Abstract
Objective: To evaluate the efficacy of subcutaneous specific immunotherapy (SCIT) for allergic rhinitis (AR) combined with asthma. Methods: A retrospective analysis of clinical data from 93 patients with AR combined with asthma admitted to our hospital from January 2022 to January 2023 was conducted. Based on the treatment interventions received, the patients were divided into a control group (n=46, receiving sublingual specific immunotherapy [SLIT]) and an observation group (n=47, receiving SCIT). Clinical treatment response, lung function, levels of immune indicators, levels of inflammatory indicators, and occurrence of adverse reactions were compared between the two groups. Results: The total response rate was 95.74% in the observation group and 84.78% in the control group (P > 0.05). In terms of scores for symptom assessment, Total Nasal Symptom Score (TNSS), Depression Anxiety Stress Scale (DASS), and Nasal Allergy Symptom Score (NASS) scores in both groups decreased after treatment, with greater decreases in the observation group (P < 0.05). In addition, lung function was improved in both groups after treatment as reflected by increased Forced Expiratory Volume in one second to Forced Vital Capacity ratio (FEV1/FVC) and Peak Expiratory Flow (PEF) levels, with greater increases found in the observation group (P < 0.05). Among the immune and inflammatory indicators, Cluster of Differentiation 14 (CD14) and Interleukin-33 (IL-33) levels decreased, while Secretory Protein D-1 (SPD-1), serum Immunoglobulin G4 (sIgG4), Interferon-γ (INF-γ), and Interleukin-27 (IL-27) levels increased in both groups after treatment, with greater changes observed in the observation group (P < 0.05). There was no significant difference in the incidence of adverse reactions between the observation group (14.89%) and the control group (21.74%) (P > 0.05). Conclusion: In the treatment of AR combined with asthma, SCIT can better alleviate clinical symptoms, improve lung function, regulate immune and inflammatory responses in patients, and does not increase the risk of adverse reactions compared to SLIT.
Keywords: Subcutaneous specific immunotherapy, sublingual specific immunotherapy, allergic rhinitis, asthma, immunity, inflammation, efficacy evaluation
Introduction
Allergic rhinitis (AR) and asthma are common co-occurring allergic respiratory disorders that significantly impact patients’ quality of life and healthcare utilization [1,2]. These conditions are characterized by high susceptibility, chronicity, and recurrent exacerbations, posing substantial burdens on affected individuals and the healthcare system [1,2]. The pathogenesis of AR and asthma involves complex immune reactions and inflammatory processes [3]. Upon allergen exposure, the body mounts excessive immune responses, releasing various inflammatory mediators that lead to inflammation of the nasal mucosa and airways, manifesting clinically as symptoms such as sneezing, nasal congestion, rhinorrhea, cough, chest tightness, and dyspnea [3].
The current clinical methods for treating this condition primarily include pharmacotherapy, allergen-specific immunotherapy (ASIT), and environmental control measures. Pharmacotherapy involves the use of medications such as corticosteroids, antihistamines, leukotriene receptor antagonists, and bronchodilators to alleviate symptoms and control inflammation in both the upper and lower airways. However, traditional pharmacological treatments for combined AR and asthma often fail to achieve satisfactory long-term control, and prolonged use may result in drug resistance and adverse effects [4]. ASIT, on the other hand, aims to desensitize the immune system to specific allergens through gradual exposure, leading to reduced symptoms and medication usage. Immunomodulatory therapies, particularly subcutaneous specific immunotherapy (SCIT) and sublingual specific immunotherapy (SLIT), have gained attention as targeted approaches addressing the underlying immunological mechanisms [5]. SLIT, for instance, involves the oral administration of allergen extracts to induce mucosal immune regulation and therapeutic effects [5]. SCIT, on the other hand, gradually increases the patient’s tolerance to specific allergens by subcutaneous injection, thereby alleviating allergic and inflammatory reactions. Both immunotherapies have achieved certain efficacy in treating AR and asthma, but their efficacy and safety are still controversial [6]. Environmental control measures focus on minimizing allergen exposure in the patient’s surroundings.
However, these current methods have their disadvantages. Some patients may not achieve optimal symptom control, leading to ongoing symptoms and impaired quality of life. Compliance with long-term medication use, and allergen immunotherapy can be challenging, and treatment side effects may impact patient satisfaction and adherence. Therefore, there is a need for innovative research to address these limitations and develop more effective, safe, and patient-friendly approaches for managing AR combined with asthma. This study aims to investigate the effects of SLIT and SCIT to find the optimal treatment strategies that offer better symptom control, enhanced patient adherence, and reduced side effects.
