Abstract
Objective: To evaluate the efficacy of Erbium-doped: yttrium-aluminum garnet (Er:YAG) laser combined with minocycline hydrochloride (Mino-HCL) in treating combined endodontal-periodontal lesions (CEPLs), and to provide a clinical reference for its use. Methods: A total of 114 patients with CEPLs, admitted to Aerospace Center Hospital from October 2021 to October 2023, were retrospectively analyzed. Of these, 53 patients were treated with Er:YAG laser (control group) and 61 patients received Er:YAG laser combined with Mino-HCL (research group). Clinical efficacy and pain severity were compared between the two groups. Measurements included plaque index (PLI), sulcus bleeding index (SBI), probing depth (PD), inflammatory factors, and oxidative stress, with adverse effects recorded. A 6-month follow-up assessed the quality of life and oral health outcomes in both groups. Results: The research group demonstrated superior clinical efficacy, reduced pain, and improved management of inflammatory responses and oxidative stress compared to the control group (all P<0.05). Additionally, the research group had lower SBI, PLI, and PD values post-treatment (all P<0.05). There was no significant difference in the incidence of adverse reactions between the two groups (P>0.05). Follow-up results indicated better quality of life and oral health in the research group. Conclusion: The combination of Er:YAG laser and Mino-HCL is effective for treating CEPLs.
Keywords: Erbium-doped: yttrium-aluminum garnet laser, minocycline hydrochloride, combined endodontal-periodontal lesions, inflammatory response, stress response
Introduction
Combined endodontal-periodontal lesions (CEPLs) are complex oral diseases involving both pulpitis and periodontal destruction, with ahigh clinical incidence [1]. These lesions arise due to the shared tissue origin (mesodermal or ectodermal) and similar biologic factors, such as anaerobic bacteria and immune mechanisms, which lead to interactions and pathologic overlap between the dental pulp and periodontal tissues [2]. Clinically, CEPLs present as gingival bleeding, periapical disease, and extensive edema of surrounding soft tissues. If untreated, CEPLs can progress to tooth defects, loss, and even structural changes in the oral skeleton, possibly causing oral dysfunction [3]. Statistics indicate that periodontal disease affects about 80% of adults, with CEPLs comprising approximately 6.4-8.7% of these cases and their prevalence increasing [4,5]. Research has shown that CEPLs are primarily associated with the loss of oral immune function and heightened inflammatory responses following plaque infection [6]. Therefore, mitigating inflammation is crucial in CEPL treatment.
The yttrium-aluminum garnet (YAG) laser is commonly used in clinical practice for treating CEPLs. The erbium-doped: YAG (Er:YAG) laser, with a wavelength of 2,940 nm, operates in pulse mode and is effective and safe for treating periodontal soft and hard tissues. However, its shallow penetration limits its impact on deeper tissues [7,8]. Minocycline hydrochloride (Mino-HCL), a semi-synthetic tetracycline, exhibits strong antibacterial properties and adheres to tooth surfaces, providing prolonged antibacterial and anti-inflammatory effects that improve the gingival environment [9]. Previous studies have demonstrated the effectiveness of Er laser combined with Mino-HCL in treating conditions like peri-implantitis and gingival hyperplasia [10,11]. However, there is limited research on its clinical application for CEPLs.
We hypothesize that combining Er:YAG laser with Mino-HCL may offer significant benefits for treating CEPLs by enhancing anti-inflammatory effects. This study aims to evaluate and analyze this approach, offering novel insight into its clinical application and providing a new reference for CEPL treatment.
Materials and methods
Sample size calculation
Patients with CEPLs at Aerospace Center Hospital were randomly selected for this study. The sample size was calculated using the formula N= (1-P) × P× Z2/E2. We set statistic (Z) at 1.96, error (E) at 10%, and probability (P) at 0.5, based on a 95% confidence interval. The calculation resulted in N=96.
