced hypertension syndrome. Exp Ther Med. 2018;16:619–622. doi: 10.3892/etm.2018.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge G, Zhang Y, Zhang M. Pregnancy-induced hypertension and retinopathy of prematurity: a meta-analysis. Acta Ophthalmol. 2021;99:e1263–e1273. doi: 10.1111/aos.14827. [DOI] [PubMed] [Google Scholar]
- 15.Shi F, Yu A, Yuan L. Clinical significance of detection of coagulation indexes, immune factors and inflammatory factors in patients with pregnancy-induced hypertension syndrome in China. Iran J Public Health. 2019;48:681–687. [PMC free article] [PubMed] [Google Scholar]
- 16.Bello NA. Ischemic and nonischemic heart failure after pregnancy-induced hypertension: another piece of the puzzle. JACC Heart Fail. 2023;11:1229–1230. doi: 10.1016/j.jchf.2023.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Socha MW, Malinowski B, Puk O, Dubiel M, Wicinski M. The NLRP3 inflammasome role in the pathogenesis of pregnancy induced hypertension and preeclampsia. Cells. 2020;9:1642. doi: 10.3390/cells9071642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y, Gu S, Zhang X. Effects of nifedipine and labetalol combined with magnesium sulfate on blood pressure control, blood coagulation function, and maternal and infant outcome in patients with pregnancy-induced hypertension. Comput Math Methods Med. 2022;2022:9317114. doi: 10.1155/2022/9317114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Mary S, Small H, Herse F, Carrick E, Flynn A, Mullen W, Dechend R, Delles C. Preexisting hypertension and pregnancy-induced hypertension reveal molecular differences in placental proteome in rodents. Physiol Genomics. 2021;53:259–268. doi: 10.1152/physiolgenomics.00160.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pop VJM, Boekhorst MGBM, Deneer R, Oei G, Endendijk JJ, Kop WJ. Psychological distress during pregnancy and the development of pregnancy-induced hypertension: a prospective study. Psychosom Med. 2022;84:446–456. doi: 10.1097/PSY.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Li H, Guo W, Zhao H, Zheng N, Huang Y. Predictive value of coagulation function and D-dimer for pregnancy outcome in pregnancy-induced hypertension. Am J Transl Res. 2023;15:1150–1158. [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, Tian Y, Li J, Hu M, Hou J, Zhang M. Coagulation index and pregnancy outcome in gestational diabetes mellitus. Clin Lab. 2021;67 doi: 10.7754/Clin.Lab.2020.200336. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Chen L, Wang X, Liu Y. Association between serum beta-human chorionic gonadotropin and inflammation, oxidative stress in pregnancy-induced hypertension. Microvasc Res. 2021;135:104130. doi: 10.1016/j.mvr.2020.104130. [DOI] [PubMed] [Google Scholar]
- 24.Areda BG, Gizaw ST, Berdida DH, Kebalo AH. Evaluation of serum lipid profiles, uric acid, and high sensitivity C-reactive protein levels between pregnancy-induced hypertension and normotensive pregnant women attending Ambo University Referral Hospital, Ambo, Ethiopia, 2020: a case-control study. Health Sci Rep. 2022;5:e806. doi: 10.1002/hsr2.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhutani N, Jethani V, Jethani S, Ratwani K. Coagulation profile and platelet parameters in pregnancy induced hypertension cases and normotensive pregnancies: a cross-sectional study. Ann Med Surg (Lond) 2022;80:104124. doi: 10.1016/j.amsu.2022.104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng L, Liao C. Multivariate logistic regression analysis of preeclampsia in patients with pregnancy induced hypertension and the risk predictive value of monitoring platelet, coagulation function and thyroid hormone in pregnant women. Am J Transl Res. 2022;14:6805–6813. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Sun J, Zhang M, Lin Y, Fang L, Fang X, Mai W, Yin Z. The significance of combined detection of CysC, urinary mAlb and beta(2)-MG in diagnosis of the early renal injury in pregnancy-induced hypertension syndrome. Saudi J Biol Sci. 2019;26:1982–1985. doi: 10.1016/j.sjbs.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang PY, Tsai YL, Chang YJ, Wang PH. Comparisons of urine protein-to-creatinine ratios and their dynamic change patterns during labor at term between normal pregnant women and women with pregnancy induced hypertension. Int J Med Sci. 2022;19:1473–1481. doi: 10.7150/ijms.72926. [DOI] [PMC free article] [PubMed] [Google Scholar]