Data and methods
Basic information
A retrospective analysis was conducted on the clinical data of 93 patients with concomitant AR and asthma admitted to our hospital from January 2022 to January 2023. Patients were eligible if they were 18 years old or older, met the diagnostic criteria for AR and asthma [7,8], had dust mites as the identified allergen, had strong willingness for treatment and completed 1 year of treatment and follow-up with good adherence to the prescribed medication regimen, and had complete clinical data. Patients were excluded if they had a history of alcohol or drug dependence, concurrent sensitivity to other inhaled allergens besides dust mites, severe organ dysfunction, abnormalities in immune or coagulation function, malignancies, severe infections, bleeding tendencies, recent (within 1 month) use of immunomodulatory drugs or antihistamines, recent history of typical asthma attacks, allergic reactions or contraindications to the study interventions, and concurrent cognitive impairment, consciousness disorders, or mental illness. Participants were divided into a control group (n=46) and an observation group (n=47) based on the treatment interventions. This study was approved by the Medical Ethics Committee of Renmin Hospital, Hubei University of Medicine.
Methods
Control group
The control group received SLIT, with the following specifics: The patients were administered sublingual drops containing dust mite allergens (manufactured by Zhejiang Wumei Biotechnology Co., Ltd., with Chinese Medical Device Registration No. S20060012). The medication comprised 5 specifications, numbered 1 to 5. The treatment duration was 1 year, and hospitalization was not required. In the first 3 weeks, patients were orally administered dust mite drops No. 1 to No. 3 sublingually daily, with the dosage gradually increasing. The specific dosages were as follows: 1, 2, 3, 4, 6, 8, and 10 drops per day each week, increasing sequentially. From the 4th to the 5th week, patients switched to dust mite drops No. 4, with 3 drops each time, once daily. Starting from the 6th week until the end of treatment, patients took dust mite drops No. 5, with 2 drops each time, once daily. During the treatment, if any adverse reactions occurred, they were to inform the doctor immediately by phone for timely management.
Observation group
The observation group received SCIT, with the following specifics: The standardized house dust mite allergen preparation produced by ALK-AbelloA/S was used for treatment, with a treatment duration of 1 year. The injection site was the outer third of the upper arm for the subcutaneous injection, with the dosage gradually increasing. In the first week, the injection dosage was 20 Standardized Quality Unit (SQ-U), which was gradually increased every week until reaching the maximum dosage. The specific incremental dosages were as follows: 40 SQ-U, 80 SQ-U, 200 SQ-U, 400 SQ-U, 800 SQ-U, 2,000 SQ-U, 4,000 SQ-U, 8,000 SQ-U, 10,000 SQ-U, 20,000 SQ-U, 40,000 SQ-U, 60,000 SQ-U, and 80,000 SQ-U. From the 15th week, the dosage was adjusted to 100,000 SQ-U, and injections were administered every 6 weeks after the 16th week. Thirty minutes before injection, the patient was given antihistamine medication to reduce the occurrence of adverse reactions. After injection, the patient was observed for 30 minutes to ensure no abnormalities before leaving.
Outcome measures
(1) Clinical treatment response: The efficacy was evaluated based on the changes in patient symptoms (sum of nasal symptom score and asthma symptom score), where significant effect was defined as a reduction of symptoms score by more than 65%, effective as a reduction in the range of 25% to 65%, and ineffective as a reduction of less than 25% in symptoms score after treatment.
(2) Scores of clinical symptoms: Before and after treatment, nasal symptoms were assessed using the Total Nasal Symptom Score (TNSS) [9], where sneezing, rhinorrhea, nasal congestion, and nasal itching were scored as 0, 1, 2, and 3 respectively for none, mild, moderate, and severe symptoms, with a total score ranging from 0 to 12. Asthma symptoms were assessed using the Daytime Asthma Symptom Score (DASS) and the Nighttime Asthma Symptom Score (NASS) [10]. For DASS, no symptoms, mild asthma, frequent attacks, and continuous attacks were scored as 0, 1, 2, and 3 respectively. For NASS, no symptoms, waking up once due to asthma symptoms, waking up frequently, continuous attacks causing sleep disturbances, and unable to lie flat were scored as 0, 1, 2, 3, and 4 respectively.