Study subjects
A total of 114 patients with CEPLs admitted to Aerospace Center Hospital from October 2021 to October 2023 were retrospectively analyzed. Among them, 53 patients treated with Er:YAG laser were classified as the control group, while 61 patients receiving Er:YAG laser combined with Mino-HCLconstituted the research group. The study was approved by the Ethics Committee of Aerospace Center Hospital (Approval No. 20210716-009y) and conducted in strict accordance with the Declaration of Helsinki.
Eligibility and exclusion criteria
Inclusion criteria: (1) Patients aged 18-60 years diagnosed with CEPLs via X-rays and pulp vitality tests. (2) Patients had not received recent relevant treatments and met the indications for laser therapy [12,13].
Exclusion criteria: (1) Patients with drug allergies, teeth with no preservation value, simple pulpitis, periapical periodontitis, autoimmune disorders. (2) Pregnant or lactating women. (3) Patients withdrew from the study.
Methods
Control group: Patients first underwent root canal treatment. The pulp chamber of the affected tooth was accessed, and the pulp was removed. A root canal was established, and the working length was measured. ProTaper nickel-titanium files were used for root canal preparation, with irrigation using 3% sodium hypochlorite. After preparation, the root canal was treated with an Er:YAG laser (Fotona, Germany) in photon-initiated photoacoustic streaming mode, with a pulse power of 0.3 W and a frequency of 15 Hz. The canal was then filled with AH-Plus root canal sealer and warm vertical compaction of gutta-percha. Periodontal pockets were irradiated with a laser at 0.8 W and 20 Hz. The treatment was performed once a week for 4 weeks. Research group: In addition to the above treatment, Mino-HCL was applied. After each laser treatment, Mino-HCL ointment (Sunstar INC, H20100244) was gently injected into the periodontal pocket until it overflowed.
Clinical efficacy evaluation
Efficacy was evaluated based on the treatment guidelines for CEPLs [14]. A “marked response” was defined as the complete resolution of clinical symptoms, absence of periodontal abscess and discharge, normalization of the periodontal pocket, and disappearance of tooth mobility. A “response” was characterized by a reduction in tooth mobility, significant alleviation of pain symptoms, and a decrease in probing pocket depth. If symptoms were unchanged or worsened, it was classified as a “non-response”. The overall response rate was calculated as (marked response + response) cases/total number of cases × 100%.
Prognostic follow-up
All patients underwent a 6-month follow-up with monthly reviews. At the final follow-up, quality of life and oral health were assessed using the MOS 36-Item Short Form Health Survey (SF-36) [15] and the Oral Health Impact Profile (OHIP-14) [16]. The SF-36 includes eight domains, and higher scores reflect better quality of life. The OHIP-14, with a maximum score of 56, indicates worse oral health with higher scores.
Primary outcome measures
Clinical efficacy: Evaluated as described above.
Plaque index (PLI), sulcus bleeding index (SBI), and probing depth (PD):
PLI: After drying the tooth surface with an air gun, the amount and thickness of dental plaque were assessed with a probe and recorded on a scale from 0 to 3, where higher scores indicated more plaque.
SBI: The periodontal probe was used to gently probe the periodontal pocket. Bleeding was observed 10-15 seconds after probe removal and scored from 0 to 3, with higher scores indicating more bleeding.
PD: The distance from the gingival margin to the bottom of the gingival sulcus was measured.
Secondary outcome measures
Visual Analogue Scale (VAS) [17]: Pain was assessed before treatment, 3 hours after treatment, and 3 days after treatment. Higher scores indicate more pronounced pain.
Gingival crevicular fluids: The gingival crevicular fluids were collected before and after treatment for enzyme-linked immunosorbent assays (ELISAs) to measure hypersensitive C-reactive protein (hs-CRP), tumor necrosis factor-α (TNF-α), interleukin-1β/6 (IL-1β/6), superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) levels. Kits were purchased from Shanghai Guangrui Biotech.
