Abstract
Objective: Acute ST-segment elevation myocardial infarction (STEMI) remains a major contributor to morbidity and mortality worldwide. The no-reflow phenomenon following percutaneous coronary intervention (PCI) complicates the clinical outcome of STEMI. This study aimed to identify a valuable predictor for no-reflow phenomenon. Methods: This retrospective study analyzed clinical data from 378 STEMI patients with metabolic syndrome who underwent PCI between January 2023 and December 2023. Patients were divided into normal reflow (n = 311) and no-reflow (n = 67) groups based on post-PCI coronary angiography results. Data collected included patient demographics, medication usage, lipid profiles, cardiac biomarkers, and the triglyceride-glucose (TyG) index. Results: Patients in the no-reflow group were older (59.98 ± 3.45 vs. 58.69 ± 3.57 years, P = 0.007), with higher fasting glucose (118.57 ± 7.23 vs. 113.59 ± 7.62 mg/dL, P < 0.001) and triglycerides (185.36 ± 10.17 vs. 176.56 ± 10.38 mg/dL, P < 0.001). The TyG index was notably higher in the no-reflow group (8.97 ± 1.15 vs. 7.49 ± 1.17, P < 0.001), showing the strongest correlation with no-reflow (r = 0.420, P < 0.001). Receiver Operating Characteristic (ROC) analysis identified the TyG index as the best predictor, with an AUC of 0.818 at a threshold of 8.1. Multivariable logistic regression identified TyG index ≥ 8.1 as the strongest independent predictor of no-reflow (OR, 9.591; 95% CI, 4.469-20.587, P < 0.001). The AUC of the TyG for predicting no-reflow was 0.869, with specificity and sensitivity of 0.891 and 0.791, respectively. Conclusion: The TyG index is a powerful predictor of the no-reflow phenomenon in STEMI patients with metabolic syndrome undergoing PCI. Its robust sensitivity and specificity underscore its utility for risk stratification, enabling clinicians to identify high-risk patients and tailor preventive strategies.
Keywords: Myocardial infarction, no-reflow phenomenon, percutaneous coronary intervention, metabolic syndrome, triglyceride-glucose index, risk stratification
Introduction
Cardiovascular diseases, including myocardial infarction (MI), remain one of the primary contributors to morbidity and mortality worldwide [1]. Acute ST-segment elevation myocardial infarction (STEMI) involves an abrupt and prolonged interruption of blood supply to a segment of the heart muscle. This condition requires immediate medical action, typically through procedures like percutaneous coronary intervention (PCI), to restore blood flow to the heart and reduce the risk of tissue damage [2]. Among the risk factors for cardiovascular disease, metabolic syndrome (MetS) has garnered significant attention due to its rising prevalence and substantial impact on cardiovascular outcomes [3]. MetS is marked by a cluster of metabolic abnormalities, such as excess abdominal fat, dyslipidemia, hypertension, and hyperglycemia, which collectively elevate the risk of cardiovascular disease [4].
Research has consistently demonstrated that individuals with MetS who experience a myocardial infarction tend to experience poorer clinical outcomes, including increased mortality rates, a greater incidence of recurrent ischemic events, and an elevated risk of complications related to revascularization procedures [5,6]. Among these complications, the no-reflow phenomenon is particularly noteworthy due to its significant correlation with MetS and its harmful effect on myocardial recovery after PCI. No-reflow is a complex process influenced by multiple factors, including endothelial dysfunction, inflammation, oxidative stress, and microvascular obstruction [7]. The occurrence of no-reflow complicates the clinical management of STEMI and poses a significant challenge for clinicians [8]. As such, identifying reliable predictors is crucial for risk stratification and implementing preemptive therapeutic strategies to mitigate it.
In patients with STEMI, MetS can intensify the risk of no-reflow following PCI. This effect is likely mediated by processes related to endothelial dysfunction and inflammation [9]. The triglyceride-glucose (TyG) index has been recognized as a new indicator of insulin resistance and metabolic abnormalities [10]. Calculated from fasting plasma levels of triglycerides and glucose, the TyG index correlates well with traditional measures of insulin resistance, offering a practical and integrative approach to assessing metabolic risk in clinical settings [11].
Insulin resistance, a hallmark of MetS, plays a critical role in the pathogenesis of no-reflow [12]. It is characterized by a diminished ability of cells to respond to insulin, leading to hyperglycemia and hyperinsulinemia [13]. These metabolic disruptions are associated with endothelial cell dysfunction, increased oxidative stress, and heightened inflammatory responses, which collectively impair microvascular integrity and function [14]. Elevated triglyceride levels, often seen in insulin-resistant states, exacerbate these effects by promoting lipotoxicity and contributing to a pro-atherogenic lipid profile [15]. These pathophysiologic alterations underline the significance of assessing insulin resistance through accessible surrogate markers such as TyG index.
Recent research highlights the significance of lipid profiles, including total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and very-low-density lipoprotein (VLDL) cholesterol, in assessing the risk of cardiovascular diseases [16]. However, the independent and combined predictive value of these parameters in relation to the no-reflow phenomenon in metabolically impaired populations remains underexplored [17]. The TyG index, which reflects both glycemic and lipid derangements, offers a holistic measure of metabolic dysfunction and may serve as a valuable prognostic tool in the STEMI setting [18].
Considering the established association between MetS and the no-reflow phenomenon, this study seeks to explore how metabolic indicators, particularly the TyG index, can predict the likelihood of no-reflow in STEMI patients with MetS. By clarifying the predictive capability of TyG for no-reflow, we aim to pinpoint high-risk individuals who may benefit from customized preventive and treatment approaches to mitigate the negative impact of no-reflow.
Materials and methods
Study design
This retrospective study analyzed the clinical data of patients with STEMI complicated by MetS who underwent PCI at Baoji People’s Hospital between January 2023 and December 2023. Patients were divided into a normal reflow group (n = 311) and a no-reflow group (n = 67) based on the presence or absence of reflow after PCI. The study was approved by the Institutional Review Board and Ethics Committee of Baoji People’s Hospital, and informed consent was waived given the exclusive use of de-identified patient data for this retrospective analysis.
