Abstract
Objective: To evaluate the effects of atorvastatin calcium on clinical manifestations and serum inflammatory markers in elderly patients with hypertension. Methods: This retrospective cohort study included 68 elderly patients with hypertension admitted to the Chongming Branch of Shanghai Tenth People’s Hospital between March 2022 and June 2023. Patients were matched into two groups, each comprising 34 participants: a control group receiving amlodipine besylate and an experimental group receiving additional atorvastatin calcium. The study compared blood pressure, lipid profiles, and serum inflammatory markers before and after treatment between the groups. Biomarkers measured included tumor necrosis factor-alpha (TNF-α), high-sensitivity C-reactive protein (hs-CRP), and interleukin-6 (IL-6). Indices of target organ function measured were urinary microalbumin (mAlb), endogenous creatinine clearance (Ccr), and various cardiac structural indices, including left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), and left ventricular posterior wall thickness (LVPW). Adverse reactions post-treatment were also recorded. Results: Post-treatment assessments indicated significant reductions in both diastolic and systolic blood pressure (DBP and SBP), as well as improvements in lipid profiles (total cholesterol, triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol) in both groups. The experimental group exhibited greater improvements in 24-hour mean diastolic and systolic blood pressures after 4, 8, and 12 weeks of treatment compared to the control group (all P < 0.05). Additionally, serum inflammatory markers, including TNF-α, hs-CRP, and IL-6, were significantly lower in the experimental group than in the control group (all P < 0.05). The experimental group also showed superior improvements in mAlb and Ccr levels (both P < 0.05). Cardiac function indicators improved, with the experimental group showing greater increases in LVEF and more pronounced reductions in LVEDD and LVPW (both P < 0.05) compared to the control group. The incidence of adverse reactions was slightly lower in the experimental group (11.76%) than that in the control group (14.71%), although this difference was not statistically significant (P > 0.05). Conclusion: Adding atorvastatin calcium to amlodipine therapy significantly improves clinical outcomes and reduces serum inflammatory marker levels in elderly patients with hypertension. This combination therapy is considered safe and may be recommended for broader clinical application.
Keywords: Atorvastatin calcium, hypertension, elderly patients, clinical symptoms, serum inflammatory markers
Introduction
Hypertension, characterized by sustained elevation of both systolic and diastolic blood pressure, is a prevalent systemic disorder in elderly populations [1]. Extensive research has elucidated various physiological and pathological alterations associated with this condition in older adults, including increased peripheral vascular resistance, a progressive decline in cardiac output, enhanced stiffness of large vessels, reduced elasticity, diminished vascular compliance, increased internal pressure gradients, impaired renal perfusion and creatinine clearance, reduced sodium excretion, heightened salt sensitivity, and decreased activity of the renin-angiotensin-aldosterone system (RAAS) [2-4].
Chronic inflammation is common in elderly patients with hypertension [5]. Hypertension can induce a pro-inflammatory state by activating the renin-angiotensin system (RAS), leading to the production of pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) [6]. This inflammatory response can be attributed to several factors, including age-related changes in the immune system, increased oxidative stress, and endothelial dysfunction. Atorvastatin calcium, primarily known as a cholesterol-lowering drug, also possesses anti-inflammatory properties. It inhibits the synthesis of isoprenoid intermediates necessary for the prenylation and activation of RAS-related proteins involved in inflammatory pathways.
Amlodipine, a calcium channel blocker of the dihydropyridine class, plays a pivotal role in managing hypertension in the elderly [7,8]. This medication not only facilitates diuresis and sodium excretion but also exerts anti-atherosclerotic effects. By directly dilating blood vessels, amlodipine effectively reduces peripheral vascular resistance, thereby achieving a smooth reduction in blood pressure [9-11]. Additionally, atorvastatin calcium improves endothelial function, reduces oxidative stress, and downregulates the expression of pro-inflammatory cytokines, thereby mitigating the inflammatory response [12]. However, amlodipine monotherapy often exhibits suboptimal efficacy in treating hypertension in elderly patients.
In response to these limitations, this study included 68 elderly individuals diagnosed with hypertension who were admitted to our hospital. Participants were divided into control and experimental groups, with the latter receiving combination therapy that included atorvastatin calcium to evaluate its therapeutic efficacy. Atorvastatin calcium, a lipid-lowering agent, potentially inhibits the progression of atherosclerosis and cardiovascular disease by reducing cholesterol synthesis [13].
The primary objective of this study was to assess the effectiveness of atorvastatin calcium in conjunction with amlodipine and to examine its impact on both physiological indices and pathological states in elderly patients with hypertension. This study aimed to provide new insights into managing hypertension in the elderly and to investigate the feasibility and efficacy of combined atorvastatin calcium and amlodipine therapy. These findings may facilitate the development of more individualized and precise treatment strategies in clinical practice.
Materials and methods
Research participants
This study employed a retrospective analytical approach to assemble a cohort of 68 elderly patients with hypertension admitted to the Chongming Branch of Shanghai Tenth People’s Hospital. The inclusion period spanned from March 2022 to June 2023. Participants were matched into experimental and control groups, each comprising 34 individuals.