Adverse reactions: Incidents such as tooth loosening and edema were recorded, and the total incidence was calculated.
Prognostic SF-36 and OHIP-14 Scores: These scores were analyzed as described above.
Statistical analysis
Data were analyzed using SPSS version 23.0. Categorical data, such as gender and clinical efficacy, were reported as [n (%)] and compared using the chi-square test. Continuous data, such as age and inflammatory factors, were presented as (x±sd), and compared between groups using independent sample t-tests and within groups using paired t-tests. Multiple group comparisons were performed using repeated measures ANOVA and Least-Significant Difference (LSD) tests. Statistical significance was set at P<0.05.
Results
Comparison of clinical baseline data
As shown in Table 1, there were no significant differences between the two groups in terms of age, sex, disease duration, and other baseline characteristics (all P>0.05), confirming their comparability.
Table 1.
Comparison of clinical baseline information
| Group | n | Age | Course of disease (months) | Male | Female | BMI (kg/m2) | Smoking | Non-smoking |
|---|---|---|---|---|---|---|---|---|
| Control | 53 | 43.60±4.50 | 3.62±1.02 | 29 (54.72) | 24 (45.28) | 24.48±2.34 | 22 (41.51) | 31 (58.49) |
| Research | 61 | 43.38±6.23 | 3.77±0.82 | 26 (42.62) | 35 (57.38) | 24.08±1.35 | 24 (39.34) | 37 (60.66) |
| t (or χ2) | 0.220 | 0.854 | 1.661 | 1.158 | 0.055 | |||
| P | 0.827 | 0.395 | 0.197 | 0.249 | 0.814 | |||
Note: BMI, Body mass index.
Comparison of clinical efficacy
Table 2 illustrates that the research group had an overall response rate of 91.80%, significantly higher than the 77.36% observed in the control group (P<0.05).
Table 2.
Comparison of clinical efficacy
| Group | n | Marked response | Response | Non-response | Overall response rate |
|---|---|---|---|---|---|
| Control | 53 | 19 (35.85) | 22 (41.51) | 12 (22.64) | 77.36 |
| Research | 61 | 25 (40.98) | 31 (50.82) | 5 (8.20) | 91.80 |
| χ2 | 4.664 | ||||
| P | 0.031 |
Comparison of pain levels
Table 3 indicates that there was no significant difference in VAS scores between the two groups before treatment (P>0.05). VAS scores increased for both groups compared to baseline, with no notable inter-group difference, 3 hours after treatment (P>0.05). However, 3 days after treatment, VAS scores decreased for both groups, with the research group showing a significantly lower score than the control group (P<0.05).
Table 3.
Comparison of pain levels according to VAS score
| Group | n | Before treatment | 3 h after treatment | 3 d after treatment | F | P |
|---|---|---|---|---|---|---|
| Control | 53 | 4.68±1.25 | 6.21±1.35* | 3.45±1.12*,# | 65.240 | <0.001 |
| Research | 61 | 4.57±0.85 | 6.28±1.08* | 2.54±0.81*,# | 252.400 | <0.001 |
| t | 0.533 | 0.312 | 5.032 | |||
| P | 0.595 | 0.756 | <0.001 |
indicates P<0.05 compared to before treatment;
indicates P<0.05 compared to 3 h after treatment.
VAS, Visual Analogue Scale.
Comparison of periodontal condition
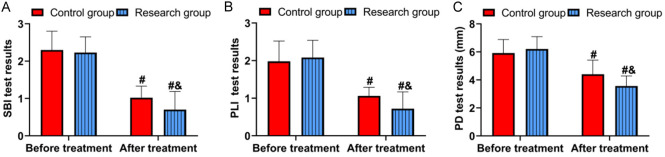
Figure 1 shows that both groups had similar SBI, PLI, and PD before treatment (all P>0.05). After treatment, improvements were observed in both groups, with the research group showing lower SBI, PLI, and PD compared to the control group (all P<0.05).