Eligibility and grouping criteria
Inclusion criteria: an age of 18 years old or above; have no history of mental illness; have normal cognitive function, and be able to cooperate with various treatments and examinations-- meeting the diagnostic criteria for acute STEMI as per the third global definition of MI. They must also be experiencing their first onset; undergoing a PCI following an emergency diagnosis at a chest pain center; and and satisfying the MetS criteria established by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III). This entails having at least three of the following risk factors: a waist circumference of 102 cm or greater for men and 88 cm or more for women (using European thresholds); triglyceride levels of 150 mg/dL or higher, or being treated for dyslipidemia; HDL cholesterol levels below 40 mg/dL for men or below 50 mg/dL for women, or being treated for this lipid issue; blood pressure readings of 130/85 mm Hg or higher, or treatment for previously diagnosed hypertension; and fasting glucose levels of 100 mg/dL or above, or a prior diagnosis of type 2 diabetes mellitus.
Exclusion criteria: patients with unstable vital signs, such as irregular heart rate, body temperature, or blood pressure; patients with severe cognitive impairment, visual or auditory dysfunction, or a history of mental illness who could not cooperate with treatment or examinations; patients with a MMSE score less than 24; patients who had received fibrinolytic treatment within the last 24 hours; patients with active infections or documented systemic inflammatory diseases, known malignancies, or terminal liver or renal failure.
Grouping criteria: The study analyzed TIMI blood flow grading and myocardial blush grading (MBG) using coronary angiography. The TIMI blood flow grades are divided into: Grade 0 (no perfusion), Grade 1 (partial contrast agent passage through the occluded region without filling the distal vessels), Grade 2 (complete filling of the distal artery by the contrast agent, but with slower flow than normal), and Grade 3 (normal perfusion, where the contrast agent fully and quickly fills and clears the distal vessels). The MBG levels are categorized as: level 0 (no contrast density), level 1 (minimal contrast density), level 2 (moderate but below normal contrast density), and level 3 (normal contrast density). After PCI, a TIMI blood flow grade of ≤ 2 or a grade of 3 with an MBG of ≤ 2 indicates a no-reflow phenomenon, as previously defined by Gupta et al. [19]. Based on the presence or absence of this no-reflow phenomenon post-PCI, patients were divided into two groups: the Normal Reflow Group (n = 311) and the No-Reflow Group (n = 67). The experimental design process is shown in Figure 1. Using Gpower (version 3.1.9.7), the statistical power of the sample size in this study was calculated to be 0.959, assuming an effect size of 0.5, an alpha level of 0.05, and a two-tailed test. This result suggested the sample size in this study was reasonable.
Figure 1.
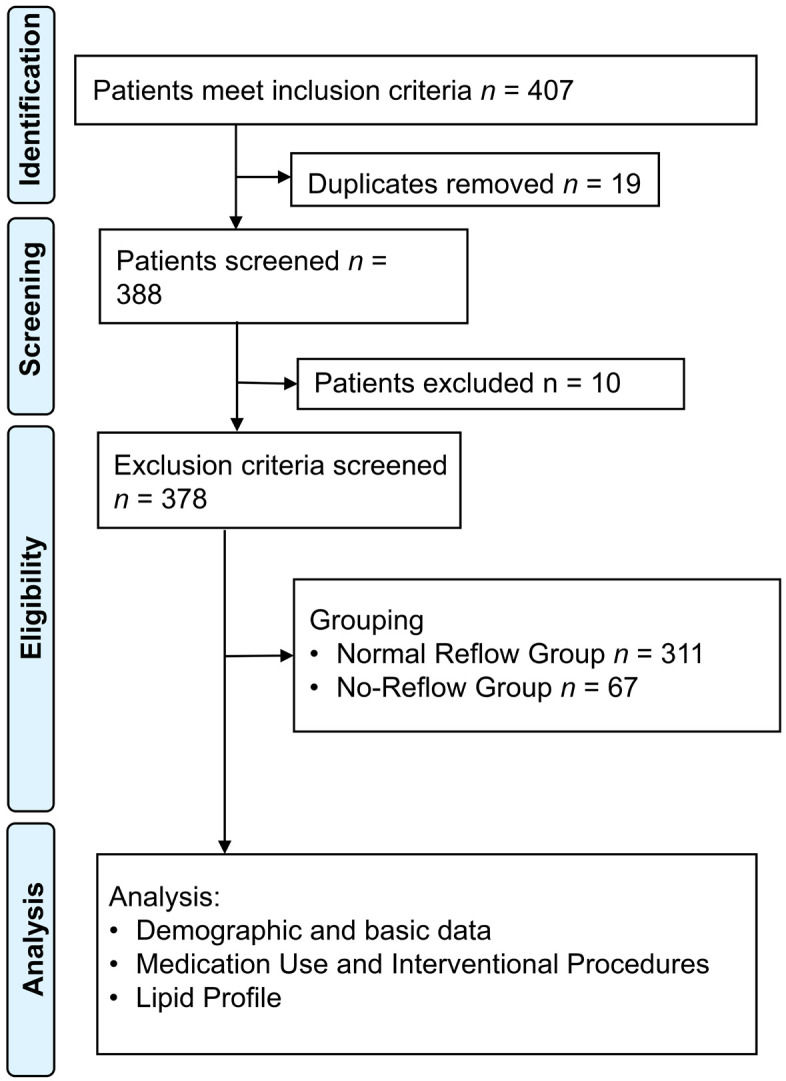
Experimental design flow chart.
Data collection
This research gathered patient demographic data by a case management system. The analysis focused on various aspects including patient baseline characteristics, medication usage, interventional procedures, levels of inflammatory markers, lipid profiles, cardiac biomarkers, coagulation profiles, hematological parameters, and echocardiographic measures. Fasting blood samples (5 ml) were collected on the day of PCI surgery for examining metabolic parameters. A blood lipid/glucose linked test panel (Alere Inc., USA) was used to determine total cholesterol (TC), HDL cholesterol, LDL-C, VLDL-C, non-HDL cholesterol, and TyG index.
