Abstract
Objective: This retrospective study aimed to evaluate the impact of intraoperative dexmedetomidine on myocardial injury markers (heart-type fatty acid binding protein [H-FABP], creatine kinase-mb [CK-MB], and cardiac troponin I [cTnI]) and postoperative delirium in patients undergoing heart valve replacement. Methods: Clinical data from 160 cardiac patients who underwent heart valve replacement with cardiopulmonary bypass (CPB) between January 2019 and January 2024 were analyzed. Patients were divided into an observation group (n = 82) receiving dexmedetomidine and a control group (n = 78) without dexmedetomidine. After propensity score matching, both groups comprised 53 patients each. Outcome measures included myocardial injury markers, postoperative delirium incidence, and perioperative parameters. Results: The observation group showed shorter postoperative recovery durations, reduced intraoperative sufentanil requirements, and lower myocardial injury markers 1 day postoperatively (all P < 0.001). No significant differences were found in midazolam dosages (all P > 0.05), while propofol and sufentanil dosages were lower in the observation group (both P < 0.001). The incidence of postoperative delirium was significantly lower in the observation group (P = 0.014), with cardioversion time and propofol dosage identified as delirium risk factors. Conclusion: Dexmedetomidine use during heart valve replacement surgery was associated with improved postoperative outcome, including reduced myocardial injury and lower postoperative delirium incidence. These findings suggest a potential role for dexmedetomidine in enhancing patient recovery, although further research is needed to explain its impact on postoperative delirium.
Keywords: Dexmedetomidine, heart valve replacement, heart-type fatty acid binding protein, creatine kinase-MB, cardiac troponin I, postoperative delirium
Introduction
Socioeconomic development has increased the global public health challenge of cardiovascular diseases, particularly heart valve diseases, which are on the rise, including degenerative and ischemic conditions [1,2]. The American Heart Association reports that heart valve disease affects approximately 2.5% of the U.S. population, with 1.8% suffering from moderate to severe forms [3]. Notably, the incidence of heart valve disease significantly increases with age.
Surgical intervention remains a primary treatment for heart valve disease, with heart valve replacement and cardiac valvuloplasty being key options [4]. Heart valve replacement is often preferred for severe cases of mitral valve disease, aortic stenosis, and tricuspid valve lesions, effectively alleviating symptoms, improving compromised cardiac function, enhancing quality of life, and extending life expectancy [5,6].
Cardiac surgeries, especially those involving extracorporeal circulation such as cardiopulmonary bypass (CPB), are frequently associated with perioperative cardiac ischemia, potentially leading to postoperative myocardial dysfunction and arrhythmias [7]. In this context, biomarkers like cardiac troponin I (cTnI), heart-type fatty acid binding protein (H-FABP), and creatine kinase isoenzyme (CK-MB) are crucial for assessing myocardial injury and predicting patient outcome [8]. cTnI is a sensitive marker for myocardial infarction, associated with increased complications and mortality; H-FABP is an early indicator of myocardial injury, responding swiftly to cardiomyocyte damage; while CK-MB, although less specific, remains a vital marker for assessing myocardial damage [9].
Dexmedetomidine’s role in anesthetic management and enhancing postoperative recovery in heart valve replacement surgeries has garnered significant attention. This α2-adrenergic agonist provides sedation, analgesia, and mitigates stress and inflammation, addressing critical aspects of patient care [10]. Postoperative delirium, a prevalent issue in cardiovascular surgeries, notably affects recovery, leading to cognitive impairment and increased healthcare costs [11,12]. Understanding the interaction between dexmedetomidine and postoperative delirium outcomes is vital for optimizing recovery strategies and improving patient prognosis.
Current research into dexmedetomidine’s impact on heart valve replacement surgery patients has identified its benefits in reducing cardiac injury markers and delirium incidence [13]. However, existing studies often exhibit limitations such as a lack of diversity in patient populations or short follow-up periods, which may limit the generalizability of findings. To address these constraints, this study adopted different methods to enhance the generalizability and reliability of its results. Additionally, the specific mechanisms by which dexmedetomidine influences cognitive outcome post-surgery remain underexplored, with most studies focusing on immediate postoperative effects rather than long-term recovery.
The present study aimed to address these gaps by evaluating the combined effect of dexmedetomidine on biomarkers of cardiac injury (H-FABP, CK-MB, cTnI) and postoperative delirium. By providing a more comprehensive analysis of how these drugs interact to impact patient outcome, the study aims to offer new insight into anesthesia management and recovery strategies, deepening our understanding of dexmedetomidine’s role in improving postoperative cognitive function and overall patient care.