(3) Pulmonary function: Before and after treatment, forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and peak expiratory flow rate (PEF) were measured using a spirometer (MS-IOS type), and the ratio of FEV1 to FVC was calculated.
(4) Levels of immunological indictors: Before and after treatment, 5 mL of fasting venous blood was collected from the elbow in patients, and serum levels of CD14, soluble programmed cell death ligand 1 (SPD-L1), and specific immunoglobulin G4 (sIgG4) were measured using enzyme-linked immunosorbent assay (ELISA) (Echelon Biosciences, CAT#K-1200).
(5) Levels of inflammatory factors: Before and after treatment, blood specimens were collected from patients (using the same method as above), and interleukin-33 (IL-33), interferon-gamma (INF-γ), and interleukin-27 (IL-27) levels were measured using ELISA (Echelon Biosciences, CAT#K-1200).
(6) Occurrence of adverse reactions: Gastrointestinal discomfort, nausea/vomiting, skin reactions, worsening of rhinitis, and worsening of asthma were recorded as adverse reactions. All these indices were tested three months after the completion of the treatment.
(7) Prognosis: To assess the prognosis, symptoms, including running nose, rhinocnesmus, sneezing, and nasal congestion, were recorded in both groups. Each symptom was scored on a scale of 0-3 points, ranging from a normal state (0 points) to unbearable symptoms (3 points). The severity of symptoms was directly proportional to the score, and the prognosis was classified into favorable and unfavorable.
Statistical analysis
GraphPad Prism 8 was used for graphical visualization, and SPSS 22.0 was used for data analysis. Measurement data were described using (x̅ ± s) and analyzed using the t-test, while count data were described as n (%) and analyzed using the chi-square test. Logistics regression was conducted to analyze the risk factors affecting the prognosis. P < 0.05 indicated statistical significance.
Results
There were no statistically significant differences between the control and observation groups in terms of sex distribution, age, course of disease, Body Mass Index (BMI), and severity of illness. This indicates that the two groups were well-matched in terms of baseline characteristics, laying a solid foundation for the effective comparison of the study outcomes (Table 1).
Table 1.
Basic information (x̅ ± s, n [%])
| Control (n=46) | Observation (n=47) | t/x2 | P | |
|---|---|---|---|---|
| Sex | - | - | 0.258 | 0.611 |
| Male | 26 (56.52) | 29 (61.70) | - | - |
| Female | 20 (43.48) | 18 (38.30) | - | - |
| Age (years) | 24.79±3.27 | 25.43±3.56 | 0.902 | 0.369 |
| Course of disease (years) | 1.65±0.42 | 1.79±0.47 | 1.513 | 0.133 |
| BMI (kg/m2) | 23.68±1.35 | 23.26±1.44 | 1.450 | 0.150 |
| Severity of illness | - | - | 0.408 | 0.522 |
| Moderate | 35 (76.09) | 33 (70.21) | - | - |
| Severe | 11 (23.91) | 14 (29.79) | - | - |
Comparison of clinical treatment response
The total response rate was 95.74% in the observation group and the 84.78% in the control group, showing no significant difference between groups (P > 0.05). See Table 2.
Table 2.
Comparison of clinical treatment response [n (%)]
| Group (n) | Significant effect | Effective | Ineffective | Total response rate |
|---|---|---|---|---|
| Control (n=46) | 17 (36.96) | 22 (47.83) | 7 (15.22) | 39 (84.78) |
| Observation (n=47) | 25 (53.19) | 20 (42.55) | 2 (4.26) | 45 (95.74) |
| X2 | - | - | - | 2.064 |
| P | - | - | - | 0.150 |
Comparison of clinical symptoms
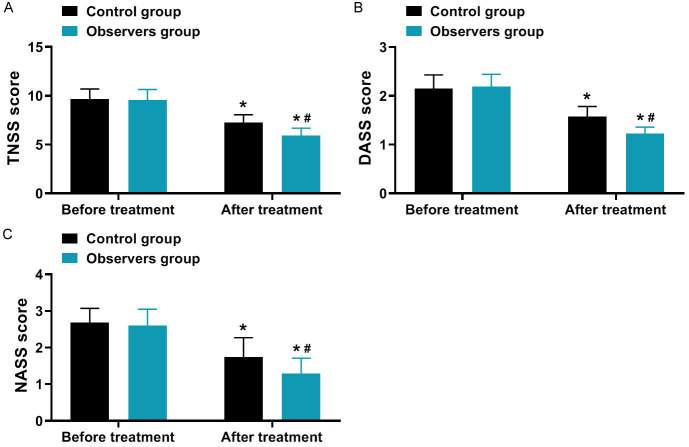
As shown in Figure 1, the TNSS score, DASS score, and NASS score decreased in both groups after treatment compared to before treatment, with a greater magnitude of changes observed in the observation group (all P < 0.001).