Statistical analysis
Data were analyzed using SPSS version 29.0 (SPSS Inc., Chicago, IL, USA). Continuous variables following a normal distribution were expressed as mean ± standard deviation (SD), while categorical variables were summarized using frequencies and percentages. To illustrate the correlation between metabolic values and the no-reflow phenomenon, a correlation heatmap was created. The Student’s t-test was utilized to compare continuous variables between the normal reflow and no-reflow groups. For categorical variables, either the Chi-square test or Fisher’s exact test was employed for comparisons. Additionally, Pearson’s correlation coefficient was used to analyze the relationships between metabolic values and the no-reflow phenomenon.
Logistic regression analysis was conducted to identify the independent predictors associated with the no-reflow phenomenon. Initially, univariate logistic regression was used to calculate the crude odds ratios (ORs) for each variable. Following this, a multivariable logistic regression analysis was carried out to account for potential confounding variables and to compute adjusted ORs. Variables with p-values below 0.1 from the univariate analysis were included in the multivariable model. In this multivariate analysis, adjustment was made for various covariates to mitigate the effects of confounding that could affect the association between metabolic values and the no-reflow phenomenon. The covariates considered included age, sex, smoking habits, a history of hypertension, diabetes, dyslipidemia, and chronic kidney disease, based on their established or potential links with both metabolic values and the no-reflow phenomenon. These covariates were incorporated into the regression model as independent variables alongside the metabolic values being evaluated. Additionally, ROC curve analysis was performed to evaluate the predictive accuracy of the metabolic values regarding the no-reflow phenomenon, with the area under the curve (AUC) calculated to measure the discriminatory power of each value. A significance level of P < 0.05 was set for all statistical tests conducted.
Results
Demographic and basic data
The study included a total of 378 patients, consisting of 311 in the normal reflow group and 67 in the no-reflow group. Notable differences in various baseline characteristics were identified between the two cohorts (Table 1). The mean age of patients in the no-reflow group was significantly higher than that of the normal reflow group (59.98 ± 3.45 years vs. 58.69 ± 3.57 years) (P = 0.007). Additionally, fasting glucose level and triglyceride level were significantly higher in the no-reflow group than those in the normal reflow group (FG: (118.57 ± 7.23 mg/dL vs. 113.59 ± 7.62 mg/dL); Triglyceride: (185.36 ± 10.17 mg/dL vs. 176.56 ± 10.38 mg/dL) (all P < 0.001)). However, there were no significant differences between the two groups regarding body mass index, gender distribution, smoking habits, prevalence of hypertension, prevalence of diabetes, or family history of coronary artery disease (CAD) (all P > 0.05).
Table 1.
Baseline characteristics of study participants
| Value | Normal reflow group (n = 311) | No-reflow group (n = 67) | t/χ2 | P Value |
|---|---|---|---|---|
| Age (years) | 58.69 ± 3.57 | 59.98 ± 3.45 | 2.76 | 0.007 |
| Body Mass Index | 27.22 ± 2.98 | 27.96 ± 3.19 | 1.725 | 0.088 |
| Gender (M/F) | 200/111 | 48/19 | 1.009 | 0.315 |
| Smoking (%) | 35.05% (109) | 41.79% (28) | 0.812 | 0.367 |
| Hypertension (%) | 45.16% (140) | 53.73% (36) | 1.351 | 0.245 |
| Diabetes (%) | 30.23% (94) | 38.81% (26) | 1.498 | 0.221 |
| Family History of CAD (%) | 20.26% (63) | 23.88% (16) | 0.246 | 0.620 |
| Fasting Glucose (mg/dL) | 113.59 ± 7.62 | 118.57 ± 7.23 | 5.071 | P < 0.001 |
| Triglycerides (mg/dL) | 176.56 ± 10.38 | 185.36 ± 10.17 | 6.401 | P < 0.001 |
Note: CAD: coronary artery disease.
Medication use and interventional procedures
An examination of the use of medications and interventional procedures showed no significant differences between the two groups (Table 2).
Table 2.
Medication use and interventional procedures
| Parameter | Normal reflow group (n = 311) | No-reflow group (n = 67) | χ2 | P Value |
|---|---|---|---|---|
| Aspirin (%) | 84.89% (264) | 82.09% (55) | 0.15 | 0.699 |
| Clopidogrel (%) | 78.14% (243) | 76.12% (51) | 0.039 | 0.843 |
| Heparin (%) | 70.10% (218) | 67.17% (45) | 0.107 | 0.744 |
| Statins (%) | 64.95% (202) | 59.70% (40) | 0.451 | 0.502 |
| Beta-blockers (%) | 59.81% (186) | 55.22% (37) | 0.308 | 0.579 |
| ACE inhibitors/ARBs (%) | 49.84% (155) | 47.76% (32) | 0.030 | 0.862 |
| Thrombectomy (%) | 8.04% (25) | 11.94% (8) | 0.620 | 0.431 |
| Stent type (DES/BMS) | 69.77% (217)/30.23% (94) | 64.18% (43)/35.82% (24) | 0.564 | 0.452 |
Note: ACE: angiotensin converting enzyme; ARB: Angiotensin Receptor Blocker; DES: drug-eluting stent; BMS: bare mental stent.
Lipid profile
The lipid profile analysis demonstrated significant differences between the normal reflow and no-reflow groups (Table 3). Patients in no-reflow group had significantly higher total cholesterol level and LDL-C level compared to those in normal reflow group [TC: (200.70 ± 18.82 mg/dL) vs. (195.16 ± 15.47 mg/dL) (P = 0.027); LDL-C: (117.67 ± 11.62 mg/dL) vs. (113.52 ± 10.58 mg/dL) (P = 0.008)]. Conversely, HDL-C was significantly lower in the no-reflow group (41.39 ± 4.43 mg/dL) than that of the normal reflow group (43.22 ± 4.38 mg/dL) (P = 0.003). Non-HDL cholesterol levels were higher in patients with no-reflow (169.46 ± 8.92 mg/dL) versus normal reflow (165.69 ± 7.73 mg/dL) (P = 0.002). Similarly, VLDL cholesterol was elevated in the no-reflow group (25.65 ± 3.58 mg/dL) compared to the normal reflow group (24.12 ± 3.41 mg/dL) (P = 0.01). Notably, the TyG index was substantially higher in the no-reflow group (8.97 ± 1.15) compared to the normal reflow group (7.49 ± 1.17) (P < 0.001).
