Abstract
Background: To evaluate the prognostic value of echocardiography parameters, T lymphocyte subpopulations, NF-κB, and CD64 levels in neonatal sepsis. Methods: A retrospective analysis was conducted on 78 neonates treated for sepsis between January 2018 and December 2022, comprising 64 with poor prognosis and 14 with good prognosis. Among them, 51 were critically ill and 27 were non-critically ill. Echocardiographic parameters, T-lymphocyte subpopulations, NF-κB, and CD64 levels were compared across different prognosis and severity groups. Factors influencing prognosis were identified through multivariate logistic regression analysis. Results: The left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), CD3+, and CD4+ T lymphocyte levels in critically ill neonates were (61.15±8.22)%, (32.26±6.61)%, (45.56±7.12)%, and (26.61±6.80)%, respectively, significantly lower than those of non-critically ill neonates (all P < 0.05). The levels of NF-κB and CD64 in critically ill neonates were (18.11±2.61) mg/L and (7.42±1.15)%, respectively, significantly higher than those of non-critically ill neonates (all P < 0.05). Logistic regression analysis showed that LVEF, LVFS, CD4+, CD64, and disease severity were the factors influencing prognosis in neonatal sepsis (all P < 0.05). The area under the ROC curve for the logistic regression equation in predicting prognosis in neonatal sepsis was 0.878, with sensitivity and specificity of 85.30% and 84.10%, respectively. Conclusion: Echocardiography parameters, T lymphocyte subpopulations, NF-κB, and CD64 levels are associated with neonatal sepsis severity. LVEF, LVFS, CD4+ T lymphocytes, CD64, and disease severity are linked to prognosis, suggesting their potential as prognostic indicators for neonatal sepsis.
Keywords: Echocardiography, T lymphocyte subsets, nuclear factor-κB, CD64
Introduction
Neonatal sepsis is a prevalent and severe infectious condition that significantly endangers the health and lives of newborns [1-3]. It is a systemic infection caused by pathogens such as bacteria, viruses, or fungi. This unfavorable condition is particularly concerning due to its high incidence and mortality rate in newborns. Previous research indicated that the global incidence of neonatal sepsis is approximately 2,202 cases per 100,000 live births, with a mortality rate ranging between 11% and 19% [4]. These figures underscore neonatal sepsis as a critical threat to infant health. Despite advancements in medical technology, neonatal sepsis still poses a risk of poor outcome, especially in severe cases. Therefore, identifying reliable and effective methods to predict the prognosis of neonatal sepsis is crucial, as they could enable timely intervention toimprove survival rates [4,5].
Echocardiography has emerged as a promising diagnostic tool in this context. It is a non-invasive, repeatable, and visual method extensively used in diagnosing and assessing cardiovascular diseases [6]. Current research has revealed a correlation between echocardiographic parameters related to myocardial injury and the severity of neonatal sepsis, suggesting that echocardiography could be a valuable tool for assessing the severity and prognosis of neonatal sepsis [7].
In addition to echocardiography, specific immune biomarkers have been identified as significant for evaluating the prognosis of various diseases. Among these, peripheral blood T lymphocyte subsets, nuclear factor-κB (NF-κB), and CD64 have been extensively studied and are recognized for their prognostic significance [8-10]. However, there are few reports on their application value for neonatal sepsis.
Our study aims to address this gap by comparing echocardiographic parameters and levels of T lymphocyte subsets, NF-κB, and CD64 in infants exhibiting varying severities and prognoses of neonatal sepsis. Through comprehensive analysis of these biomarkers, we seek to identify key indicators that can enhance the accuracy of prognosis in neonatal sepsis cases. This research could provide valuable insight into managing and treating of this severe condition.
Methods
This retrospective study was approved by the institutional review board of Beijing Anzhen Hospital and conducted in accordance with the ethical guidelines outlined in the Declaration of Helsinki.
General information
Neonates treated for sepsis in Beijing Anzhen Hospital from January 2018 to December 2022 were screened. Inclusion criteria: (1) diagnosis consistent with the “Expert Consensus on the Diagnosis and Treatment of Neonatal Sepsis” [10]; (2) age < 28 days; (3) informed consent from the guardians. Exclusion criteria: (1) congenital or hereditary metabolic diseases; (2) other severe diseases such as malignant tumors or immune system diseases.
Sample collection
Peripheral venous blood samples were collected from all enrolled neonates in a fasting state.