Figure 1.
Comparison of clinical symptom scores (x̅ ± s, score). A: TNSS score; B: DASS score; C: NASS score. Note: Compared to before treatment, *P < 0.05; between groups in the After treatment, #P < 0.05. TNSS, Total Nasal Symptom Score; DASS, Depression Anxiety Stress Scale; NASS, Nasal Allergy Symptom Score.
Comparison of pulmonary function
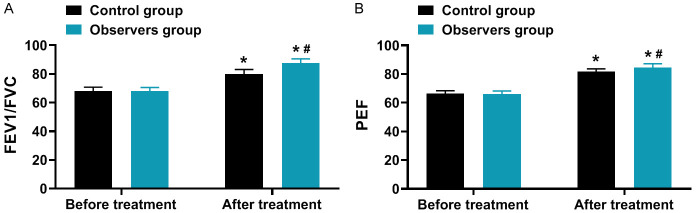
As illustrated in Figure 2, the FEV1/FVC and PEF levels increased in both groups after treatment compared to before treatment, with a greater magnitude of changes observed in the observation group (all P < 0.001).
Figure 2.
Comparison of pulmonary function (x̅ ± s, %). A: FEV1/FVC; B: PEF. Note: Compared to before treatment, *P < 0.05; between groups in the After treatment, #P < 0.05. FEV1/FVC, Forced Vital Capacity ratio; PEF, Peak Expiratory Flow.
Comparison of immune and inflammatory levels
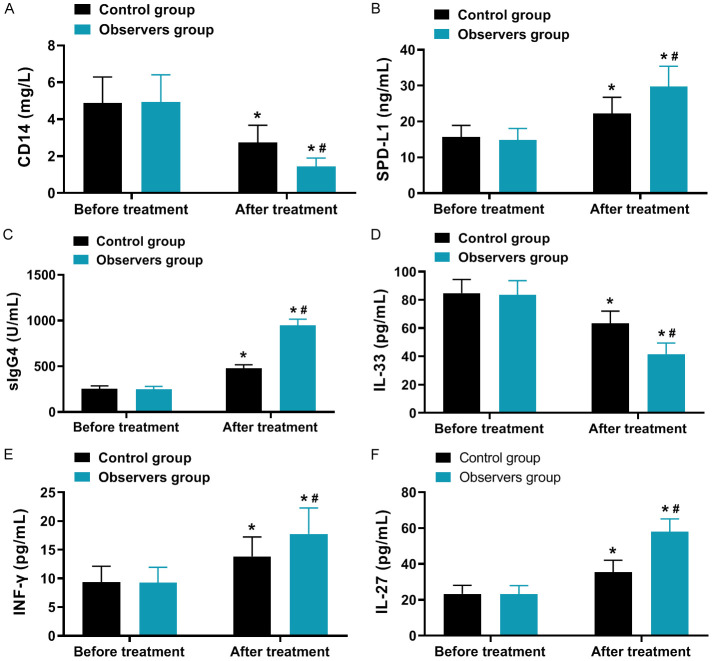
As depicted in Figure 3, the levels of CD14 and IL-33 decreased in both groups after treatment compared to before treatment, while the levels of SPD-1, sIgG4, INF-γ, and IL-27 increased, with a greater magnitude of changes observed in the observation group (all P < 0.001).
Figure 3.
Comparison of immune and inflammatory levels (x̅ ± s). A: CD14; B: SPD-L1; C: slgG4; D: IL-33; E: INF-γ; F: IL-27. Note: Compared to before treatment, *P < 0.05; between groups in the After treatment, #P < 0.05. CD14, Cluster of Differentiation 14; IL-33, Interleukin-33; SPD-1, Secretory Protein D-1; sIgG4, serum Immunoglobulin G4; INF-γ, Interferon-γ; IL-27, Interleukin-27.
Comparison of adverse reactions
The occurrence rate of adverse reactions in the observation group (14.89%) did not significantly differ from that in the control group (21.74%) (P=0.393), as shown in Table 3.
Table 3.