Table 3.
Lipid profile
| Parameter | Normal reflow group (n = 311) | No-reflow group (n = 67) | t | P Value |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 195.16 ± 15.47 | 200.70 ± 18.82 | 2.251 | 0.027 |
| LDL cholesterol (mg/dL) | 113.52 ± 10.58 | 117.67 ± 11.62 | 2.694 | 0.008 |
| HDL cholesterol (mg/dL) | 43.22 ± 4.38 | 41.39 ± 4.43 | 3.073 | 0.003 |
| Non-HDL cholesterol (mg/dL) | 165.69 ± 7.73 | 169.46 ± 8.92 | 3.211 | 0.002 |
| VLDL cholesterol (mg/dL) | 24.12 ± 3.41 | 25.65 ± 3.58 | 2.634 | 0.010 |
| TyG index | 7.49 ± 1.17 | 8.97 ± 1.15 | 9.549 | P < 0.001 |
Note: LDL: low density lipoprotein; HDL: high-density lipoprotein; VLDL: very low-density lipoprotein; TyG: triglyceride-glucose.
Correlation analysis between metabolic values and no-reflow
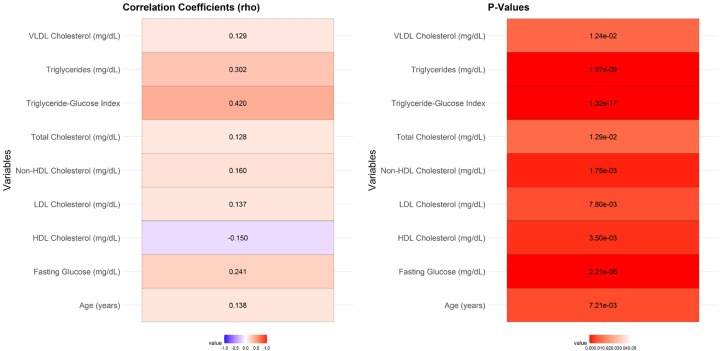
Correlation analysis demonstrated a significant relationship between several values and the no-reflow phenomenon (Figure 2). Age showed a modest positive correlation with no-reflow (r = 0.138, P = 0.007). Fasting glucose levels (r = 0.241, P < 0.001), triglycerides (r = 0.302, P < 0.001), and the TyG index (r = 0.420, P < 0.001) were strongly correlated with no-reflow. Similarly, total cholesterol (r = 0.128, P = 0.013), LDL cholesterol (r = 0.137, P = 0.008), non-HDL cholesterol (r = 0.160, P = 0.002), and VLDL cholesterol (r = 0.129, P = 0.012) exhibited significant positive correlations with no-reflow. Conversely, HDL cholesterol had a significant negative correlation with no-reflow (r = -0.150, P = 0.004).
Figure 2.
Correlation analysis between significant values no-reflow. Note: LDL: low density lipoprotein; HDL: high-density lipoprotein; VLDL: very low-density lipoprotein.
ROC analysis
ROC analysis of metabolic values indicated varying degrees of predictive accuracy for the no-reflow phenomenon (Table 4). The TyG index demonstrated the highest AUC at 0.818, with a sensitivity of 0.806 and specificity of 0.707 at the optimal threshold of 8.1, resulting in a Youden index of 0.513. Triglycerides also showed a relatively high AUC of 0.729, with a sensitivity of 0.627 and specificity of 0.781 at a threshold of 183.855, yielding a Youden index of 0.408. Fasting glucose had an AUC of 0.682, sensitivity of 0.642, and specificity of 0.669 at a threshold of 116.515, producing a Youden index of 0.311. Other data such as age (AUC = 0.604), total cholesterol (AUC = 0.597), LDL cholesterol (AUC = 0.603), HDL cholesterol (AUC = 0.613), non-HDL cholesterol (AUC = 0.621), and VLDL cholesterol (AUC = 0.597) demonstrated lower predictive values, with Youden indices ranging from 0.194 to 0.236. These findings suggest that the TyG index is the most robust predictor of no-reflow among the assessed metabolic values.
Table 4.
ROC analysis between metabolic values and no-reflow
| Value | Best threshold | Sensitivity | Specificity | AUC | Youden index |
|---|---|---|---|---|---|
| Age (years) | 59.76 | 0.627 | 0.608 | 0.604 | 0.235 |
| Fasting Glucose (mg/dL) | 116.515 | 0.642 | 0.669 | 0.682 | 0.311 |
| Triglycerides (mg/dL) | 183.855 | 0.627 | 0.781 | 0.729 | 0.408 |
| Total cholesterol (mg/dL) | 199.39 | 0.612 | 0.617 | 0.597 | 0.229 |
| LDL cholesterol (mg/dL) | 118.94 | 0.522 | 0.714 | 0.603 | 0.236 |
| HDL cholesterol (mg/dL) | 40.465 | 0.463 | 0.768 | 0.613 | 0.231 |
| Non-HDL cholesterol (mg/dL) | 169.295 | 0.522 | 0.672 | 0.621 | 0.194 |
| VLDL cholesterol (mg/dL) | 26.005 | 0.493 | 0.723 | 0.597 | 0.216 |
| TyG index | 8.1 | 0.806 | 0.707 | 0.818 | 0.513 |
Note: LDL: low density lipoprotein; HDL: high-density lipoprotein; VLDL: very low-density lipoprotein; TyG: triglyceride-glucose.