In the experimental group, there were 21 males and 13 females, with ages ranging from 60 to 85 years and an average age of 69.25 ± 2.46 years. The mean duration of hypertension was 6.28 ± 1.85 years. This group included 16 patients with grade I hypertension and 18 patients with grade II hypertension. The control group consisted of 20 males and 14 females, aged between 61 and 86 years, with an average age of 70.18 ± 2.53 years. The average duration of hypertension in this group was 6.25 ± 1.96 years, with an equal distribution of 17 cases of grade I and 17 cases of grade II hypertension. The baseline characteristics of both groups were statistically comparable, with no significant differences observed (P > 0.05), confirming the appropriateness of the group assignments for comparative analysis. This study was approved by the Ethics Committee of the Chongming Branch of Shanghai Tenth People’s Hospital.
Inclusion and exclusion criteria
Inclusion criteria: Participants were diagnosed with hypertension according to the Chinese Guidelines for the Prevention and Treatment of Hypertension (2023 edition) [14]. Participants were aged 60 years or older. Participants had cognitive clarity, and the ability to communicate effectively.
Exclusion criteria: Patients with severe hypertension-related complications, significant lesions in other critical organs. Patients with a history of drug abuse, concurrent infectious diseases, or severe impairment of immune function.
Methods
Control group treatment: Patients in the control group received amlodipine besylate (Pfizer Pharmaceutical Co., Ltd., State Pharmaceutical License No. H10950224) at a dose of 5 mg/day, administered orally.
Experimental group treatment: In addition to the control treatment, the experimental group received atorvastatin calcium (Zhejiang Haizheng Pharmaceutical Co., Ltd., State Drug Permit H20103631) at a dose of 20 mg/day, taken orally at bedtime.
Duration of treatment: Both groups followed their respective treatment protocols for two months.
Outcome measurements
This study aimed to compare the efficacy of different treatment regimens in controlling blood pressure, managing blood lipid levels, reducing serum inflammatory markers, and affecting target organ indices before and after treatment in both groups. The specific indices measured were as follows.
Blood pressure control: Systolic blood pressure (SBP), 24-hour average SBP, diastolic blood pressure (DBP), and 24-hour average DBP.
Lipid control: Total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
Inflammatory markers: Tumor necrosis factor-alpha (TNF-α; 88-7324, Thermo Fisher Scientific), high-sensitivity C-reactive protein (hs-CRP; E-EL-H5134, Elabscience), and interleukin-6 (IL-6; D6050, R&D Systems), measured using enzyme-linked immunosorbent assays.
Target organ indices: Urinary microalbumin (mAlb) and endogenous creatinine clearance (Ccr) were derived from 2 mL of fasting venous blood and 5 mL of mixed urine collected over 24 hours. Cardiac structural indices, such as left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), and left ventricular posterior wall thickness (LVPW), were also evaluated.
Adverse reactions: Any post-treatment adverse reactions were recorded for both groups.
Statistical processing
Data analysis was performed using SPSS software version 26.0. Quantitative data, including changes in blood pressure, blood lipid levels, and inflammatory factor levels, were expressed as mean ± standard deviation (Mean ± SD) and analyzed using the t-test. Qualitative data, such as the incidence of adverse reactions, were expressed as percentages and analyzed using the chi-squared test. A P-value < 0.05 was considered statistically significant, indicating meaningful differences between pre- and post-treatment values.
Results
Comparison of general data
In the experimental group, there were 21 males and 13 females, aged 60 to 85 years, with an average age of 69.25 ± 2.46 years. The mean duration of hypertension in this group was 6.28 ± 1.85 years. This group included 16 patients with grade I hypertension and 18 patients with grade II hypertension. The control group consisted of 20 males and 14 females, aged 61 to 86 years, with an average age of 70.18 ± 2.53 years. The mean duration of hypertension in this group was 6.25 ± 1.96 years, with 17 cases of grade I hypertension and 17 cases of grade II hypertension. Baseline characteristics between the two groups were statistically comparable, with no significant differences observed (all P > 0.05), confirming the suitability of the group assignments for comparative analysis (Table 1).
Table 1.
Comparison of general data
| Male (n) | Female (n) | Age (year) | Duration of hypertension (year) | Grade I hypertension (n) | Grade II hypertension | |
|---|---|---|---|---|---|---|
| Control group (n=34) | 20 | 14 | 70.18 ± 2.53 | 6.25 ± 1.96 | 16 | 18 |
| Experimental group (n=34) | 21 | 13 | 69.25 ± 2.46 | 6.28 ± 1.85 | 17 | 17 |
| t-value | 1.537 | 0.0649 | ||||
| P-value | > 0.05 | > 0.05 | 0.1291 | 0.9484 |
Comparison of blood pressure improvement between the two groups
Post-treatment, both patient groups showed a significant reduction in blood pressure compared to their respective baseline measurements. Before treatment, the experimental group had a mean DBP of 98.03 ± 8.37 mmHg and SBP of 163.19 ± 11.26 mmHg. The control group had similar baseline values, with a DBP of 97.84 ± 9.16 mmHg and an SBP of 163.48 ± 10.95 mmHg. After the intervention, the experimental group demonstrated enhanced blood pressure control, with post-treatment values of 74.96 ± 4.25 mmHg for DBP and 123.27 ± 9.06 mmHg for SBP. In contrast, the control group showed post-treatment values of 87.52 ± 5.38 mmHg for DBP and 136.26 ± 10.13 mmHg for SBP. The reduction in both DBP and SBP was significantly greater in the experimental group, with statistical analysis confirming these differences (P < 0.05) (Figures 1 and 2).