Laboratory analysis
T lymphocyte subsets and CD64 levels were measured using a flow cytometer (Epies-XI4, single laser, 4-color flow cytometer, Beckman Coulter, USA) with kits provided by Beckman Coulter (Lot Number: TLYM20240801). NF-κB levels were detected using the enzyme-linked immunosorbent assay (ELISA) method, with kits purchased from Shanghai Roche Company (Lot Number: ELISANKB20240801). Cardiac ultrasound examination was conducted using a Doppler ultrasound diagnostic apparatus (EPIQ 7C, Philips), equipped with an S5-1 probe (frequency 1.0-5.0 MHz). The examination was performed in a calm state to measure left ventricular fractional shortening (LVFS) and calculate left ventricular ejection fraction (LVEF). All examinations were conducted by the same experienced sonographer to ensure consistency.
Severity assessment
The severity of the neonates’ condition was assessed using the Neonatal Critical Illness Score (NCIS), which assessed ten parameters (heart rate, blood pressure, respiration, PaO2, pH value, Na ions, K ions, Cr/BUN, hematocrit, and gastrointestinal performance). A score ≤ 90 indicated critical illness, while a score > 90 indicated non-critical illness.
Outcome classification
Neonates were categorized into Good Prognosis and Poor Prognosis groups based on clinical outcomes at discharge. A good prognosis was defined by survival, stabilization of vital signs, and absence of severe complications, while a Poor Prognosis was defined by the presence of severe complications, prolonged hospitalization, or death.
Statistical analysis
Statistical analysis was performed using SPSS software version 22.0 (IBM et al., USA). Quantitative data were expressed as mean ± standard deviation, with group differences analyzed by t-tests. Categorical data were reported as frequencies or percentages, and group differences were evaluated using the chi-square test. Logistic regression analysis was employed for multifactor analysis. The predictive value was analyzed using the Receiver Operating Characteristic (ROC) curve. A P-value of less than 0.05 was deemed significant.
Results
Comparison of echocardiographic parameters and T lymphocyte subsets between neonates of different genders
There were no significant differences in echocardiographic parameters or T lymphocyte subsets between neonates of different genders (all P > 0.05), as shown in Table 1.
Table 1.
Comparison of echocardiographic parameters and T lymphocyte subsets in neonates of different genders
| Indicator | Male (n = 50) | Female (n = 28) | t/χ2 | P |
|---|---|---|---|---|
| LVEF (%) | 71.92±8.10 | 72.60±6.82 | -0.376 | 0.708 |
| Ascending aortic diameter (mm) | 8.11±1.03 | 8.19±1.10 | -0.321 | 0.749 |
| LVFS (%) | 36.53±6.80 | 36.90±6.11 | -0.239 | 0.812 |
| CD3+ (%) | 47.83±7.28 | 48.82±6.01 | -0.612 | 0.543 |
| CD4+ (%) | 30.10±6.92 | 31.40±7.22 | -0.784 | 0.436 |
| CD8+ (%) | 18.45±3.12 | 18.38±3.00 | 0.096 | 0.923 |
| NF-κB (mg/L) | 15.54±2.80 | 15.02±2.11 | 0.855 | 0.395 |
| CD64 (%) | 6.06±1.15 | 6.33±1.09 | -1.013 | 0.314 |
Notes: LVEF: left ventricular ejection fraction. LVFS: left ventricular fractional shortening.
Comparison of echocardiographic parameters and T lymphocyte subsets between neonates of different gestational ages
There were no significant differences in echocardiographic parameters or T lymphocyte subsets between neonates of different gestational ages (all P > 0.05), as shown in Table 2.
Table 2.
Comparison of echocardiographic parameters and T lymphocyte subsets in neonates of different gestational ages
| Indicator | Gestational Age < 37 weeks (n = 50) | Gestational Age ≥ 37 weeks (n = 28) | t/χ2 | P |
|---|---|---|---|---|
| LVEF (%) | 71.12±8.90 | 72.39±7.10 | -0.648 | 0.519 |
| Ascending aortic diameter (mm) | 8.14±1.02 | 8.21±1.10 | -0.283 | 0.778 |
| LVFS (%) | 36.10±6.23 | 37.12±6.95 | -0.665 | 0.508 |
| CD3+ (%) | 48.92±7.04 | 50.01±6.90 | -0.661 | 0.511 |
| CD4+ (%) | 29.10±6.99 | 30.04±7.81 | -0.546 | 0.587 |
| CD8+ (%) | 18.20±3.10 | 18.28±2.99 | -0.111 | 0.912 |
| NF-κB (mg/L) | 16.65±2.90 | 15.50±2.60 | 1.742 | 0.086 |
| CD64 (%) | 6.50±1.10 | 6.04±1.09 | 1.777 | 0.08 |
Notes: LVEF: left ventricular ejection fraction. LVFS: left ventricular fractional shortening.