Maerials and methods
Clinical information
This retrospective study analyzed clinical data from 160 cardiac patients who underwent CPB heart valve replacement at the First Affiliated Hospital of Xi’an Jiaotong University Yulin Hospital between January 2019 and January 2024. The research was approved by the Medical Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University Yulin Hospital (Ethical lot number: LL2024012 (A), approval date: 31 January 2024).
Inclusion exclusion criteria
Inclusion criteria comprised patients aged 50 years and older, eligible for CPB heart valve replacement without symptoms of rheumatic fever, with complete clinical data, and who had not recently received targeted therapy. Patients were required to undergo surgery and have an outcome evaluation afterward.
Exclusion criteria included discontinuation of anticoagulants less than three days pre-surgery, history of secondary cardiac surgery, hypertension (systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 110 mmHg), anemia (hemoglobin < 7.0 g/L), hypovolemia, hypoproteinemia (protein level < 30 g/L), severe heart, liver, kidney diseases, or malignant tumors.
Propensity score matching (PSM)
The study used PSM to balance baseline characteristics between the observation and control groups, ensuring comparability. Each patient’s probability of receiving dexmedetomidine was calculated using a multivariate logistic regression model with covariates such as age and gender. Optimal matching was performed with a tolerance of 0.02, using non-replacement sampling and random case sequencing to minimize bias and enhance execution efficiency. SPSS software version 26.00 facilitated data processing and the PSM process, resulting in successful matching of 53 patient pairs.
From the eligible cases, 160 patients were selected and divided into two groups: an observation group that received dexmedetomidine (n = 82) and a control group without dexmedetomidine (n = 78). After PSM, both groups comprised 53 patients each. In the study, patients in the observation group received dexmedetomidine hydrochloride injection intravenously at a rate of 0.3-0.6 μg/(kg-h). The control group followed a conventional anesthetic protocol.
Anesthesia induction in both groups included 0.02 mg/kg imipramine, 0.6 μg/kg sufentanil, and 0.2 mg/kg etomidate. Mechanical ventilation with 70% fraction of inspiratory O2 maintained partial pressure of carbon dioxide (PaCO2) at 35-45 mmHg post-tracheal intubation. Bispectral index (BIS) was kept within 40 to 60, and blood pressure fluctuations were limited to 20%. Standard hypothermic CPB conditions (30-32°C) were used during surgery with appropriate perfusion parameters (activated clotting time > 480 seconds, hematocrit 20-25%, perfusion flow 2.0-2.4 L/(m-min), pH 7.35-7.45, and PaCO2 35-45 mmHg). Rewarming occurred at a rate of 0.20 to 0.25°C/min. Dopamine and dobutamine were infused to maintain stable blood pressure post-aortic opening. These steps ensured procedure safety and efficacy. Notably, the inclusion of dexmedetomidine in this study was based on institutional protocol changes starting January 2023 for heart valve replacement surgeries, reflecting emerging evidence of its perioperative benefits. This study evaluates dexmedetomidine’s clinical value by comparing patient outcomes before and after the protocol change.
Data collection
Based on intraoperative and medical records, we collected various clinical data, perioperative records, laboratory tests, and documented adverse reactions. Clinical data encompassed age, gender, body mass index, disease duration, education level, American Society of Anesthesiologists classification, New York Heart Association (NYHA) classification, and the number of surgeries undergone [14]. The NYHA classification, diabetes mellitus, and hypertension were considered regarding etiology [15]. Perioperative records included postoperative awakening time, cardiac resuscitation time, ICU stay duration, extubation time, hospitalization time, midazolam, sufentanil, and propofol dosages, heart rate (HR), and mean arterial pressure (MAP). Laboratory parameters included cTnI, H-FABP, and CK-MB. Adverse reactions such as respiratory depression, vomiting, bradycardia, and delirium were documented. Laboratory tests were conducted using enzyme-linked immunosorbent assay. HR and MAP were monitored using a BENEVISION N15 patient monitor. The Confusion Assessment Method for the Intensive Care Unit assessed awakening scores post-anesthesia [16]. Postoperative delirium diagnosis relied on these criteria: (1) acute mental status change, (2) inattention, (3) disorganized thinking, and (4) altered consciousness level. A diagnosis was made if specific criteria (1) + (2) + (3) or (1) + (2) + (4) were met. Hemodynamic indicators were measured preoperatively and post-CPB, while cardiac injury markers were assessed preoperatively and 1 day postoperatively.
Outcome measures
Main outcome measures: We analyzed changes in myocardial injury indicators before and after surgery in both patient groups. Additionally, we used logistic regression to analyze risk factors influencing the incidence of postoperative delirium within 1-7 days.