Figure 1.
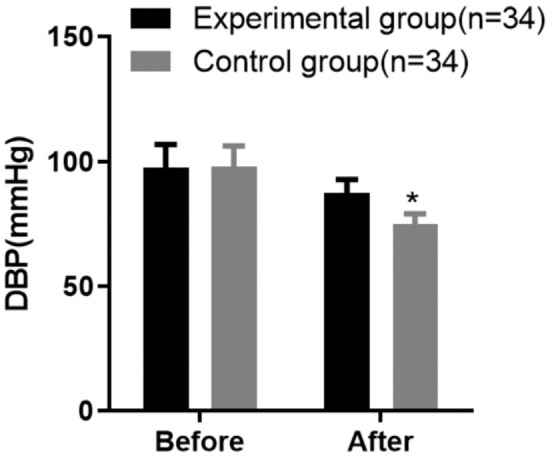
Comparison of diastolic blood pressure (DBP) improvement between the two groups of patients. Note: Compared with the control group after treatment, *P < 0.05.
Figure 2.
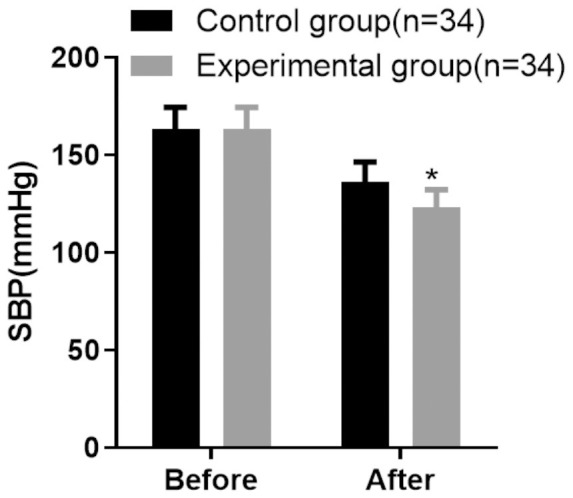
Comparison of systolic blood pressure (SBP) improvement between the two groups of patients. Note: Compared with the control group after treatment, *P < 0.05.
Comparison of 24-hour average DBP levels at different time points between the two groups
Twenty-four hour average DBP levels showed a significant sequential decrease from baseline at 4, 8, and 12 weeks after treatment in both the experimental and control groups. Notably, the reduction in 24-hour average DBP was more pronounced in the experimental group than in the control group at both 8 and 12 weeks post-treatment (P < 0.05). These findings are detailed in Table 2 and illustrated in Figure 3.
Table 2.
Comparison of 24-hour average DBP levels at different time points between the two groups (Mean ± SD, mmHg)
| Group | n | Before treatment | 4 weeks after treatment | 8 weeks after treatment | 12 weeks after treatment |
|---|---|---|---|---|---|
| Experimental group | 34 | 100.05 ± 5.73 | 91.14 ± 4.38 | 83.18 ± 5.61 | 78.08 ± 4.93 |
| Control group | 34 | 99.86 ± 5.92 | 92.26 ± 4.75 | 88.25 ± 5.84 | 84.63 ± 5.21 |
| t-value | 0.1345 | 1.011 | 3.651 | 5.325 | |
| P-value | 0.8934 | 0.3158 | 0.0005 | < 0.001 |
DBP: diastolic blood pressure.
Figure 3.
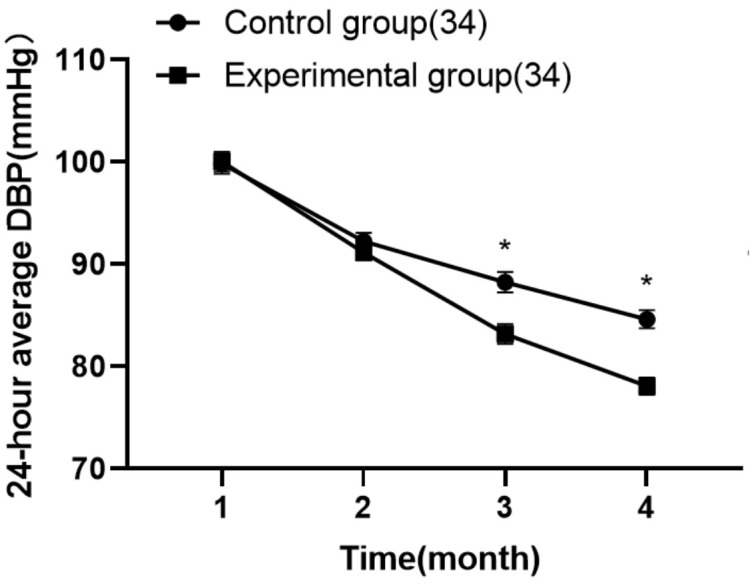
The 24-hour average DBP levels at different time points. Note: *P < 0.05 compared with the control group after treatment. DBP: diastolic blood pressure.