Univariate logistic regression analysis
Univariate logistic regression analysis identified several parameters significantly associated with the no-reflow phenomenon (Table 5). Age ≥ 60 years was significantly associated with no-reflow (OR, 2.603; 95% CI, 1.520-4.538; P < 0.001). Elevated fasting glucose levels (≥ 116.515 mg/dL) were also strongly associated (OR, 3.618; 95% CI, 2.100-6.364; P < 0.001). Patients with triglyceride levels ≥ 183.855 mg/dL had a markedly higher likelihood of no-reflow (OR, 6.004; 95% CI, 3.444-10.667; P < 0.001). Total cholesterol ≥ 199.39 mg/dL (OR, 2.544; 95% CI, 1.489-4.418; P < 0.001), LDL cholesterol ≥ 118.94 mg/dL (OR, 2.728; 95% CI, 1.593-4.694; P < 0.001), and non-HDL cholesterol ≥ 169.295 mg/dL (OR, 2.241; 95% CI, 1.313-3.839; P = 0.003) were also significantly associated with no-reflow. Conversely, HDL cholesterol levels ≥ 40.465 mg/dL were inversely associated with no-reflow (OR, 0.350; 95% CI, 0.202-0.607; P < 0.001). VLDL cholesterol ≥ 26.005 mg/dL showed a significant positive association (OR, 2.539; 95% CI, 1.479-4.365; P < 0.001). The most substantial predictor was the TyG index a significant positive association (OR, 2.539; 95% < 0.001).
Table 5.
Univariate Logistic regression analysis
| Value | Coefficient | Std Error | Wald | P Value | OR | CI Lower | CI Upper |
|---|---|---|---|---|---|---|---|
| Age (years) ≥ 60 | 0.957 | 0.278 | 3.440 | < 0.001 | 2.603 | 1.520 | 4.538 |
| Fasting glucose (mg/dL) ≥ 116.515 | 1.286 | 0.282 | 4.563 | < 0.001 | 3.618 | 2.100 | 6.364 |
| Triglycerides (mg/dL) ≥ 183.855 | 1.792 | 0.287 | 6.235 | < 0.001 | 6.004 | 3.444 | 10.667 |
| Total cholesterol (mg/dL) ≥ 199.39 | 0.934 | 0.277 | 3.377 | < 0.001 | 2.544 | 1.489 | 4.418 |
| LDL cholesterol (mg/dL) ≥ 118.94 | 1.004 | 0.275 | 3.651 | < 0.001 | 2.728 | 1.593 | 4.694 |
| HDL cholesterol (mg/dL) ≥ 40.465 | -1.050 | 0.279 | 3.758 | < 0.001 | 0.350 | 0.202 | 0.607 |
| Non-HDL cholesterol (mg/dL) ≥ 169.295 | 0.807 | 0.273 | 2.958 | 0.003 | 2.241 | 1.313 | 3.839 |
| VLDL cholesterol (mg/dL) ≥ 26.005 | 0.932 | 0.275 | 3.385 | < 0.001 | 2.539 | 1.479 | 4.365 |
| TyG index ≥ 8.1 | 2.307 | 0.333 | 6.925 | < 0.001 | 10.042 | 5.378 | 20.035 |
Note: LDL: low density lipoprotein; HDL: high-density lipoprotein; VLDL: very low-density lipoprotein; TyG: triglyceride-glucose.
Variable screen and multivariable logistic regression analysis
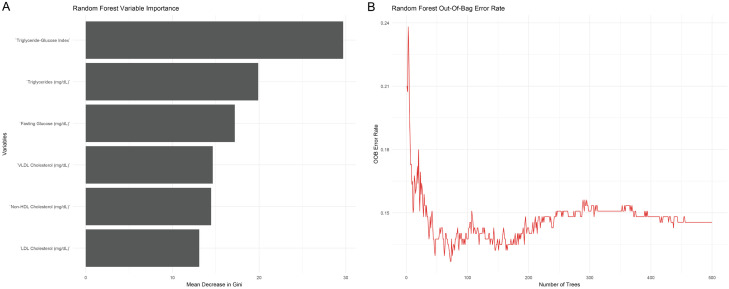
Using random forest variable importance analysis (Figure 3), 6 parameters including fasting glucose, triglycerides, LDL cholesterol, total cholesterol, Non-HDL cholesterol, VLDL cholesterol, and TyG Index were identified with a mean decrease in Gini ≥ 10. The random forest out-of-bag error rate stabilized between 0.1 to 0.15, confirming the robustness of these variables for further analysis. As a result, these parameters were used to establish multivariable Logistic regression analysis.
Figure 3.
Random forest variable importance analysis. A. Variables with Mean Decrease in Gini ≥10; B. Random forest out-of-bag error rate with different number of decision-making trees. Note: LDL: low density lipoprotein; HDL: high-density lipoprotein; VLDL: very low-density lipoprotein.
Multivariate logistic regression analysis identified that several metabolic values were independently associated with the no-reflow phenomenon post-PCI (Table 6). Fasting glucose levels ≥ 116.515 mg/dL were significantly associated with no-reflow (OR, 3.750; 95% CI, 1.844-7.626; P < 0.001). Elevated triglycerides .001). ElevadL also showed a strong independent association (OR, 6.073; 95% CI, 2.989-12.339; P < 0.001). Total cholesterol ≥ 199.39 mg/dL was independently predictive of no-reflow (OR, 2.343; 95% CI, 1.166-4.710; P = 0.017), as was LDL cholesterol ≥ 118.94 mg/dL (OR, 2.446; 95% CI, 1.207-4.955; P = 0.013). Additionally, non-HDL cholesterol levels as was LDL dL (OR, 2.895; 95% CI, 1.416-5.920; P = 0.004) and VLDL cholesterol ≥ 26.005 mg/dL (OR, 2.400; 95% CI, 1.193-4.830; P = 0.014) were significant predictors of no-reflow. The TyG index 400; 95% CI, was the most potent independent predictor, with an OR of 9.591 (95% CI, 4.469-20.587; P < 0.001).
Table 6.