Secondary outcome measures: We compared the time for clinical symptoms to disappear between the two patient groups, recorded the dosage of anesthetic drugs administered to patients, compared changes in patients’ hemodynamic indexes, and compiled data on the incidence of adverse reactions in patients (Figure 1).
Figure 1.
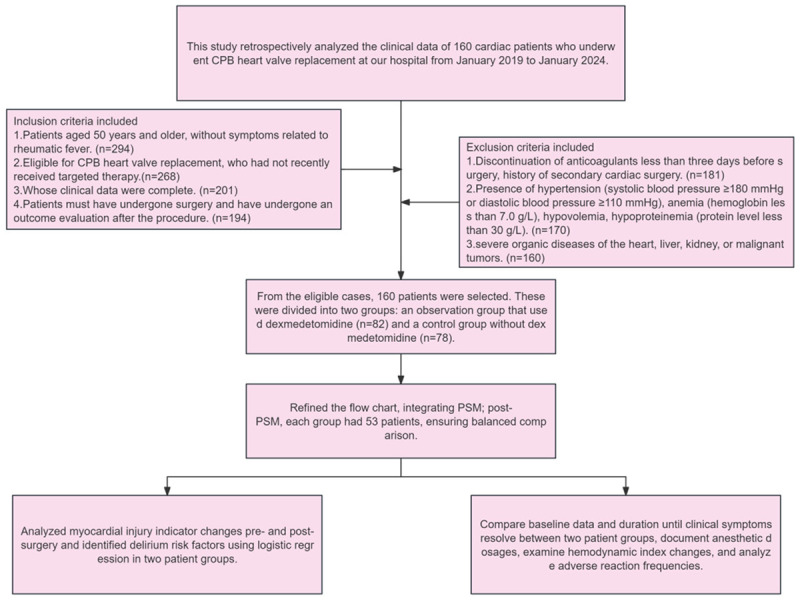
Flow chart of patient sample inclusion and study design.
Statistical analysis
We utilized SPSS 26.0 software for data processing. For normally distributed measured data, we employed the K-S test. Average distributed data were expressed as mean ± standard deviation (x ± sd), and analyzed using the t-test for intergroup comparisons; independent samples t-test for between-group comparisons, and paired t-test for within-group comparisons. Non-normally distributed data were expressed as quartiles P50 [P25, P75], analyzed using the rank sum test. Repeated measures analysis of variance was used for multi-time point data, followed by the Bonferroni test for post hoc analysis. Chi-square test was used for data comparison. Logistic regression was employed for univariate and multivariate analyses, and the dichotomous cutoff value of measured data was determined using the receiver operating characteristic curve. A p-value < 0.05 indicated a significant difference.
Results
Comparison of baseline information
A comparison of baseline data between the two groups revealed no significant difference (all P > 0.05, Table 1).
Table 1.
Baseline information
| Data | n | Control group (n = 53) | Observation group (n = 53) | t/Z/χ2 values | P-value |
|---|---|---|---|---|---|
| Age (years) | 63.00 [58.00, 65.00] | 62.00 [58.00, 67.00] | 0.013 | 0.992 | |
| Gender | |||||
| Male | 57 | 28 | 29 | 0.038 | 0.846 |
| Women | 49 | 25 | 24 | ||
| BMI (kg/m2) | 5.00 [5.00, 6.00] | 5.00 [4.00, 7.00] | -0.370 | 0.708 | |
| Duration of illness (years) | 5.00 [4.25, 6.00] | 5.00 [4.00, 6.00] | -0.317 | 0.747 | |
| Educational attainment | |||||
| ≥ High School | 31 | 15 | 16 | 0.046 | 0.831 |
| < High School | 75 | 38 | 37 | ||
| ASA classification | |||||
| II | 50 | 25 | 25 | 0.001 | > 0.999 |
| III | 56 | 28 | 28 | ||
| NYHA Classification | |||||
| II | 55 | 26 | 29 | 0.340 | 0.560 |
| III | 51 | 27 | 24 | ||
| Etiology | |||||
| Degenerative valve disease | 23 | 12 | 11 | 0.056 | 0.814 |
| Rheumatic Valve Disease | 83 | 41 | 42 | ||
| Diabetes | |||||
| Yes | 17 | 9 | 8 | 0.070 | 0.791 |
| No | 89 | 44 | 45 | ||
| High blood pressure | |||||
| Yes | 22 | 11 | 11 | 0.001 | > 0.999 |
| No | 84 | 42 | 42 |
Note: BMI, Body Mass Index; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association Functional Classification.