Comparison of 24-hour average SBP levels at different time points between the two groups
Similarly, 24-hour average SBP levels showed significant reductions at each post-treatment time point in both groups. The experimental group demonstrated more substantial improvements in 24-hour average SBP, particularly at the 8-week and 12-week time points (P < 0.05), underscoring the effectiveness of the treatment protocol in this group. Detailed data are presented in Table 3 and depicted in Figure 4.
Table 3.
Comparison of 24-hour average SBP levels at different time points between the two groups (Mean ± SD, mmHg)
| Group | n | Before treatment | 4 weeks after treatment | 8 weeks after treatment | 12 weeks after treatment |
|---|---|---|---|---|---|
| Experimental group | 34 | 166.89 ± 6.76 | 146.25 ± 4.13 | 130.39 ± 3.16 | 125.84 ± 3.15 |
| Control group | 34 | 165.32 ± 7.75 | 148.32 ± 4.29 | 139.63 ± 3.41 | 132.63 ± 3.52 |
| t-value | 0.8902 | 0.998 | 11.59 | 8.382 | |
| P-value | 0.3766 | 0.556 | < 0.0001 | < 0.0001 |
SBP: systolic blood pressure.
Figure 4.
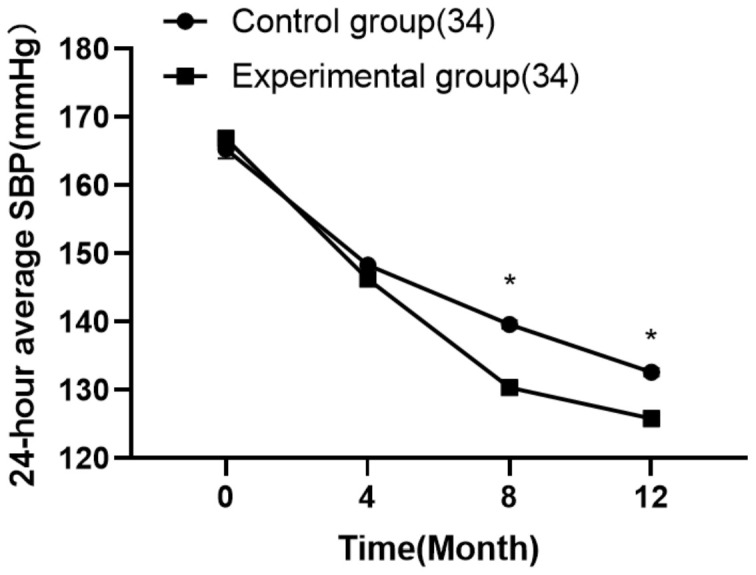
The 24-hour average systolic blood pressure SBP levels at different time points. Note: *P < 0.05 when compared with the control group after treatment.
Comparison of lipid profile improvement before and after treatment between the two groups
Following treatment, both groups showed significant improvements in lipid profiles, with reductions in TC, TG, and LDL-C, along with an increase in HDL-C levels. Notably, the lipid control achieved in the experimental group was significantly superior to that in the control group (P < 0.05). The results are summarized in Table 4.
Table 4.
Comparison of the improvement of blood lipids before and after treatment in the two groups (Mean ± SD, mmol/L)
| Group | n | Total Cholesterol | Triglyceride | Low-density lipoprotein cholesterol | High-density lipoprotein cholesterol | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||
| Experimental group | 34 | 6.31 ± 1.16 | 3.49 ± 0.43 | 4.13 ± 1.22 | 2.01 ± 0.19 | 4.35 ± 1.25 | 1.49 ± 0.41 | 1.29 ± 0.37 | 2.25 ± 0.24 |
| Control group | 34 | 6.29 ± 1.18 | 5.03 ± 0.78 | 4.11 ± 1.18 | 3.94 ± 0.28 | 4.32 ± 1.27 | 3.02 ± 0.86 | 1.28 ± 0.36 | 1.68 ± 0.12 |
| t-value | 0.0705 | 10.08 | 0.0687 | 33.26 | 0.0982 | 9.364 | 0.1130 | 12.39 | |
| P-value | 0.9440 | < 0.0001 | 0.9454 | < 0.0001 | 0.9221 | < 0.0001 | 0.9104 | < 0.0001 | |
Comparison of inflammatory factor levels before and after treatment between the two groups
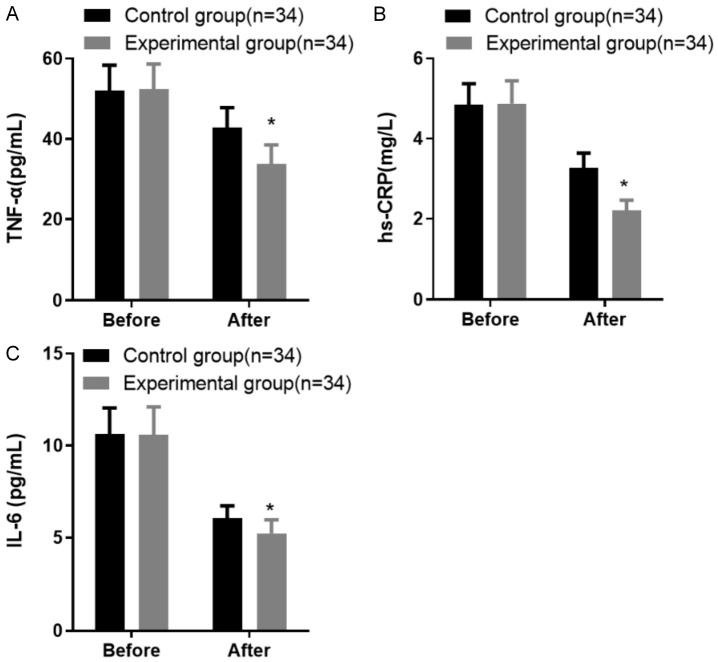
Both groups exhibited significant improvements in inflammatory factor levels after treatment (P < 0.05). Initially, the experimental group had levels of TNF-α, hs-CRP, and IL-6 at 52.63 ± 6.29 pg/mL, 4.87 ± 0.54 mg/L, and 10.63 ± 1.42 pg/mL, respectively. The corresponding pre-treatment values in the control group were 52.05 ± 6.31 pg/mL, 4.84 ± 0.53 mg/L, and 10.58 ± 1.53 pg/mL.