Multivariable Logistic regression analysis
| Value | Coefficient | Std Error | Wald | P Value | OR | OR CI Lower | OR CI Upper |
|---|---|---|---|---|---|---|---|
| Fasting glucose (mg/dL) ≥ 116.515 | 1.322 | 0.362 | 3.650 | < 0.001 | 3.750 | 1.844 | 7.626 |
| Triglycerides (mg/dL) ≥ 183.855 | 1.804 | 0.362 | 4.988 | < 0.001 | 6.073 | 2.989 | 12.339 |
| Total cholesterol (mg/dL) ≥ 199.39 | 0.851 | 0.356 | 2.390 | 0.017 | 2.343 | 1.166 | 4.710 |
| LDL cholesterol (mg/dL) ≥ Lg/dLt | 0.894 | 0.360 | 2.483 | 0.013 | 2.446 | 1.207 | 4.955 |
| Non-HDL Cholesterol (mg/dL) ≥ Ln-HDL | 1.063 | 0.365 | 2.913 | 0.004 | 2.895 | 1.416 | 5.920 |
| VLDL cholesterol (mg/dL) ≥ 26.005 | 0.875 | 0.357 | 2.454 | 0.014 | 2.400 | 1.193 | 4.830 |
| TyG index ≥ nde | 2.261 | 0.390 | 5.802 | < 0.001 | 9.591 | 4.469 | 20.587 |
Note: LDL: low density lipoprotein; HDL: high-density lipoprotein; VLDL: very low-density lipoprotein; TyG: triglyceride-glucose.
Model evaluation
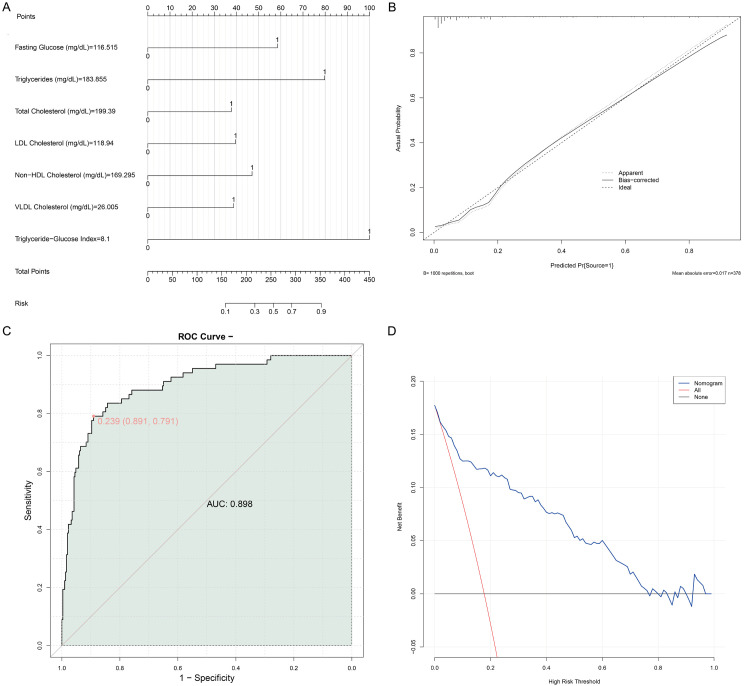
A nomogram revealed the relationship between several continuous independent variables (fasting blood glucose, triglycerides, total cholesterol, LDL cholesterol, non-HDL cholesterol, and VLDL cholesterol) and the no reflow event (Figure 4A). The nearly coincided calibrate plot suggested the model can accurately predict the probability of no reflow (Figure 4B). The value of AUC of the model was 0.869, with specificity being 0.891 and sensitivity being 0.791, indicating good differentiation ability (Figure 4C). The decision curve analysis (DCA) plot indicated that the multivariable logistic regression model added value across different decision thresholds compared to no intervention (Figure 4D).
Figure 4.
Verification of the model’s predictive performance. A. Nomogram; B. Calibrate plot; C. ROC curve; D. DCA plot.
Discussion
The present study demonstrates that the TyG index is a significant prognostic indicator for predicting the no-reflow phenomenon in STEMI patients with MetS undergoing PCI. This finding has substantial clinical implications, given the adverse outcomes associated with no-reflow [20], such as increased infarct size, reduced left ventricular function, and higher mortality rate.
First, we observed a robust association between elevated TyG index and the no-reflow phenomenon. The biochemical basis for this observation ties into the pathophysiology of both insulin resistance and endothelial dysfunction, which are pivotal in the development of no-reflow post-PCI [21]. The TyG index, calculated from fasting triglyceride and glucose measurements, serves as a surrogate marker for insulin resistance. Insulin resistance, in turn, exacerbates atherogenesis and fosters an environment conducive to pro-inflammatory, pro-thrombotic states, and endothelial dysfunction, all of which contribute to the no-reflow phenomenon [22].
Insulin resistance plays a key role in the development of small, dense LDL particles, which are especially harmful in promoting atherosclerosis [23]. These particles penetrate the endothelial layer more easily, get oxidized, and initiate a cascade of inflammatory responses that promote plaque formation and instability, which are critical factors in the context of acute coronary syndromes [24]. The increased level of inflammatory markers in the no-reflow cohort supports the concept that systemic inflammation leads to microvascular obstructions through various mechanisms such as endothelial swelling, intramyocardial hemorrhage, and microvascular embolization [25].
Elevated fasting glucose and triglyceride levels were also independently predictive of no-reflow. Hyperglycemia has been documented to impair endothelial function through multiple mechanisms, including oxidative stress, the formation of advanced glycation end products (AGEs), and the activation of protein kinase C (PKC) pathway, all of which contribute to endothelial dysfunction and microvascular complications [12,15]. Similarly, hypertriglyceridemia may induce endothelial dysfunction through increased production of reactive oxygen species (ROS), which causes oxidative stress and inflammation, thereby exacerbating microvascular thrombogenesis and reducing coronary flow reserve [26].
Our findings are consistent with the gluco-lipotoxicity theory, which posits that elevated levels of glucose and lipids lead to cellular dysfunction and apoptosis in various tissues, including endothelial cells [25,27]. This dysregulation is particularly relevant in MetS, which is characterized by concomitant hyperglycemia, dyslipidemia, hypertension, and central obesity, all of which synergistically impair vascular function and increase susceptibility to no-reflow post-PCI [7,28].