Comparison of time to disappearance of clinical symptoms
In comparing the time for clinical symptoms to disappear between the two groups, significant differences were observed. The observation group exhibited shorter postoperative awakening time, cardiac resuscitation time, ICU stay duration, postoperative extubation time, and postoperative hospitalization time compared to the control group (all P < 0.001, Table 2).
Table 2.
Time to the disappearance of clinical symptoms
| Cluster | Postoperative awakening time (h) | Cardiac resuscitation time (s) | ICU stay duration (h) | Postoperative extubation time (h) | Hospitalization time (d) |
|---|---|---|---|---|---|
| Control group (n = 53) | 109.66±8.36 | 29.00 [27.00, 33.00] | 17.00 [16.00, 21.00] | 19.00 [15.00, 22.00] | 109.66±8.36 |
| Observation group (n = 53) | 93.26±6.79 | 27.00 [25.00, 29.00] | 16.00 [14.00, 17.00] | 17.00 [13.00, 19.00] | 93.26±6.79 |
| t/Z value | -11.081 | -4.031 | -4.069 | -3.45 | -11.081 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Comparison of anesthetic drug usage
Analysis of anesthetic drug usage during surgery showed that patients in the observation group received lower doses of sufentanil and propofol compared to the control group (both P < 0.001). . However, there was no statistical difference in midazolam usage between the two groups (P = 0.755, Table 3).
Table 3.
Comparison of anesthesia drug dosage
| Cluster | Midazolam (mg) | Sufentanil (μg) | Propofol (mg) |
|---|---|---|---|
| Control group (n = 53) | 15.52±1.31 | 264.72±19.96 | 776.37±80.37 |
| Observation group (n = 53) | 15.44±1.19 | 239.25±22.17 | 720.23±51.85 |
| t-value | -0.313 | -6.215 | 4.273 |
| P value | 0.755 | < 0.001 | < 0.001 |
Comparison of hemodynamic indices
Before anesthesia induction and at the end of CPB, hemodynamic indicators such as HR and MAP were measured in both groups. There were no significant differences in HR before anesthesia induction or at the end of CPB between the groups (both P > 0.05, Figure 2). However, upon CPB cessation, the MAP in the observation group was significantly higher than that of the control group (P = 0.008).
Figure 2.
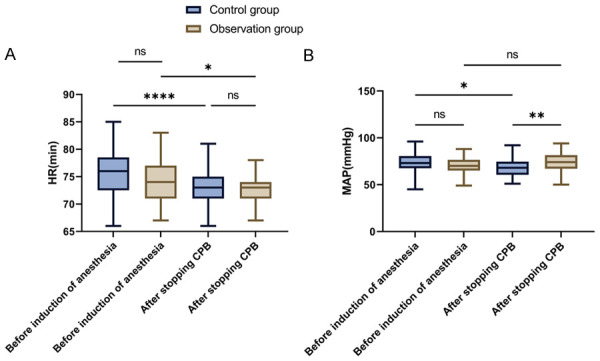
Changes in hemodynamic indexes in patients before induction of anesthesia and at the time of cessation of CPB. A. Comparison of HR changes in patients before anesthesia induction and during CPB cessation. B. Comparison of changes in MAP before induction of anesthesia and at cessation of CPB in the patient. Note: HR, heart rate; MAP, mean arterial pressure; CPB, cardiopulmonary bypass; ns P > 0.05, *P < 0.05, **P < 0.01, ****P < 0.0001.
Comparison of myocardial injury indicators
Preoperative and 1-day postoperative myocardial injury markers were evaluated in both groups. Preoperative levels of H-FABP, CK-MB, and cTnI showed no statistical difference between the groups (Figure 3, all P > 0.05). However, postoperative 1-day levels of H-FABP, CK-MB, and cTnI were significantly lower in the observation group compared to the control group (all P < 0.001).
Figure 3.
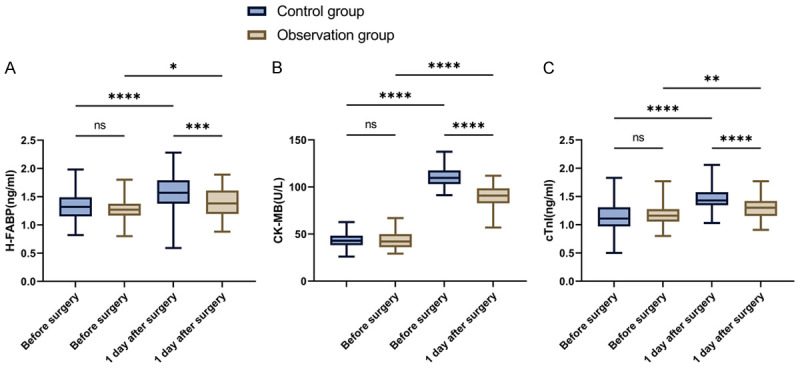
Changes in myocardial injury indexes in patients postoperatively and 1 d postoperatively. A. Comparison of changes in H-FABP. B. Comparison of CK-MB changes. C. Comparison of cTnI changes. Note: cTnl, Cardiac troponin I; H-FABP, heart-type fatty acid binding protein; CK-MB, creatine kinase isoenzyme; nsP > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Statistics on postoperative adverse reaction
Our analysis of adverse reactions in both groups showed no significant difference in respiratory depression (P = 0.558), vomiting (P = 0.646), or bradycardia (P = 0.558) in the control group (Table 4). However, the incidence of postoperative delirium in the observation group was significantly lower than in the control group (P = 0.014) (Table 4).