Post-treatment, the experimental group showed a marked reduction in these markers, with TNF-α at 33.79 ± 4.16 pg/mL, hs-CRP at 2.21 ± 0.25 mg/L, and IL-6 at 5.23 ± 0.74 pg/mL. In contrast, the control group had post-treatment levels of 42.75 ± 5.03 pg/mL for TNF-α, 3.26 ± 0.38 mg/L for hs-CRP, and 6.05 ± 0.69 pg/mL for IL-6. The reduction in inflammatory markers was significantly greater in the experimental group, as shown in Figure 5.
Figure 5.
Comparison of inflammatory factor levels before and after treatment in the two groups of patients. A: Tumor necrosis factor-alpha (TNF-α) levels; B: Hypersensitive C-reactive protein (hs-CRP) levels; C: Interleukin-6 (IL-6) levels. Note: Compared with the control group after treatment, *P < 0.05.
Comparison of target organ indices before and after treatment between the two groups
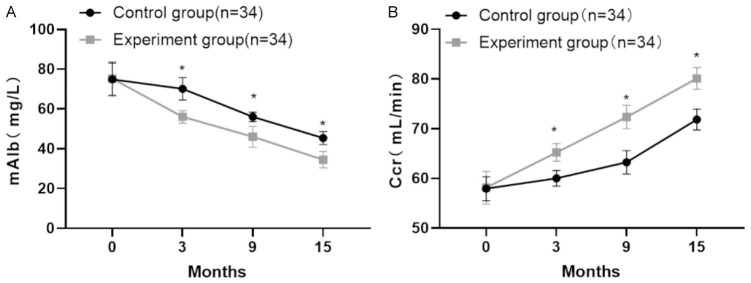
Post-treatment analysis revealed significant reductions in mAlb levels in both the experimental and control groups. The experimental group had a significantly lower mAlb level of 34.47 ± 6.13 mg/L compared to 40.14 ± 6.25 mg/L in the control group (P < 0.05). Similarly, endogenous Ccr significantly increased in both groups, with the experimental group showing a higher Ccr level of 80.02 ± 2.16 mL/min compared to 71.85 ± 2.09 mL/min in the control group (P < 0.05). These outcomes are detailed in Table 5 and illustrated in Figure 6.
Table 5.
Comparison of target organ indices before and after treatment in the two groups (Mean ± SD)
| Group | n | Microalbumin (mAlb, mg/L) | Creatinine Clearance (Ccr, ml/min) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Before treatment | After treatment | Before treatment | After treatment | ||
| Experimental group | 34 | 75.32 ± 8.36 | 34.47 ± 4.13 | 57.94 ± 2.41 | 80.02 ± 2.16 |
| Control group | 34 | 74.87 ± 8.27 | 40.14 ± 3.25 | 58.14 ± 3.28 | 71.85 ± 2.09 |
| t-value | 1.139 | 8.174 | 0.908 | 9.962 | |
| P-value | 0.402 | 0.003 | 0.486 | < 0.0001 | |
Figure 6.
Comparison of target organ indices before and after treatment between the two groups of patients. A: Urinary microalbumin levels (mAlb) levels; B: Creatine clearance (Ccr) levels. Note: Compared with the control group after treatment, *P < 0.05.
Comparison of cardiac structural indices before and after treatment between the two groups
A comparative analysis of cardiac structural indices, including LVEF, LVEDD, and LVPW, was conducted between the two groups before and after treatment. Both groups experienced improvements, characterized by an increase in LVEF levels and reductions in LVEDD and LVPW measurements post-treatment. The experimental group showed a more pronounced increase in LVEF and greater reductions in LVEDD and LVPW compared to the control group (P < 0.05). These findings are presented in Table 6.
Table 6.