Figure 1.
Comparison of periodontal conditions. A. Comparison of SBI. B. Comparison of PLI. C. Comparison of PD. Comparison to before treatment, #P<0.05; comparison to control group, &P<0.05. PLI, Plaque index; SBI, sulcus bleeding index; PD, probing depth.
Comparison of inflammation levels
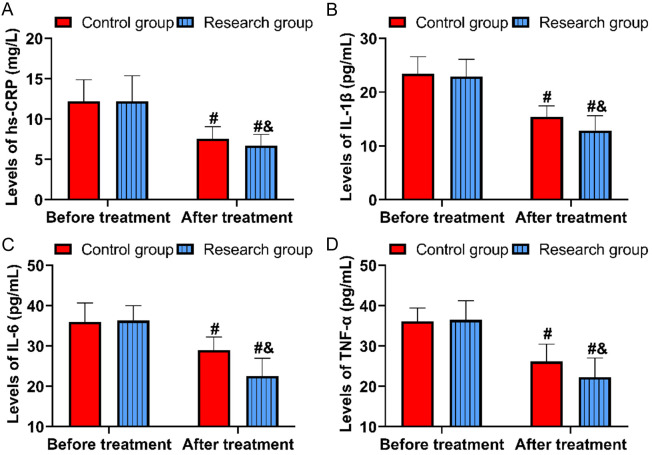
Figure 2 demonstrates that before treatment, levels of hypersensitive C-reactive protein (hs-CRP), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were similar between the groups (all P>0.05). After treatment, these levels decreased significantly in the research group (hs-CRP: 6.72±1.36 mg/L, IL-1β: 12.89±2.74 ng/mL, IL-6: 22.57±4.37 ng/mL, TNF-α: 22.31±4.70 ng/mL) compared to the control group (all P<0.05).
Figure 2.
Comparison of inflammation levels. A. Comparison of hs-CRP. B. Comparison of IL-1β. C. Comparison of IL-6. D. Comparison of TNF-α. Comparison to before treatment, #P<0.05; comparison to control group, &P<0.05. hs-CRP, hypersensitive-C reactive protein; TNF-α, tumor necrosis factor-α; IL-1β/6, interleukin-1β/6.
Comparison of oxidative stress
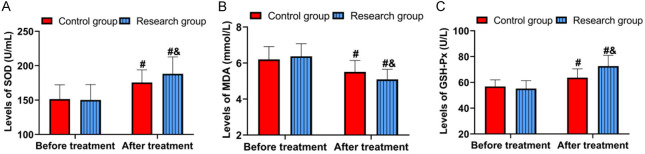
Figure 3 indicates that before treatment, there were no significant differences in oxidative stress markers between the groups (P>0.05). After treatment, the research group had higher levels of SOD (188.20±24.56 U/mL) and GSH-Px (72.63±8.26 U/L) compared to the control group (SOD: 175.52±18.32 U/mL, GSH-Px: 63.65±6.84 U/L) (both P<0.05). Additionally, MDA levels decreased in both groups after treatment, with the research group showing a lower MDA level (5.09±0.56 mmol/L) compared to the control group (5.51±0.64 mmol/L) (P<0.05).
Figure 3.
Comparison of oxidative stress. A. Comparison of SOD. B. Comparison of MDA. C. Comparison of GSH-Px. Comparison to before treatment, #P<0.05; comparison to control group, &P<0.05. SOD, Superoxide dismutase; MDA, malondialdehyde; GSH-Px, glutathione peroxidase.
Comparison of adverse reactions
Table 4 reports that the total incidence of adverse reactions was 11.48% in the research group and 20.75% in the control group, with no significant difference between the groups (P>0.05).
Table 4.