Table 4.
Adverse reaction data
| Cluster | Respiratory depression | Vomiting | Bradycardia | Delirium |
|---|---|---|---|---|
| Control group (n = 53) | 2 | 3 | 1 | 19 |
| Observation group (n = 53) | 1 | 2 | 2 | 8 |
| χ2-value | 0.343 | 0.209 | 0.343 | 6.013 |
| P value | 0.558 | 0.646 | 0.558 | 0.014 |
Risk factors for delirium occurrence
To explore the factors contributing to delirium, logistic regression analysis was performed. Univariate analysis identified treatment regimen (P = 0.017), cardiac resuscitation time (P = 0.023), and propofol dosage (P < 0.001) as influencing delirium occurrence (Figure 4A). Multifactorial logistic regression further revealed that cardioversion time (P = 0.025) and propofol dosage (P < 0.001) were independent risk factors for delirium development (Figure 4B).
Figure 4.
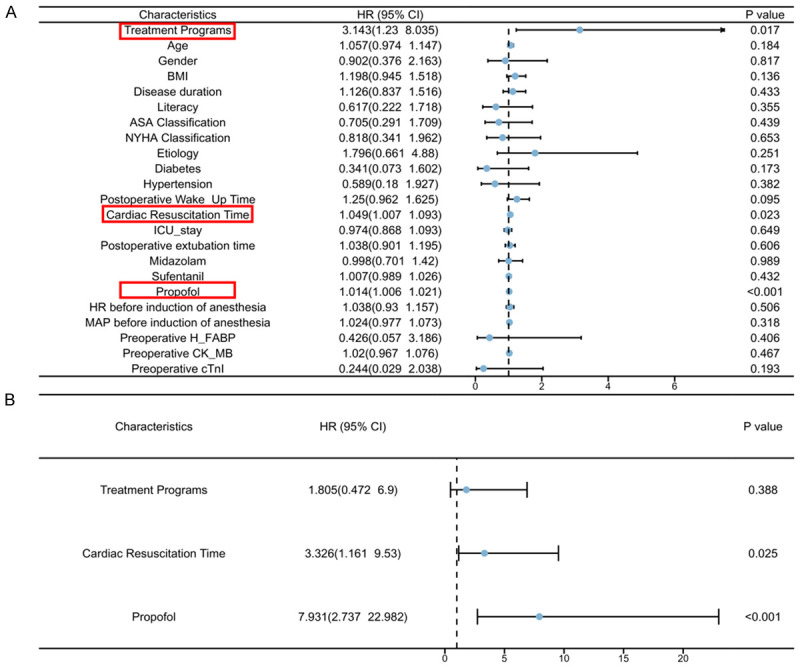
Risk factors for occurrence of delirium. A. Logistic regression single-factor analysis. B. Logistic regression with multifactorial analysis. Note: Indicators with one-factor differences are marked in red. HR, heart rate.
Discussion
In this study, we investigated the effects of intraoperative dexmedetomidine in patients undergoing heart valve replacement, particularly focusing on H-FABP, CK-MB, cTnI levels, and postoperative delirium. We observed significantly reduced dosages of intraoperative midazolam, sufentanil, and propofol in patients receiving dexmedetomidine compared to those not receiving it. Dexmedetomidine was also effective in controlling heart rate and mean arterial pressure. Additionally, patients who received dexmedetomidine showed lower oxidative stress levels and better cognitive function recovery in the postoperative period. While there was a difference in the incidence of postoperative delirium between the two groups, the specific anesthesia regimen was not identified as an independent risk factor for delirium in our patient cohort.