Comparison of cardiac structural indexes before and after treatment in the two groups (Mean ± SD, n=34)
| Group | LVEF (%) | LVEDD (mm) | LVPW (mm) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Before treatment | After Treatment | Before treatment | After Treatment | Before treatment | After treatment | |
| Experimental group (n=34) | 45.29 ± 4.15 | 56.92 ± 3.47 | 58.82 ± 3.47 | 52.13 ± 2.96 | 15.64 ± 2.13 | 11.84 ± 1.52 |
| Control group (n=34) | 45.14 ± 4.23 | 52.46 ± 3.31 | 58.09 ± 3.56 | 54.85 ± 3.08 | 15.48 ± 2.16 | 13.95 ± 1.62 |
| t-value | 0.1476 | 5.423 | 0.8562 | 3.713 | 0.3075 | 5.538 |
| P-value | 0.883 | < 0.0001 | 0.3950 | 0.0004 | 0.7594 | < 0.0001 |
LVEF: left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter; LVPW: left ventricular posterior wall thickness.
Comparison of incidence rates of post-treatment adverse reactions between the two groups
The total incidence rates of post-treatment adverse reactions were 11.76% (4/34) in the experimental group and 14.71% (5/34) in the control group, with no statistically significant difference between the two groups (P > 0.05). See Table 7.
Table 7.
Comparison of adverse reactions after treatment in the two groups [n (%)]
| Group | n | Headache and dizziness | Edema | Nausea | Constipation | Total incidence |
|---|---|---|---|---|---|---|
| Experimental group | 34 | 1 (2.94) | 1 (2.94) | 1 (2.94) | 1 (2.94) | 4 (11.76) |
| Control group | 34 | 2 (5.89) | 1 (2.94) | 1 (2.94) | 1 (2.94) | 5 (14.71) |
| χ2 value | 2.376 | |||||
| P-value | 0.148 |
Discussion
Elderly patients with hypertension often present with increased SBP, decreased DBP, elevated pulse pressure, significant blood pressure variability, and abnormalities in circadian blood pressure rhythms. The primary management strategy typically involves long-term, consistent pharmacotherapy aimed at normalizing blood pressure [15-18]. In this study, patients in the control group were administered amlodipine, a calcium channel blocker that lowers blood pressure by inhibiting calcium ion influx into vascular smooth muscle, leading to vasodilation and reduced myocardial contractility [19-21]. However, the efficacy of amlodipine as a stand-alone treatment warrants further investigation.
Atorvastatin calcium is widely used in patients with hypertension to effectively reduce blood lipids, manage glucose levels, treat atherosclerosis, and prevent cardiovascular events [22,23]. It has a favorable safety profile with minimal adverse effects. Atorvastatin calcium works by inhibiting HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis, thereby lowering blood lipid levels. When used in hypertensive patients, it can reduce cholesterol, stabilize atherosclerotic plaques, and provide therapeutic benefits in managing hypertension [24,25]. This study’s findings demonstrate that both groups of elderly hypertensive patients experienced reductions in DBP and SBP following treatment. Notably, the experimental group showed more favorable outcomes, with post-treatment DBP and SBP readings of 74.96 ± 4.25 mmHg and 123.27 ± 9.06 mmHg, respectively, compared to 87.52 ± 5.38 mmHg and 136.26 ± 10.13 mmHg in the control group. Additionally, improvements in 24-hour average DBP) and SBP were observed over 4, 8, and 12 weeks, with the experimental group displaying superior control at the 8- and 12-week intervals. These results suggest that atorvastatin calcium significantly enhances blood pressure control in hypertensive patients.
Further analysis revealed reductions in blood lipid profiles (TC, TG, and LDL-C) and an increase in HDL-C post-treatment in both groups. The experimental group demonstrated superior lipid management, indicating that atorvastatin calcium effectively improves blood lipid profiles. This improvement suggests enhanced vascular endothelial function and reduced vascular inflammatory responses.
Moreover, the study showed significant post-treatment reductions in serum inflammatory markers (TNF-α, hs-CRP, and IL-6) in both groups, with the experimental group achieving lower levels of 33.79 ± 4.16 pg/mL for TNF-α, 2.21 ± 0.25 mg/L for hs-CRP, and 5.23 ± 0.74 pg/mL for IL-6. These markers are crucial, as hs-CRP, synthesized by the liver, serves as a nonspecific marker reflecting the acute phase of systemic inflammation and is considered a primary risk factor for predicting cardiovascular and cerebrovascular diseases [26,27]. Patients with hypertension typically exhibit persistent elevations in hs-CRP levels, and increased levels of TNF-α and IL-6 can trigger chronic vascular inflammation, potentially leading to elevated blood pressure [28-30].
This study demonstrated that the combined treatment of amlodipine and atorvastatin calcium effectively reduces the inflammatory response in patients, contributing to lower blood pressure levels. Post-treatment, urinary mAlb levels decreased significantly in both the experimental and control groups, with the experimental group showing lower levels (34.47 ± 6.13 mg/L) compared to the control group (40.14 ± 6.25 mg/L). Similarly, endogenous Ccr levels increased, with the experimental group achieving higher levels (80.02 ± 11.16 mL/min) versus the control group (71.85 ± 11.09 mL/min). These findings indicate that the combination of amlodipine with atorvastatin calcium provides better protection of target organs in elderly patients with hypertension compared to amlodipine alone.