Comparison of adverse reactions
| Groups | n | Repeated bleeding | Infection | Edema | Inflammation | Total adverse reaction rate |
|---|---|---|---|---|---|---|
| Control | 53 | 3 (5.66) | 2 (3.77) | 1 (1.89) | 2 (3.77) | 20.75 |
| Research | 61 | 2 (3.28) | 1 (1.64) | 2 (3.28) | 2 (3.28) | 11.48 |
| χ2 | 1.837 | |||||
| P | 0.175 |
Comparison of prognostic oral health
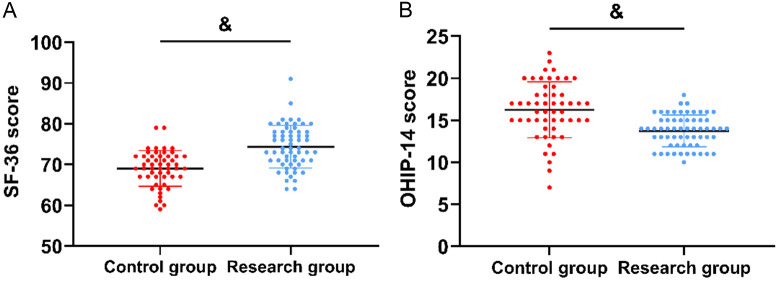
Figure 4 shows that at follow-up, the research group had higher SF-36 scores (74.38±5.24) and lower OHIP-14 scores (13.74±1.89) compared to the control group (both P<0.05).
Figure 4.
Comparison of prognosis. A. Comparison of SF-36 scores. B. Comparison of OHIP-14 scores. Comparison to control group, &P<0.05. SF-36, Mos 36-item Short Form Health Survey; OHIP-14, Oral Health Impact Profile.
Discussion
The pathogenesis of CEPLs is complex, involving anaerobic bacterial infections in both the pulp and periodontal pocket. These infections lead to simultaneous damage to the pulp and periodontium, resulting in dual damage to the teeth [18]. Due to the complex anatomy of the teeth, treating a single cause often fails to address both the pulp and periodontium effectively, making it difficult to fully control infections and leading to a high rate of tooth extractions [19]. Thus, a comprehensive treatment approach is essential to manage CEPLs, suppress inflammatory responses, and control infections, which is crucial for improving prognosis and maintaining periodontal health. This study demonstrates that Er:YAG laser combined with Mino-HCL effectively inhibits inflammation in CEPLs and offers a promising new approach for future treatment.
First, our results show that the research group had a higher overall response rate compared to the control group, indicating that Er:YAG laser combined with Mino-HCL provides superior therapeutic effectiveness for CEPLs. This finding aligns with previous studies on the use of Er:YAG laser and Mino-HCL in treating periodontal diseases such as peri-implantitis [20,21], confirming its valuable clinical application. The Er laser, with a wavelength of 2,940 nm, is highly absorbed by water molecules - an essential component of bacteria-leading to a “micro-explosion” effect that deconstructs bacterial structures and achieves sterilization [22,23]. Mino-HCL, which inhibits collagenase activity and promotes periodontal tissue regeneration, is commonly used in treating periodontitis and peri-implantitis [24]. Combined, these treatments enhance antibacterial effects, providing a reliable therapeutic option for CEPLs.
Additionally, the research group showed lower post-treatment SBI, PLI, and PD compared to the control group, further supporting the effectiveness of the combined treatment in improving periodontal function. The research group also experienced less pain post-treatment, as indicated by lower VAS scores, suggesting that the combination of Er:YAG laser and Mino-HCL alleviates pain more effectively. Pain is a common issue with CEPLs, often exacerbated by bacterial infection and residual bacteria in the root canal [25]. The inclusion of Mino-HCL likely reduces bacterial re-invasion, mitigates infection-induced gingival swelling and pain, and helps correct periodontal pathology. Furthermore, Mino-HCL has demonstrated notable analgesic effects, which contribute to pain relief following laser treatment [26]. Elevated VAS scores 3 hours after treatment in both groups could be attributed to the transient increase in pain due to laser-induced damage to periodontal tissues, which subsides as the anesthetic effect wears off.