Comparison of adverse reactions [n (%)]
| Adverse Reactions | Control (n=46) | Observation (n=47) | X2 | P |
|---|---|---|---|---|
| Gastrointestinal discomfort | 3 (6.52) | 2 (4.26) | - | - |
| Nausea and vomiting | 2 (4.35) | 2 (4.26) | - | - |
| Skin reactions | 2 (4.35) | 1 (2.13) | - | - |
| Aggravation of rhinitis | 1 (2.17) | 1 (2.13) | - | - |
| Aggravation of asthma | 2 (4.35) | 1 (2.13) | - | - |
| Total occurrence rate | 10 (21.74) | 7 (14.89) | 0.729 | 0.393 |
Assessment of prognosis
Based on the clinical response to treatment, the patients were categorized into a favorable prognosis group and an unfavorable prognosis group. Factors influencing prognosis were analyzed, and SPD-1 and sIgG4 were found to have a strong association with treatment efficacy (P < 0.05, Table 4). These significant indicators were further examined using multivariate logistic regression analysis, which revealed that SPD-1 and sIgG4 were independent risk factors affecting prognosis (P < 0.05, Table 5).
Table 4.
Univariate analysis
| Factors | Favorable prognosis group (n=70) | Unfavorable prognosis group (n=23) | t/x2 | P |
|---|---|---|---|---|
| Sex | 0.098 | 0.753 | ||
| Male | 40 | 14 | ||
| Female | 30 | 9 | ||
| Age (years) | 0.006 | 0.935 | ||
| ≥ 20 | 45 | 15 | ||
| < 20 | 25 | 8 | ||
| Course of disease (years) | 0.014 | 0.905 | ||
| ≥ 1.5 | 55 | 19 | ||
| < 1.5 | 15 | 4 | ||
| BMI (kg/m2) | 0.031 | 0.860 | ||
| ≥ 20 | 38 | 12 | ||
| < 20 | 32 | 11 | ||
| Severity of illness | 0.053 | 0.818 | ||
| Moderate | 50 | 17 | ||
| Severe | 20 | 6 | ||
| TNSS score | 8.01±2.22 | 9.06±2.82 | 1.836 | 0.069 |
| DASS score | 1.52±0.04 | 1.53±0.03 | 1.100 | 0.274 |
| NASS score | 1.23±0.31 | 1.25±0.25 | 0.281 | 0.779 |
| CD14 | 1.72±0.11 | 1.77±0.12 | 1.849 | 0.068 |
| IL-33 | 30.23±1.22 | 29.77±1.32 | 1.537 | 0.128 |
| SPD-1 | 1022.33±35.21 | 450.27±20.23 | 73.843 | 0.001 |
| sIgG4 | 42.83±1.25 | 70.22±1.27 | 90.817 | 0.001 |
| INF-γ | 15.25±0.56 | 15.04±0.22 | 1.749 | 0.084 |
| IL-27 | 58.99±1.69 | 58.32±1.72 | 1.642 | 0.104 |
| FEV1/FVC | 80.22±2.68 | 79.87±2.25 | 0.564 | 0.574 |
| PEF | 82.01±2.22 | 81.34±2.62 | 1.200 | 0.233 |
TNSS, Total Nasal Symptom Score; DASS, Depression Anxiety Stress Scale; NASS, Nasal Allergy Symptom Score; FEV1/FVC, Forced Vital Capacity ratio; PEF, Peak Expiratory Flow; CD14, Cluster of Differentiation 14; IL-33, Interleukin-33; SPD-1, Secretory Protein D-1; sIgG4, serum Immunoglobulin G4; INF-γ, Interferon-γ; IL-27, Interleukin-27.
Table 5.
Logistics regression analysis of risk factors affecting prognosis
| Factor | Β | Standard error | Chi square value | P-value | OR value | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Upper limit | ||||||
| SPD-1 | -1.846 | 0.718 | 6.613 | 0.01 | 0.149 | 0.029 | 0.635 |
| sIgG4 | -2.269 | 0.721 | 9.701 | 0.003 | 0.116 | 0.026 | 0.415 |
SPD-1, Secretory Protein D-1; sIgG4, serum Immunoglobulin G4.