The superior protection is likely due to atorvastatin calcium’s ability to maintain renal function and positively impact renal health by improving blood pressure control and enhancing endothelial function.
Furthermore, comparisons of cardiac structural indices, including LVEF, LVEDD, and LVPW, between the two groups before and after treatment revealed significant improvements. Both groups showed increased LVEF levels and decreased LVEDD and LVPW after treatment. Notably, the experimental group exhibited more substantial improvements in these indices than the control group. This suggests that the combination of amlodipine and atorvastatin calcium not only improves left ventricular function but also enhances overall cardiac function in elderly patients with hypertension, further supporting the benefits of this combined therapeutic approach.
It is important to note that this study employed a retrospective analysis, and the sample size, while informative, is insufficient to make conclusive generalizations based on these findings. Further research with a larger cohort is necessary to corroborate these results.
In conclusion, the combination of atorvastatin calcium and amlodipine significantly enhances the clinical management of hypertension in elderly patients. This therapeutic approach not only markedly improves clinical symptoms but also reduces inflammatory factor levels in the body. Additionally, this combination therapy has demonstrated a commendable safety profile, making it a valuable treatment option worthy of broader adoption.
Disclosure of conflict of interest
None.
References
- 1.Angeli F, Verdecchia P, Masnaghetti S, Vaudo G, Reboldi G. Treatment strategies for isolated systolic hypertension in elderly patients. Expert Opin Pharmacother. 2020;21:1713–1723. doi: 10.1080/14656566.2020.1781092. [DOI] [PubMed] [Google Scholar]
- 2.Tsounis D, Bouras G, Giannopoulos G, Papadimitriou C, Alexopoulos D, Deftereos S. Inflammation markers in essential hypertension. Med Chem. 2014;10:672–681. doi: 10.2174/1573406410666140318111328. [DOI] [PubMed] [Google Scholar]
- 3.Saheera S, Krishnamurthy P. Cardiovascular changes associated with hypertensive heart disease and aging. Cell Transplant. 2020;29:963689720920830. doi: 10.1177/0963689720920830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17:639–654. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzik TJ, Nosalski R, Maffia P, Drummond GR. Immune and inflammatory mechanisms in hypertension. Nat Rev Cardiol. 2024;21:396–416. doi: 10.1038/s41569-023-00964-1. [DOI] [PubMed] [Google Scholar]
- 6.Totoń-Żurańska J, Mikolajczyk TP, Saju B, Guzik TJ. Vascular remodelling in cardiovascular diseases: hypertension, oxidation, and inflammation. Clin Sci (Lond) 2024;138:817–850. doi: 10.1042/CS20220797. [DOI] [PubMed] [Google Scholar]
- 7.Ma W, Sun N, Duan C, Zhao L, Hua Q, Sun Y, Dang A, Gao P, Qu P, Cui W, Zhao L, Dong Y, Cui L, Qi X, Jiang Y, Xie J, Li J, Wu G, Du X, Huo Y, Chen P for LEADER Study Group. Effectiveness of levoamlodipine maleate for hypertension compared with amlodipine besylate: a pragmatic comparative effectiveness study. Cardiovasc Drugs Ther. 2021;35:41–50. doi: 10.1007/s10557-020-07054-1. [DOI] [PubMed] [Google Scholar]
- 8.Simon A, Dézsi CA. Treatment of hypertensive and hypercholesterolaemic patients with the triple fixed combination of atorvastatin, perindopril and amlodipine: the results of the CORAL study. Adv Ther. 2019;36:2010–2020. doi: 10.1007/s12325-019-01002-8. [DOI] [PubMed] [Google Scholar]
- 9.Lin CP, Hsiao FC, Wu CT, Lin YS, Chen SW, Chu PH. Beneficial effects of fixed-dose combination of amlodipine and atorvastatin in patients with concomitant hypertension and hypercholesterolemia: a multi-institutional cohort study. Acta Cardiol Sin. 2022;38:736–750. doi: 10.6515/ACS.202211_38(6).20220529A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar G, Gaikwad VB, Sharma A, Halder SK, Kumar DA, Anand J, Agrawal S, Kumbhar A, Kinholkar B, Mathur R, Doshi M, Bachani D, Mehta S. Fixed-dose combination of metoprolol, telmisartan, and chlorthalidone for essential hypertension in adults with stable coronary artery disease: phase III study. Adv Ther. 2022;39:923–942. doi: 10.1007/s12325-021-01971-9. [DOI] [PubMed] [Google Scholar]