The use of CPB during heart valve replacement surgery can induce cardiac damage due to various factors, including direct heart manipulation, blood exposure to the external environment, and the physiologic stresses of artificial circulation [17]. These factors can lead to myocardial ischemia and reperfusion injury, resulting in cardiomyocyte dysfunction and possible cell death. Biomarkers such as H-FABP, CK-MB, and cTnI play crucial roles in assessing myocardial damage and diagnosing post-surgical cardiac injury [18]. H-FABP, characterized by its rapid release into the bloodstream after injury due to its small molecular weight, serves as an early indicator of myocardial injury, typically exhibiting elevated levels within 1-3 hours post-damage [19]. Conversely, CK-MB has traditionally been a marker for myocardial injury, especially in diagnosing myocardial infarction, due to its high specificity [20]. cTnI is considered the gold standard for diagnosing myocardial infarctions, with elevated levels indicating significant myocardial damage [21].
In our study, we observed significantly lower levels of H-FABP, CK-MB, and cTnI in patients treated with dexmedetomidine compared to the control group one day postoperatively, indicating dexmedetomidine’s cardioprotective role in heart valve replacement surgery. This aligns with findings by Yang et al. [22], who demonstrated that dexmedetomidine preconditioning could protect the heart from ischemia/reperfusion injury by reducing inflammation and apoptosis through downregulation of the endoplasmic reticulum stress pathway. Furthermore, a meta-analysis [23] showed that dexmedetomidine could lower CK-MB and cTn-I levels and shorten ICU stay duration in CPB cardiac surgery patients, which supports our results. Therefore, the application of dexmedetomidine in heart valve replacement surgery offers significant cardioprotective effects [24], highlighting its potential in reducing myocardial ischemia and reperfusion injury associated with CPB.
The increased occurrence of delirium following cardiac surgery with CPB significantly impacts patient outcomes [25]. This issue may arise from various CPB-related factors, such as alterations in cerebral perfusion, hemodilution, and the influence of artificial components like membrane oxygenators, creating a unique physiologic state [26]. This condition can disrupt cerebral blood flow and oxygen metabolism, possibly leading to microvascular embolism, decreased cerebral perfusion, cerebral tissue hypoxia, and ultimately, postoperative delirium [27]. Our findings suggest that cardioversion time and propofol dosage are independent predictors for delirium. Prolonged cardioversion times may indicate the complexity and challenges of the surgery, increasing the risk of postoperative delirium. Similarly, prolonged exposure to or high doses of propofol, a critical anesthetic, can deepen anesthesia, delay recovery, and impair cognitive function post-surgery, particularly affecting elderly patients or those with pre-existing cognitive issues.
In our study, the use of dexmedetomidine in reducing postoperative delirium among elderly patients undergoing heart valve replacement surgery aligns with previous research, highlighting its potential benefits. Notably, other studies have also shown promising results, such as one demonstrating a reduction in in-hospital delirium with postoperative dexmedetomidine and intravenous acetaminophen [28], and another by Shafa et al. [29] indicating that lower doses of isoproterenol minimized delirium and adverse effects. These findings support our results and underscore the importance of dexmedetomidine in enhancing postoperative outcome. They also suggest that combining dexmedetomidine with other medications could further reduce delirium risk. Our study contributes to the growing body of evidence supporting dexmedetomidine’s efficacy, indicating that its strategic use, possibly in combination with other treatments, offers a promising approach to reducing delirium in elderly cardiac surgery patients. This synergy not only reinforces the value of dexmedetomidine in postoperative care but also paves the way for further research into optimized dosing and combined therapeutic strategies to enhance patient recovery and minimize delirium incidence.
In this study, we did not identify the anesthesia protocol as an independent risk factor for postoperative delirium. This finding may indicate that the anesthesia protocol has a smaller direct effect on postoperative delirium than expected, or that other more significant factors are masking its effect. However, this does not imply that the choice of anesthetic regimen has no indirect impact on the risk of postoperative delirium. Anesthesia protocols may indirectly reduce this risk by adjusting the type and dosage of medications and managing the patient’s physiological fluctuations during surgery, such as maintaining stable blood pressure and heart rate. Therefore, although the anesthetic regimen did not emerge as a significant independent risk factor in this study, its role in postoperative management still warrants attention.
Research limitations
While this study has provided valuable insight, it has some limitations. For instance, we only collected and analyzed 160 cases, limiting the generalizability of our results. Additionally, being a single-center study, more data are needed to validate our findings. We aim to conduct further experiments and gather more samples in future studies to enhance the robustness of our conclusions.
In conclusion, our study indicates that patients treated with dexmedetomidine during heart valve replacement surgery experienced reduced intraoperative midazolam, sufentanil, and propofol dosages, along with lower levels of postoperative myocardial injury biomarkers (H-FABP, CK-MB, and cTnI). Although the anesthesia regimen was not identified as an independent risk factor for postoperative delirium, its impact on delirium incidence varied. These findings suggest that dexmedetomidine plays a positive role in reducing myocardial injury and improving postoperative recovery in heart valve replacement surgery.