CEPLs are chronic inflammatory diseases associated with biofilms, and the progression of oral inflammation is crucial to disease advancement [27]. Thus, inhibiting oral inflammatory responses is a fundamental step in managing CEPLs. In this study, the research group demonstrated significantly lower levels of hs-CRP, IL-1β, IL-6, and TNF-α after treatment compared to the control group, indicating that the Er:YAG laser combined with Mino-HCL has a more pronounced anti-inflammatory effect on CEPLs. This enhanced anti-inflammatory effect is primarily attributed to Mino-HCL. Mino-HCL inhibits both aerobic and anaerobic bacteria by interfering with bacterial protein synthesis, offering broad-spectrum antibacterial properties with reduced resistance; its anti-inflammatory effects are largely due to its capacity to enhance tissue antibacterial ability [28]. Pharmacological studies have shown that Mino-HCL also has significant immunomodulatory effects, such as increasing macrophage phagocytosis, enhancing lymphocyte activity, and promoting immunoglobulin synthesis [29]. These actions collectively mitigate inflammatory reactions and resist the damaging effects of inflammatory mediators on cells, thereby comprehensively inhibiting tissue inflammation. Furthermore, Mino-HCL’s inhibition of collagenase and metalloproteinase activities helps prevent alveolar bone resorption, promotes the transformation of periodontal ligament cells into osteoblasts, and aids in periodontal tissue regeneration and adhesion [30]. The higher levels of SOD and GSH-Px and the lower level of MDA in the research group also support reduced stress injury, reflecting the more effective alleviation of periodontal inflammation achieved with Mino-HCL. Previous studies have consistently validated Mino-HCL’s anti-inflammatory effects in conditions such as cerebral ischemia-reperfusion injury and periodontitis [31,32], which align with our findings. Despite these benefits, Mino-HCL alone cannot address the damaged periodontal root canal in CEPL patients, underscoring the need for its use in conjunction with Er:YAG laser treatment.
The comparison of adverse reactions between the two groups showed no significant differences, indicating that Mino-HCL does not increase the risk of side effects, thereby supporting its safety profile. The prognostic follow-up revealed higher SF-36 scores and lower OHIP-14 scores in the research group, suggesting that the combination of Er:YAG laser and Mino-HCL is more effective in improving patient outcome. This improvement in prognostic health is attributed to the overall positive effects of the combined treatment on oral function.
However, since this study was a single-center retrospective analysis with a limited sample size and short follow-up period, further research is needed. Future studies should involve larger sample sizes and extended follow-up durations to confirm the efficacy of Er:YAG laser combined with Mino-HCL for CEPLs. Additional research is also necessary to comprehensively evaluate the therapeutic impact of this combination therapy.
In conclusion, the combination of Er:YAG laser and Mino-HCL is effective in treating CEPLs, significantly reducing oral inflammatory responses and alleviating stress injury, thereby providing reliable protection for periodontal health. This treatment regimen should be considered as a viable option for managing CEPLs.
Disclosure of conflict of interest
None.