Lipoproteins, particularly non-HDL cholesterol components like LDL and VLDL, were notably elevated in the no-reflow group. Elevated LDL cholesterol levels could directly contribute to endothelial dysfunction via increased oxidative stress and lipid peroxidation, leading to reduced nitric oxide bioavailability, which is essential for vasodilation and endothelial function [9,29]. Non-HDL cholesterol represents the sum of all atherogenic particles, and its elevation in non-reflow group enforces the hypothesis of a pro-atherogenic milieu contributing to microvascular obstruction by embolization of lipid-rich plaques during PCI [26]. On the other hand, HDL cholesterol was significantly lower in the no-reflow group, suggesting its protective role against oxidative stress and inflammation, where reduced levels might predispose individuals to endothelial dysfunction and impaired microvascular function [27,30].
Interestingly, the logistic regression analysis reinforced the independent predictive value of these lipid values, where higher levels of total cholesterol, LDL-C, non-HDL cholesterol, and VLDL cholesterol were significant predictors of no-reflow. VLDL cholesterol, though less discussed in the context of CAD compared to LDL-C, is laden with triglycerides and can deliver a significant amount of cholesterol to tissues, exacerbating atherogenic processes [10,11]. This component, reflecting the remnant lipoproteins, has been shown to be highly atherogenic and is intricately linked with insulin resistance and MetS [13].
The positive correlation between age and no-reflow implies that age-related decline in vascular flexibility and increase in inflammatory markers may contribute to the pathogenesis of microvascular dysfunction [14]. Aging is associated with endothelial senescence, characterized by reduced endothelial nitric oxide synthase (eNOS) activity and increased oxidative stress, further elevating the risk of no-reflow phenomenon post-PCI [31].
Taken together, the ROC curve and logistic regression analyses underscore the potent predictive capacity of the TyG index - based model for the no-reflow phenomenon. The model’s superior AUC accentuates its utility over traditional metabolic values. By integrating fasting glucose and triglycerides, the TyG index captures a broader metabolic dysfunction spectrum, reflecting the extensive endothelial and microvascular impairment seen in MetS.
Our findings advocate for the inclusion of the TyG index in the clinical risk stratification models for STEMI patients undergoing PCI, particularly those with MetS. Utilizing the TyG index could refine current predictive algorithms for no-reflow, facilitating early identification and tailored interventional strategies designed to mitigate this phenomenon-such strategies might include more aggressive glycemic control, lipid-lowering therapies, and anti-inflammatory approaches pre- and post-PCI.
The study’s retrospective design is a limitation that warrants cautious interpretation of causality. Prospective validation in larger, diverse cohorts would solidify the TyG index’s prognostic utility. Another limitation is the exclusive reliance on fasting glucose and triglyceride values pre-PCI; incorporating dynamic changes in these values and other biomarkers such as inflammatory cytokines and oxidative stress markers could present a more comprehensive risk assessment.
Conclusion
In conclusion, the TyG index emerges as a robust predictor of the no-reflow phenomenon post-PCI in STEMI patients with MetS. Elevated levels of fasting glucose and triglycerides, reflective of insulin resistance and metabolic dysregulation, underscore the pathophysiological mechanism of no-reflow. These insights bolster the need for integrating metabolic management inti STEMI care paradigms, aiming to mitigate the adverse effects of no-reflow and enhance clinical outcomes. Future research should endeavor to refine these predictive models and translate these findings into therapeutic interventions optimizing revascularization success.
Disclosure of conflict of interest
None.
References
- 1.Ruíz-Avalos JA, Bazán-Rodríguez L, Espinoza-Escobar G, Martínez-Villa FA, Ornelas-Aguirre JM. Predictors in no-reflow phenomenon in acute myocardial infarction with ST-segment elevation. Arch Cardiol Mex. 2022;92:461–468. doi: 10.24875/ACM.21000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalfallah M, Allaithy A, Maria DA. Impact of the total ischemia time on no-reflow phenomenon in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Anatol J Cardiol. 2022;26:382–387. doi: 10.5152/AnatolJCardiol.2021.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 4.Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the united states, 2011-2016. JAMA. 2020;323:2526–2528. doi: 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ndrepepa G, Kastrati A. Coronary no-reflow after primary percutaneous coronary intervention-current knowledge on pathophysiology, diagnosis, clinical impact and therapy. J Clin Med. 2023;12:5592. doi: 10.3390/jcm12175592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kożuch M, Południewski M, Dąbrowski EJ, Tarasiuk E, Dobrzycki S. Growth differentiation factor 15 as a predictor of the no-reflow phenomenon in patients with ST-segment elevation myocardial infarction. J Clin Med. 2022;12:245. doi: 10.3390/jcm12010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yildirim A, Kucukosmanoglu M, Koyunsever NY, Cekici Y, Belibagli MC, Kilic S. Relationship between blood viscosity and no-reflow phenomenon in ST-segment elevation myocardial infarction performed in primary percutaneous coronary interventions. Biomark Med. 2021;15:659–667. doi: 10.2217/bmm-2020-0772. [DOI] [PubMed] [Google Scholar]
- 8.Açıkgöz E, Açıkgöz SK, Çiçek G. Serum magnesium concentration may predict no-reflow phenomenon in primary angioplasty for ST-elevation myocardial infarction. Magnes Res. 2020;33:123–130. doi: 10.1684/mrh.2021.0477. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XT, Lin ZR, Zhang L, Zhao ZW, Chen LL. MELD-XI score predict no-reflow phenomenon and short-term mortality in patient with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. BMC Cardiovasc Disord. 2022;22:113. doi: 10.1186/s12872-022-02556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toprak K, Kaplangoray M, Memioğlu T, İnanır M, Omar B, Ermiş MF, Toprak İH, Acar O, Taşcanov MB, Altıparmak İH, Biçer A, Demirbağ R. The HbA1c/C-peptide ratio is associated with the no-reflow phenomenon in patients with ST-elevation myocardial infarction. Angiology. 2023 doi: 10.1177/00033197231213166. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Çelik MC, Karayiğit O, Ozkan C, Dolu AK, Kalçık M. Relationship between systemic inflammation index and no-reflow phenomenon in patients with ST-segment elevation myocardial infarction. Angiology. 2023;74:387–394. doi: 10.1177/00033197221115562. [DOI] [PubMed] [Google Scholar]