Discussion
AR and asthma are closely linked chronic respiratory conditions that significantly impact patients’ physical and mental health, as well as their quality of life. The common symptoms include wheezing, coughing, rhinorrhea, sneezing, nasal congestion, and itching [11]. While traditional symptomatic treatments can provide symptom relief, they do not alter the underlying disease course, and sufferers often require lifelong medication [12]. Specific immunotherapy, on the other hand, has the potential to modify the natural progression of these allergic diseases. By gradually exposing patients to increasing doses of allergens, specific immunotherapy aims to induce immune tolerance and reduce the occurrence of allergic symptoms [13]. Environmental allergens, particularly dust mites, are major triggers for AR and asthma [14]. The two primary forms of specific immunotherapy are SLIT and SCIT. SCIT, as the traditional approach, is recommended by authoritative guidelines, such as those from the World Allergy Organization and the Chinese Society of Allergology [15]. However, there is an ongoing debate regarding the comparative efficacy and safety of SLIT versus SCIT. Some studies have suggested that SCIT may be clinically comparable or even superior to SLIT in certain aspects [16]. Additionally, research has indicated that SCIT may be more effective than SLIT in managing patients with allergic asthma accompanied by AR [17]. However, there are large inconsistencies in the current findings among previous research.
In this study, patients in the observation group showed certain advantages in clinical efficacy and the occurrence rate of adverse reactions, but the overall difference did not reach statistical significance, which may be attributed to the relatively small sample size included in this study. The total response rate of treatment was 95.74% in the observation group (SCIT) and 84.78% in the control group (SLIT). While the difference was not statistically significant, it is important to consider the findings in the context of published studies. Several studies have reported similar rates for SCIT and SLIT in treating AR combined with asthma [17]. The mechanism underlying the clinical treatment effects of both SCIT and SLIT lies in their ability to induce immune tolerance and modulate allergen-specific immune responses. SCIT, involving subcutaneous injection, activates regulatory T cells and stimulates the production of allergen-specific IgG4 antibodies, leading to reduced allergic inflammation [16]. Similarly, SLIT stimulates regulatory cytokines and generates allergen-specific regulatory T cells, suppressing allergic responses. Thus, both SCIT and SLIT work through immune modulation mechanisms, resulting in improved clinical outcomes.
Moreover, TNSS, DASS, and NASS scores significantly decreased in both groups after treatment, with a more significant improvement observed in the observation group. This finding is consistent with previous studies that have demonstrated the superiority of SCIT over SLIT in alleviating clinical symptoms [18]. The underlying mechanism can be attributed to the different routes of administration and subsequent immune responses. SCIT, using subcutaneous injection, delivers allergens directly to antigen-presenting cells, triggering a stronger systemic immune response and better symptom control [19]. Conversely, SLIT primarily targets the mucosal immune system through oral administration, leading to local immune modulation. The systemic effects of SCIT may explain the greater improvement in clinical symptom scores observed in the observation group.
Furthermore, both groups showed improvement in lung function parameters, including FEV1/FVC and PEF levels, with more significant changes observed in the observation group. These findings align with previous studies reporting significant improvements in lung function after SCIT [20]. The mechanism behind this improvement involves the reduction of airway inflammation and hyperresponsiveness. Subcutaneous administration of allergens in SCIT induces the production of allergen-specific regulatory T cells and suppresses Th2-mediated inflammatory responses, leading to decreased airway inflammation and improved lung function [21]. The observed greater improvement in lung function in the observation group may be attributed to the stronger immunomodulatory effects of SCIT compared to SLIT.
Additionally, after treatment, the CD14 and IL-33 levels decreased, while the SPD-1, sIgG4, INF-γ, and IL-27 levels increased in both groups, with more significant changes observed in the observation group. These changes reflect the immunomodulatory effects of both SCIT and SLIT. CD14 is a marker of monocyte activation, and its decrease suggests a reduction in pro-inflammatory responses [22]. IL-33 is a pro-inflammatory cytokine associated with allergic inflammation, and its decrease indicates suppression of Th2-mediated responses. The increase in SPD-1, sIgG4, INF-γ, and IL-27 levels reflects the shift towards anti-inflammatory and regulatory immune responses [23-25]. These changes in immune and inflammatory indicators align with the known mechanisms of action of SCIT and SLIT, where they promote the development of allergen-specific regulatory T cells and the production of allergen-specific IgG4 antibodies, leading to immune tolerance and suppression of allergic inflammation.
Importantly, the incidence of adverse reactions was comparable between the observation group (14.89%) and the control group (21.74%). This finding is consistent with previous studies reporting a similar safety profile for both SCIT and SLIT [26]. Adverse reactions, such as local injection site reactions and mild systemic reactions, are generally mild and well-tolerated. The overall safety of both SCIT and SLIT is attributed to the gradual dose escalation protocols and close medical supervision during immunotherapy administration. These provide further support for the safety of both SCIT and SLIT, ensuring their appropriate use in clinical practice.