References
- 1.Fang F, Gao B, He T, Lin Y. Efficacy of root canal therapy combined with basic periodontal therapy and its impact on inflammatory responses in patients with combined periodontal-endodontic lesions. Am J Transl Res. 2021;13:14149–14156. [PMC free article] [PubMed] [Google Scholar]
- 2.Guo J, Li Y, Lin X, Yang X, Shi W, Lu X. Prognostic factors of combined periodontal and endodontic lesions: a retrospective study. Contrast Media Mol Imaging. 2022;2022:5042097. doi: 10.1155/2022/5042097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li T, He W, Jiang W, Wang X, Nie M, Wang S. Interdisciplinary management of combined periodontal-endodontic lesions with palatogingival grooves of the maxillary lateral incisors: a case report. Br Dent J. 2023;234:27–33. doi: 10.1038/s41415-022-5370-8. [DOI] [PubMed] [Google Scholar]
- 4.Dong T, Zhang Y, Li X. Time-lapse between periodontal regeneration surgery and root canal therapy in sever combined periodontal-endodontic lesions. Saudi Dent J. 2023;35:191–196. doi: 10.1016/j.sdentj.2022.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun P, Guo Z, Guo D, Wang J, Wu T, Li T, Liu J, Liu X. The microbiota profile analysis of combined periodontal-endodontic lesions using 16S rRNA next-generation sequencing. J Immunol Res. 2021;2021:2490064. doi: 10.1155/2021/2490064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YD, Lee JE, Chung Y, Lee WC, Seol YJ, Lee YM, Rhyu IC, Ku Y. Collaborative management of combined periodontal-endodontic lesions with a palatogingival groove: a case series. J Endod. 2017;43:332–337. doi: 10.1016/j.joen.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Guan R, Sun J, Hou B. Bacteria community study of combined periodontal-endodontic lesions using denaturing gradient gel electrophoresis and sequencing analysis. J Periodontol. 2014;85:1442–1449. doi: 10.1902/jop.2014.130572. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Z, Zeng Z, Wu G. Minocycline hydrochloride ointment combined with Vitapex paste is effective for middle-aged and elderly patients with combined periodontal-endodontic lesions. Am J Transl Res. 2024;16:314–322. doi: 10.62347/NLWG5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khojaste M, Navabi S, Shiezadeh F. Treatment of a hopeless tooth with combined endodontic-periodontal lesion using guided tissue regeneration: a case report with one year follow-up. Iran Endod J. 2022;17:212–215. doi: 10.22037/iej.v17i4.38667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y, Chen B, Ni YH, Yan FH. Time lapse between endodontic and periodontal treatments of combined periodontal-endodontic lesion: a systematic review. Hua Xi Kou Qiang Yi Xue Za Zhi. 2018;36:167–173. doi: 10.7518/hxkq.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia M, Qi Q. Bacterial analysis of combined periodontal-endodontic lesions by polymerase chain reaction-denaturing gradient gel electrophoresis. J Oral Sci. 2013;55:287–291. doi: 10.2334/josnusd.55.287. [DOI] [PubMed] [Google Scholar]
- 12.Vakalis SV, Whitworth JM, Ellwood RP, Preshaw PM. A pilot study of treatment of periodontal-endodontic lesions. Int Dent J. 2005;55:313–318. doi: 10.1111/j.1875-595x.2005.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wang X, Xu J, Zhou X, Xie K. The clinical study on the use of diode laser irradiation in the treatment of periodontal-endodontic combined lesions. Hua Xi Kou Qiang Yi Xue Za Zhi. 2012;30:161–164. 168. [PubMed] [Google Scholar]
- 14.Liu ZY, Zhang JD, Zhang L, Yang J, Liu XH. Analysis of minocycline hydrochloride combined with vitapex in treating senile chronic periodontal-endodontic combined lesions. Shanghai Kou Qiang Yi Xue. 2016;25:465–468. [PubMed] [Google Scholar]
- 15.Larson JS. The MOS 36-item short form health survey. A conceptual analysis. Eval Health Prof. 1997;20:14–27. doi: 10.1177/016327879702000102. [DOI] [PubMed] [Google Scholar]