- 12.Konijnenberg LSF, Damman P, Duncker DJ, Kloner RA, Nijveldt R, van Geuns RM, Berry C, Riksen NP, Escaned J, van Royen N. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res. 2020;116:787–805. doi: 10.1093/cvr/cvz301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Li Z, Quan X, Liu X, Sun T, Wei T, Pan J, Liu Z, Wang M, Dong H, Zhang Z. Strategies to attenuate myocardial infarction and no-reflow through preservation of vascular integrity by pigment epithelium-derived factor. Hum Gene Ther. 2022;33:330–345. doi: 10.1089/hum.2021.068. [DOI] [PubMed] [Google Scholar]
- 14.Güler A, Gürbak İ, Panç C, Güner A, Ertürk M. Frequency and predictors of no-reflow phenomenon in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. Acta Cardiol. 2022;77:313–321. doi: 10.1080/00015385.2021.1931638. [DOI] [PubMed] [Google Scholar]
- 15.Akbar KMA, Dharma S, Andriantoro H, Sukmawan R, Mangkuanom AS, Rejeki VG. Relationship between hemoglobin concentration at admission with the incidence of no-reflow phenomenon and in-hospital mortality in acute myocardial infarction with elevation of ST segments in patients who underwent primary percutaneous coronary intervention. Int J Angiol. 2023;32:106–112. doi: 10.1055/s-0042-1742308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaul S, Methner C, Cao Z, Mishra A. Mechanisms of the “no-reflow” phenomenon after acute myocardial infarction: potential role of pericytes. JACC Basic Transl Sci. 2023;8:204–220. doi: 10.1016/j.jacbts.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama Y, Taruya A, Kashiwagi M, Ozaki Y, Shiono Y, Tanimoto T, Yoshikawa T, Kondo T, Tanaka A. No-reflow phenomenon and in vivo cholesterol crystals combined with lipid core in acute myocardial infarction. Int J Cardiol Heart Vasc. 2022;38:100953. doi: 10.1016/j.ijcha.2022.100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buono A, Gori T. No-reflow phenomenon in acute myocardial infarction: relieve pressure from the procedure and focus attention to the patient. Int J Cardiol Heart Vasc. 2019;24:100417. doi: 10.1016/j.ijcha.2019.100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Gupta MM. No reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J. 2016;68:539–551. doi: 10.1016/j.ihj.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caiazzo G, Musci RL, Frediani L, Umińska J, Wanha W, Filipiak KJ, Kubica J, Navarese EP. State of the Art: no-reflow phenomenon. Cardiol Clin. 2020;38:563–573. doi: 10.1016/j.ccl.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal P, Rekwal L, Sinha SK, Nath RK, Khanra D, Singh AP. Predictors of no-reflow phenomenon following percutaneous coronary intervention for ST-segment elevation myocardial infarction. Ann Cardiol Angeiol (Paris) 2021;70:136–142. doi: 10.1016/j.ancard.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Gong X, Lei X, Huang Z, Song Y, Wang Q, Qian J, Ge J. D-Dimer level predicts angiographic no-reflow phenomenon after percutaneous coronary intervention within 2-7 days of symptom onset in patients with ST-segment elevation myocardial infarction. J Cardiovasc Transl Res. 2021;14:728–734. doi: 10.1007/s12265-020-09991-6. [DOI] [PubMed] [Google Scholar]
- 23.Şenöz O, Emren SV, Erseçgin A, Yapan Emren Z, Gül İ. Platelet-lymphocyte ratio is a predictor for the development of no-reflow phenomenon in patients with ST-segment elevation myocardial infarction after thrombus aspiration. J Clin Lab Anal. 2021;35:e23795. doi: 10.1002/jcla.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Huang S, Zhou Q, Dou L, Lin D. The predictive value of laboratory parameters for no-reflow phenomenon in patients with ST-elevation myocardial infarction following primary percutaneous coronary intervention: a meta-analysis. Clin Cardiol. 2024;47:e24238. doi: 10.1002/clc.24238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalfallah M, Maria DA, Allaithy A. Impact of stress hyperglycemia on no-reflow phenomenon in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Glob Heart. 2022;17:23. doi: 10.5334/gh.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Methner C, Cao Z, Mishra A, Kaul S. Mechanism and potential treatment of the “no reflow” phenomenon after acute myocardial infarction: role of pericytes and GPR39. Am J Physiol Heart Circ Physiol. 2021;321:H1030–H1041. doi: 10.1152/ajpheart.00312.2021. [DOI] [PubMed] [Google Scholar]
- 27.Annibali G, Scrocca I, Aranzulla TC, Meliga E, Maiellaro F, Musumeci G. “No-reflow” phenomenon: a contemporary review. J Clin Med. 2022;11:2233. doi: 10.3390/jcm11082233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciofani JL, Allahwala UK, Scarsini R, Ekmejian A, Banning AP, Bhindi R, De Maria GL. No-reflow phenomenon in ST-segment elevation myocardial infarction: still the Achilles’ heel of the interventionalist. Future Cardiol. 2021;17:383–397. doi: 10.2217/fca-2020-0077. [DOI] [PubMed] [Google Scholar]
- 29.Rashed MI, Saleh MA, Elfekky EM, Elmahmoudy AM. CHA2DS2 VASc score and brachial artery flow-mediated dilation as predictors for no-reflow phenomenon in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Egypt Heart J. 2022;74:13. doi: 10.1186/s43044-022-00249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Hu M, Ma S, Niu T. New R(2)-CHA(2)DS(2)-VASc score predicts no-reflow phenomenon and long-term prognosis in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Front Cardiovasc Med. 2022;9:899739. doi: 10.3389/fcvm.2022.899739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Dai R, Qin Z, Cai R, Xu Y, Su Q. LncRNA MALAT1 functions as a biomarker of no-reflow phenomenon in ST-segment elevation myocardial infarction patients receiving primary percutaneous coronary intervention. Sci Rep. 2022;12:3294. doi: 10.1038/s41598-022-06923-z. [DOI] [PMC free article] [PubMed] [Google Scholar]