This study on AR combined with asthma has made significant contributions to our understanding of these conditions. However, it is essential to acknowledge the limitations to ensure a comprehensive interpretation of the findings. One potential limitation of this study may be the relatively small sample size, which could limit the generalizability of the results to a broader population. Additionally, the study design, such as the absence of a blank control group or randomization, may restrict the establishment of causal relationships. Furthermore, the duration of follow-up in the study might have been relatively short, potentially limiting insights into the long-term effects of the intervention. It is important to consider these limitations when interpreting the study’s findings and to encourage further research to address these aspects in future investigations.
Conclusion
In the treatment of patients with AR combined with asthma, compared to SLIT, SCIT can further alleviate clinical symptoms, improve lung function, regulate immune and inflammatory responses in patients, and SCIT does not increase the risk of adverse reactions in patients.
Disclosure of conflict of interest
None.
References
- 1.Chen LC, Zeng GS, Wu LL, Zi M, Fang ZK, Fan HZ, Yu HP. Diagnostic value of FeNO and MMEF for predicting cough variant asthma in chronic cough patients with or without allergic rhinitis. J Asthma. 2021;58:326–333. doi: 10.1080/02770903.2019.1694035. [DOI] [PubMed] [Google Scholar]
- 2.Jugulete G, Luminos M, Pavelescu C, Merisescu MM. Remdesivir efficacy and tolerability in children with COVID-19-associated allergic comorbidities such as asthma, allergic rhinitis, and atopic dermatitis. Children (Basel) 2023;10:810. doi: 10.3390/children10050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andiappan AK, Puan KJ, Sio YY, Ally F, Lee B, Matta SA, Yusof N, Larbi A, Wang Y, Chew FT, Rotzschke O. Functional CTLA-4 variants associate to both allergic asthma and rhinitis potentially by modulating naïve regulatory T cells. Allergy. 2022;77:2856–2858. doi: 10.1111/all.15386. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Zhang W, Tian T, Liu Y, Bai H, Hu Q, Qi F. Latent myofascial trigger points injection therapy for adult cough variant asthma: a randomized controlled trial. Front Med (Lausanne) 2023;10:937377. doi: 10.3389/fmed.2023.937377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drazdauskaitė G, Layhadi JA, Shamji MH. Mechanisms of allergen immunotherapy in allergic rhinitis. Curr Allergy Asthma Rep. 2020;21:2. doi: 10.1007/s11882-020-00977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virchow JC, Pfaar O, Lommatzsch M. Allergen immunotherapy for allergic asthma. Allergol Select. 2024;8:6–11. doi: 10.5414/ALX02451E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui ZA, Walker A, Pirwani MM, Tahiri M, Syed I. Allergic rhinitis: diagnosis and management. Br J Hosp Med (Lond) 2022;83:1–9. doi: 10.12968/hmed.2021.0570. [DOI] [PubMed] [Google Scholar]
- 8.Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, Hilton Boon M, Kantar A, Lai K, McGarvey L, Rigau D, Satia I, Smith J, Song WJ, Tonia T, van den Berg JWK, van Manen MJG, Zacharasiewicz A. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55:1901136. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamasauskiene L, Gasiuniene E, Sitkauskiene B. Translation, adaption and validation of the total nasal symptom score (TNSS) for Lithuanian population. Health Qual Life Outcomes. 2021;19:54. doi: 10.1186/s12955-020-01659-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortescue R, Kew KM, Leung MST. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2020;9:CD011293. doi: 10.1002/14651858.CD011293.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernández CD, Casanello P, Harris PR, Castro-Rodríguez JA, Iturriaga C, Perez-Mateluna G, Farías M, Urzúa M, Hernandez C, Serrano C, Sandoval M, Hoyos-Bachiloglu R, Uauy R, Borzutzky A. Early origins of allergy and asthma (ARIES): study protocol for a prospective prenatal birth cohort in Chile. BMC Pediatr. 2020;20:164. doi: 10.1186/s12887-020-02077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jörg L, Gschwend A, Poletti SC, Caversaccio MD, Helbling A. Cough from an allergological as well as from the ENT aspect. Ther Umsch. 2021;78:165–170. doi: 10.1024/0040-5930/a001255. [DOI] [PubMed] [Google Scholar]