- 16.Campos LA, Peltomaki T, Maroco J, Campos JADB. Use of Oral Health Impact Profile-14 (OHIP-14) in different contexts. What is being measured? Int J Environ Res Public Health. 2021;18:13412. doi: 10.3390/ijerph182413412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heller GZ, Manuguerra M, Chow R. How to analyze the visual analogue scale: myths, truths and clinical relevance. Scand J Pain. 2016;13:67–75. doi: 10.1016/j.sjpain.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Sunitha V R, Emmadi P, Namasivayam A, Thyegarajan R, Rajaraman V. The periodontal - endodontic continuum: a review. J Conserv Dent. 2008;11:54–62. doi: 10.4103/0972-0707.44046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou K, Ji PH, Yu LY, Chen Q, Xu QL. Detection of anaerobes and drug sensitivity from the periodontal pockets of patients with combined periodontal-endodontic lesions. Shanghai Kou Qiang Yi Xue. 2013;22:72–76. [PubMed] [Google Scholar]
- 20.Miao H, Chen M, Otgonbayar T, Zhang SS, Hou MH, Wu Z, Wang YL, Wu LG. Papillary reconstruction and guided tissue regeneration for combined periodontal-endodontic lesions caused by palatogingival groove and additional root: a case report. Clin Case Rep. 2015;3:1042–1049. doi: 10.1002/ccr3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Zhang YW, Jiang XQ, Chen HT, Sun L. Endodontic-periodontal combined therapy for type III dens invaginatus in maxillary lateral incisor: a case report. Hua Xi Kou Qiang Yi Xue Za Zhi. 2019;37:453–456. doi: 10.7518/hxkq.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Khanna D, Kalra S. Minocycline and doxycycline: more than antibiotics. Curr Mol Pharmacol. 2021;14:1046–1065. doi: 10.2174/1874467214666210210122628. [DOI] [PubMed] [Google Scholar]
- 23.Javed S, Kohli K. Local delivery of minocycline hydrochloride: a therapeutic paradigm in periodontal diseases. Curr Drug Deliv. 2010;7:398–406. doi: 10.2174/156720110793566290. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Sun J, Ma L, Nie Z, Sai H, Cheng J, Duan J. Characterization of the interactions between minocycline hydrochloride and trypsin with spectroscopic and molecular docking technology. Molecules. 2023;28:2656. doi: 10.3390/molecules28062656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahravan A, Nekouei AH. Does ultrasonic activation of irrigation during endodontic therapy improve the clinical and microbiological effects? Evid Based Dent. 2022;23:118–119. doi: 10.1038/s41432-022-0819-9. [DOI] [PubMed] [Google Scholar]
- 26.Qiao L, Tang Q, An Z, Qi J. Minocycline relieves neuropathic pain in rats with spinal cord injury via activation of autophagy and suppression of PI3K/Akt/mTOR pathway. J Pharmacol Sci. 2023;153:12–21. doi: 10.1016/j.jphs.2023.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Walter C, Krastl G, Weiger R. Step-wise treatment of two periodontal-endodontic lesions in a heavy smoker. Int Endod J. 2008;41:1015–1023. doi: 10.1111/j.1365-2591.2008.01458.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Yang PS. Minocycline hydrochloride nanoliposomes inhibit the production of TNF-alpha in LPS-stimulated macrophages. Int J Nanomedicine. 2012;7:4769–4775. doi: 10.2147/IJN.S34036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Zhang W, Ye Z, Pei S, Zheng D, Zhu L. Safety evaluation and pharmacodynamics of minocycline hydrochloride eye drops. Mol Vis. 2022;28:460–479. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang T, Qiu Y, Song J, Zhou P, Liao H, Cheng Y, Wu X. Electrosprayed minocycline hydrochloride-loaded microsphere/SAIB hybrid depot for periodontitis treatment. Drug Deliv. 2021;28:620–633. doi: 10.1080/10717544.2021.1902020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abcouwer SF, Lin CM, Shanmugam S, Muthusamy A, Barber AJ, Antonetti DA. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflammation. 2013;10:149. doi: 10.1186/1742-2094-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Gu C, Tong X. Clinical efficacy of minocycline hydrochloride for the treatment of peri-implant disease: a systematic review with meta-analysis of randomized controlled trials. J Oral Implantol. 2023;49:245–252. doi: 10.1563/aaid-joi-D-22-00023. [DOI] [PubMed] [Google Scholar]