Disclosure of conflict of interest
None.
References
- 1.Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, Zühlke L, Prendergast BD. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18:853–864. doi: 10.1038/s41569-021-00570-z. [DOI] [PubMed] [Google Scholar]
- 2.Ajmone Marsan N, Graziani F, Meucci MC, Wu HW, Lillo R, Bax JJ, Burzotta F, Massetti M, Jukema JW, Crea F. Valvular heart disease and cardiomyopathy: reappraisal of their interplay. Nat Rev Cardiol. 2024;21:37–50. doi: 10.1038/s41569-023-00911-0. [DOI] [PubMed] [Google Scholar]
- 3.Fu JT, Popal MS, Zhang HB, Han W, Hu QM, Meng X, Ma CY. A meta-analysis of late outcomes of mitral valve repair in patients with rheumatic heart disease. J Thorac Dis. 2017;9:4366–4375. doi: 10.21037/jtd.2017.10.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wara-Aswapati S, Kaewkes D, Chotmongkol V, Sawanyawisuth K. Clinical predictive factors of coronary stenosis in patients with high-risk valvular heart disease who received diagnostic coronary angiography prior to cardiac valve surgery. Biomed Rep. 2023;20:9. doi: 10.3892/br.2023.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patanè L, Di Lenarda A, Aspromonte N, Bianca I, Capranzano P, Di Eusanio M, Di Fusco S, Di Tano G, Gabrielli D, Garatti A, Geraci G, Gerometta P, Miceli A, Montalto A, Musumeci F, Musumeci G, Nardi F, Parolari A, Pino PG, Rubino AS, Savini C, Troise G, Tarantini G, Urbinati S, Varbella F, Gulizia MM. ANMCO/GISE/SICCH Inter-Society Consensus Document: antithrombotic therapy after surgery or structural interventional procedures for valvular heart diseases: how to treat postoperative complications. G Ital Cardiol (Rome) 2019;20:149–186. doi: 10.1714/3108.30964. [DOI] [PubMed] [Google Scholar]
- 6.Jian YP, Yuan HX, Hu KH, Chen C, Li YQ, Li Y, Yang TX, Ou ZJ, Ou JS. Protein compositions changes of circulating microparticles in patients with valvular heart disease subjected to cardiac surgery contribute to systemic inflammatory response and disorder of coagulation. Shock. 2019;52:487–496. doi: 10.1097/SHK.0000000000001309. [DOI] [PubMed] [Google Scholar]
- 7.Lomivorotov VV, Shmirev VA, Efremov SM, Ponomarev DN, Moroz GB, Shahin DG, Kornilov IA, Shilova AN, Lomivorotov VN, Karaskov AM. Hypothermic versus normothermic cardiopulmonary bypass in patients with valvular heart disease. J Cardiothorac Vasc Anesth. 2014;28:295–300. doi: 10.1053/j.jvca.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Kaushik A, Kapoor A, Agarwal SK, Pande S, Kashyap S, Sinha A, Khanna R, Kumar S, Garg N, Tewari S, Goel PK. Effect of statin on perioperative myocardial injury in isolated valve surgery. Asian Cardiovasc Thorac Ann. 2021;29:369–375. doi: 10.1177/0218492320974514. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Chen Q, Guo H, Li Z, Zhang J, Lv L, Guo Y. Effects of dexmedetomidine on H-FABP, CK-MB, cTnI levels, neurological function and near-term prognosis in patients undergoing heart valve replacement. Exp Ther Med. 2017;14:5851–5856. doi: 10.3892/etm.2017.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu YQ, Zhuang LP, Wu PY, Zhong LY, Zhong XH, Chen B, Liu ZK, Luo HR, Yang LP. Effect of dexmedetomidine on postoperative renal function in patients undergoing cardiac valve surgery under cardiopulmonary bypass: a randomized clinical trial. J Cardiothorac Vasc Anesth. 2023;37:1424–1432. doi: 10.1053/j.jvca.2023.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Ho MH, Lee JJ, Lai PCK, Li PWC. Prevalence of delirium among critically ill patients who received extracorporeal membrane oxygenation therapy: a systematic review and proportional meta-analysis. Intensive Crit Care Nurs. 2023;79:103498. doi: 10.1016/j.iccn.2023.103498. [DOI] [PubMed] [Google Scholar]
- 12.Humeidan ML, Reyes JC, Mavarez-Martinez A, Roeth C, Nguyen CM, Sheridan E, Zuleta-Alarcon A, Otey A, Abdel-Rasoul M, Bergese SD. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: the neurobics randomized clinical trial. JAMA Surg. 2021;156:148–156. doi: 10.1001/jamasurg.2020.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 14.Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, Fiadjoe JE, Greif R, Klock PA, Mercier D, Myatra SN, O’Sullivan EP, Rosenblatt WH, Sorbello M, Tung A. 2022 American Society of Anesthesiologists practice guidelines for management of the difficult airway. Anesthesiology. 2022;136:31–81. doi: 10.1097/ALN.0000000000004002. [DOI] [PubMed] [Google Scholar]