- 13.Nakagome K, Nagata M. Allergen immunotherapy in asthma. Pathogens. 2021;10:1406. doi: 10.3390/pathogens10111406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson HS. Future directions in allergen immunotherapy. Allergy Asthma Proc. 2022;43:356–362. doi: 10.2500/aap.2022.43.210098. [DOI] [PubMed] [Google Scholar]
- 15.Nelson HS. 2020 updated asthma guidelines: allergen immunotherapy. J Allergy Clin Immunol. 2020;146:1286–1287. doi: 10.1016/j.jaci.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Peng H, Rao K. The secondary prevention effect and influence on serum sIgG4, IL-27 and IL-33 levels of subcutaneous immunotherapy in children with allergic rhinitis and cough variant asthma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;34:793–798. doi: 10.13201/j.issn.2096-7993.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yepes-Nuñez JJ, Guyatt GH, Gómez-Escobar LG, Pérez-Herrera LC, Chu AWL, Ceccaci R, Acosta-Madiedo AS, Wen A, Moreno-López S, MacDonald M, Barrios M, Chu X, Islam N, Gao Y, Wong MM, Couban R, Garcia E, Chapman E, Oykhman P, Chen L, Winders T, Asiniwasis RN, Boguniewicz M, De Benedetto A, Ellison K, Frazier WT, Greenhawt M, Huynh J, Kim E, LeBovidge J, Lind ML, Lio P, Martin SA, O’Brien M, Ong PY, Silverberg JI, Spergel J, Wang J, Wheeler KE, Schneider L, Chu DK. Allergen immunotherapy for atopic dermatitis: systematic review and meta-analysis of benefits and harms. J Allergy Clin Immunol. 2023;151:147–158. doi: 10.1016/j.jaci.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Fritzsching B, Contoli M, Porsbjerg C, Buchs S, Larsen JR, Elliott L, Rodriguez MR, Freemantle N. Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: results from the REACT study, a retrospective cohort study. Lancet Reg Health Eur. 2021;13:100275. doi: 10.1016/j.lanepe.2021.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo W, Hu J, Xu W, Dong J. Distinct spatial and temporal roles for Th1, Th2, and Th17 cells in asthma. Front Immunol. 2022;13:974066. doi: 10.3389/fimmu.2022.974066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong P, Liu T, Huang H, Yuan Y, Zhang W, Fu L, Chen Y. IL-27 overexpression alleviates inflammatory response in allergic asthma by inhibiting Th9 differentiation and regulating Th1/Th2 balance. Immunopharmacol Immunotoxicol. 2022;44:712–718. doi: 10.1080/08923973.2022.2077755. [DOI] [PubMed] [Google Scholar]
- 21.Saikumar Jayalatha AK, Hesse L, Ketelaar ME, Koppelman GH, Nawijn MC. The central role of IL-33/IL-1RL1 pathway in asthma: from pathogenesis to intervention. Pharmacol Ther. 2021;225:107847. doi: 10.1016/j.pharmthera.2021.107847. [DOI] [PubMed] [Google Scholar]
- 22.Hu H, Dai J, Zheng X, Wu J, Wu L, Luo W, Sun B. The relationship of D. pteronyssinus allergic component sIgE and sIgG4 in house dust mite allergic rhinitis or/and allergic asthma patients. Allergy Asthma Proc. 2023;44:100–105. doi: 10.2500/aap.2023.44.220078. [DOI] [PubMed] [Google Scholar]
- 23.Wen SL, Li F, Zhao F, Zuo JJ, Deng YQ, Zhang W, Tao ZZ. Programmed cell death protein 1 and its ligands regulate immune balance in allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;55:384–390. doi: 10.3760/cma.j.cn115330-20190618-00392. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Tan Y, Zhang L, Zheng L, Cui Y, Han L, Xie J, Zhang M, An X. Effects of budesonide plus vitamin ad on children with bronchial asthma and the effect on serum ige and c-reactive protein. J Mod Pharmacol Pathol. 2023;1:10. [Google Scholar]
- 25.Lu J, Chang T, Hao M, Liu L, Yin Z. Effect of targeted nursing on bronchoscopic alveolar lavage in the treatment of lobar pulmonary infection in children. J Mod Nurs Pract Res. 2024;4:13. [Google Scholar]
- 26.Kamel MA, Selim ES, Tantawy EA, Elgendy A, Abdulmageed A, Anis RH. Association of serum CD14 level and functional polymorphism C-159T in the promoter region of CD14 gene with allergic rhinitis. Clin Exp Med. 2023;23:4861–4869. doi: 10.1007/s10238-023-01097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]