- 11.Liang L, Kung JY, Mitchelmore B, Cave A, Banh HL. Comparative peripheral edema for dihydropyridines calcium channel blockers treatment: a systematic review and network meta-analysis. J Clin Hypertens (Greenwich) 2022;24:536–554. doi: 10.1111/jch.14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woźniak E, Broncel M, Niedzielski M, Woźniak A, Gorzelak-Pabiś P. The effect of lipid-lowering therapies on the pro-inflammatory and anti-inflammatory properties of vascular endothelial cells. PLoS One. 2023;18:e0280741. doi: 10.1371/journal.pone.0280741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takkar P, Singh B, Pani B, Kumar R. Design, synthesis and in silico evaluation of newer 1,4-dihydropyridine based amlodipine bio-isosteres as promising antihypertensive agents. RSC Adv. 2023;13:34239–34248. doi: 10.1039/d3ra06387a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin R, Yin L, Li L, Silva-Nash J, Tan J, Pan Z, Zeng J, Yan LL. Hypertension in China: burdens, guidelines and policy responses: a state-of-the-art review. J Hum Hypertens. 2022;36:126–134. doi: 10.1038/s41371-021-00570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang K, Zhang Y, Yang F, Luo X, Long W, Hou X. Effect of enalapril combined with bisoprolol on cardiac function and inflammatory indexes in patients with acute myocardial infarction. Evid Based Complement Alternat Med. 2022;2022:6062450. doi: 10.1155/2022/6062450. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Li T, Li S, Feng Y, Zeng X, Dong S, Li J, Zha L, Luo H, Zhao L, Liu B, Ou Z, Lin W, Zhang M, Li S, Jiang Q, Qi Q, Xu Q, Yu Z. Combination of dichloroacetate and atorvastatin regulates excessive proliferation and oxidative stress in pulmonary arterial hypertension development via p38 signaling. Oxid Med Cell Longev. 2020;2020:6973636. doi: 10.1155/2020/6973636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, Yang J, Jiang Y, Xu X, Wang TD, Chen Y, Li Y, Yao L, Li D, Wang L, Shen X, Yin X, Liu W, Zhou X, Zhu B, Guo Z, Liu H, Chen X, Feng Y, Tian G, Gao X, Kario K, Cai J STEP Study Group. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385:1268–1279. doi: 10.1056/NEJMoa2111437. [DOI] [PubMed] [Google Scholar]
- 18.Streit S, Poortvliet RKE, Elzen WPJD, Blom JW, Gussekloo J. Systolic blood pressure and cognitive decline in older adults with hypertension. Ann Fam Med. 2019;17:100–107. doi: 10.1370/afm.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung MH, Yi SW, An SJ, Yi JJ. Age-specific associations between systolic blood pressure and cardiovascular mortality. Heart. 2019;105:1070–1077. doi: 10.1136/heartjnl-2019-314697. [DOI] [PubMed] [Google Scholar]
- 20.Sheppard JP, Burt J, Lown M, Temple E, Lowe R, Fraser R, Allen J, Ford GA, Heneghan C, Hobbs FDR, Jowett S, Kodabuckus S, Little P, Mant J, Mollison J, Payne RA, Williams M, Yu LM, McManus RJ OPTIMISE Investigators. Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE randomized clinical trial. JAMA. 2020;323:2039–2051. doi: 10.1001/jama.2020.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Pinto R, Ferri C. Hypertension management at older age: an update. High Blood Press Cardiovasc Prev. 2019;26:27–36. doi: 10.1007/s40292-018-0290-z. [DOI] [PubMed] [Google Scholar]
- 22.Srinath S. Comparison of efficacy and safety of benidipine with amlodipine in patients with uncomplicated hypertension: a prospective study. Karpaga Vinayaga Institute of Medical Sciences and Research Centre, Kanchipuram; 2020. [Google Scholar]
- 23.Striessnig J, Ortner NJ. Encyclopedia of Molecular Pharmacology. Springer; 2022. Ca2+ channel blockers; pp. 375–383. [Google Scholar]
- 24.Almukhtar HM, Faisal IM, Merkhan MM. Acute effect of atorvastatin in comparison with rosuvastatin on glucose homeostasis in hypercholesteremic patients. Pharmacology. 2021;25:25–34. [Google Scholar]
- 25.Feingold KR. Role of glucose and lipids in the atherosclerotic cardiovascular disease in patients with diabetes. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000-2024, MDText.com, Inc.; 2000. [PubMed] [Google Scholar]
- 26.Fu J, Wang T, Li B, Zhao N. The efficacy of rosuvastatin, amlodipine, and aspirin in the treatment of hypertension with coronary heart disease and its effect on platelet aggregation. Dis Markers. 2022;2022:1111438. doi: 10.1155/2022/1111438. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Prasad EM. Is the C-Reactive Protein (CRP) test a worthy indicator of inflammation? London J Med Health Res. 2023;23:45–58. [Google Scholar]
- 28.Pastorello Y, Carare RO, Banescu C, Potempa L, Di Napoli M, Slevin M. Monomeric C-reactive protein: a novel biomarker predicting neurodegenerative disease and vascular dysfunction. Brain Pathol. 2023;33:e13164. doi: 10.1111/bpa.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akabari AH, Mistry P, Patel SK, Surati J, Patel SP, Shah U. Simultaneous estimation of fimasartan potassium trihydrate and atorvastatin calcium with greenness assessment using HPLC and UV spectrophotometric methods. Green Anal Chem. 2023;6:100067. [Google Scholar]
- 30.Gundlach K, Wolf K, Salem I, Randerath O, Seiler D. Safety of candesartan, amlodipine, and atorvastatin in combination: interaction study in healthy subjects. Clin Pharmacol Drug Dev. 2021;10:190–197. doi: 10.1002/cpdd.787. [DOI] [PubMed] [Google Scholar]