- 15.Caraballo C, Desai NR, Mulder H, Alhanti B, Wilson FP, Fiuzat M, Felker GM, Piña IL, O’Connor CM, Lindenfeld J, Januzzi JL, Cohen LS, Ahmad T. Clinical implications of the New York Heart Association classification. J Am Heart Assoc. 2019;8:e014240. doi: 10.1161/JAHA.119.014240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Santos FCM, Rêgo AS, Montenegro WS, de Carvalho STRF, Cutrim RC, Júnior AAM, Pereira FHF, Dibai-Filho AV, Bassi-Dibai D. Delirium in the intensive care unit: identifying difficulties in applying the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) BMC Nurs. 2022;21:323. doi: 10.1186/s12912-022-01103-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu T, Lai T, Chen J, Lu Y, He F, Chen Y, Xie Y. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9:e00851. doi: 10.1002/prp2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett BM, Moorthy MV, Tikkanen JT, Cook NR, Albert CM. Markers of myocardial stress, myocardial injury, and subclinical inflammation and the risk of sudden death. Circulation. 2020;142:1148–1158. doi: 10.1161/CIRCULATIONAHA.120.046947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye XD, He Y, Wang S, Wong GT, Irwin MG, Xia Z. Heart-type fatty acid binding protein (H-FABP) as a biomarker for acute myocardial injury and long-term post-ischemic prognosis. Acta Pharmacol Sin. 2018;39:1155–1163. doi: 10.1038/aps.2018.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Guo X, Yang F. Role of heart-type fatty acid binding protein in early detection of acute myocardial infarction in comparison with cTnI, CK-MB and myoglobin. J Huazhong Univ Sci Technolog Med Sci. 2004;24:449–451. 459. doi: 10.1007/BF02831105. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Xiao S, Xia Z, Cheng Y, Li Y, Tang W, Shi B, Qin C, Xu H. The diagnostic value of plasma miRNA-497, cTnI, FABP3 and GPBB in pediatric sepsis complicated with myocardial injury. Ther Clin Risk Manag. 2021;17:563–570. doi: 10.2147/TCRM.S309800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YF, Wang H, Song N, Jiang YH, Zhang J, Meng XW, Feng XM, Liu H, Peng K, Ji FH. Dexmedetomidine attenuates ischemia/reperfusion-induced myocardial inflammation and apoptosis through inhibiting endoplasmic reticulum stress signaling. J Inflamm Res. 2021;14:1217–1233. doi: 10.2147/JIR.S292263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang GR, Peng CM, Liu ZZ, Leng YF. The effect of dexmedetomidine on myocardial ischemia/reperfusion injury in patients undergoing cardiac surgery with cardiopulmonary bypass: a meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:7409–7417. doi: 10.26355/eurrev_202112_27438. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50:206–217. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 25.Patel AK, Biagas KV, Clarke EC, Gerber LM, Mauer E, Silver G, Chai P, Corda R, Traube C. Delirium in children after cardiac bypass surgery. Pediatr Crit Care Med. 2017;18:165–171. doi: 10.1097/PCC.0000000000001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ordóñez-Velasco LM, Hernández-Leiva E. Factors associated with delirium after cardiac surgery: a prospective cohort study. Ann Card Anaesth. 2021;24:183–189. doi: 10.4103/aca.ACA_43_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanson G, Khlopenyuk Y, Milocco S, Sartori M, Dreas L, Fabiani A. Delirium after cardiac surgery. Incidence, phenotypes, predisposing and precipitating risk factors, and effects. Heart Lung. 2018;47:408–417. doi: 10.1016/j.hrtlng.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam B, Shankar P, Shaefi S, Mueller A, O’Gara B, Banner-Goodspeed V, Gallagher J, Gasangwa D, Patxot M, Packiasabapathy S, Mathur P, Eikermann M, Talmor D, Marcantonio ER. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA. 2019;321:686–696. doi: 10.1001/jama.2019.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafa A, Rajabi F, Golkar K, Habibzadeh MR. Premedication propofol dose to prevent emergency delirium. Iran J Psychiatry. 2022;17:304–311. doi: 10.18502/ijps.v17i3.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]