Comparison of echocardiographic parameters and immune markers between critically ill and non-critically ill neonates
Critically ill neonates had significantly lower LVEF, LVFS, CD3+, and CD4+ T lymphocyte levels and significantly higher NF-κB and CD64 levels than non-critically ill neonates (all P < 0.05). However, there were no significant differences in gender, gestational age, ascending aortic diameter, or CD8+ T lymphocyte levels between critically ill and non-critically ill neonates (all P > 0.05), as shown in Table 3.
Table 3.
Comparison of echocardiographic parameters and T lymphocyte subsets in neonates with different severity levels
| Indicator | Critically Ill (n = 51) | Non-Critically Ill (n = 27) | t/χ2 | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 32 (62.75%) | 18 (66.67%) | 0.118 | 0.731 |
| Female | 19 (37.25%) | 9 (33.33%) | ||
| Gestational Age | ||||
| < 37 weeks | 33 (64.71%) | 17 (62.96%) | 0.023 | 0.879 |
| ≥ 37 weeks | 18 (35.29%) | 10 (37.04%) | ||
| LVEF (%) | 61.15±8.22 | 73.77±7.81 | -6.561 | 0 |
| Ascending aortic diameter (mm) | 8.03±1.12 | 8.29±1.08 | -0.987 | 0.327 |
| LVFS (%) | 32.26±6.61 | 38.73±6.80 | -4.072 | 0 |
| CD3+ (%) | 45.56±7.12 | 50.36±6.69 | -2.891 | 0.005 |
| CD4+ (%) | 26.61±6.80 | 32.97±7.70 | -3.753 | 0 |
| CD8+ (%) | 18.51±3.05 | 18.31±2.94 | 0.279 | 0.781 |
| NF-κB (mg/L) | 18.11±2.61 | 13.75±2.23 | 7.367 | 0 |
| CD64 (%) | 7.42±1.15 | 5.63±1.06 | 6.715 | 0 |
Notes: LVEF: left ventricular ejection fraction. LVFS: left ventricular fractional shortening.
Identification of factors associated with prognosis in neonates with sepsis
Neonates with poor prognoses had significantly lower LVEF, LVFS, CD3+, and CD4+ T lymphocyte levels but significantly higher CD64 levels than the neonates with good prognoses (all P < 0.05). However, there were no significant differences in gender, gestational age, ascending aortic diameter, CD8+ T lymphocyte levels, or NF-κB levels between neonates with poor and good prognosis (all P > 0.05), as shown in Table 4.
Table 4.
Comparison of echocardiographic parameters and T lymphocyte subsets in neonates with different prognoses
| Indicator | Good Prognosis (n = 64) | Poor Prognosis (n = 14) | t/χ2 | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 42 (65.63%) | 8 (57.14%) | 0.359 | 0.549 |
| Female | 22 (34.38%) | 6 (42.86%) | ||
| Gestational Age | ||||
| < 37 weeks | 41 (64.06%) | 9 (64.29%) | 0 | 0.987 |
| ≥ 37 weeks | 23 (35.94%) | 5 (35.71%) | ||
| LVEF (%) | 69.94±8.10 | 59.80±8.02 | 5.506 | 0 |
| Ascending aortic diameter (mm) | 8.08±1.10 | 8.17±1.12 | -0.355 | 0.723 |
| LVFS (%) | 36.61±4.43 | 31.77±4.20 | 4.893 | 0 |
| CD3+ (%) | 49.91±5.52 | 43.74±5.17 | 5.031 | 0 |
| CD4+ (%) | 31.14±6.15 | 25.79±6.22 | 3.791 | 0 |
| CD8+ (%) | 18.24±3.40 | 18.70±3.15 | -0.612 | 0.543 |
| NF-κB (mg/L) | 16.17±2.84 | 17.16±2.90 | -1.513 | 0.135 |
| CD64 (%) | 5.89±1.04 | 7.98±1.01 | -8.912 | 0 |
| Severity | ||||
| Critically Ill | 38 (59.38%) | 13 (92.86%) | 4.307 | 0.038 |
| Non-Critically Ill | 26 (40.63%) | 1 (7.14%) |
Notes: LVEF: left ventricular ejection fraction. LVFS: left ventricular fractional shortening.
Logistic regression analysis of factors influencing neonatal sepsis prognosis
A logistic regression analysis was performed with LVEF, LVFS, CD3+, CD4+, CD64, and severity (non-critically ill = 0, critically ill = 1) as independent variables and prognosis (good prognosis = 0, poor prognosis = 1) as the dependent variable. The assignment table is shown in Table 5. The results showed that LVEF, LVFS, CD4+, CD64, and severity were influencing factors for the prognosis of neonatal sepsis (P < 0.05), as shown in Table 6. The logistic regression equation Logit(P) = 5.541 + 0.922 × CD64 + 1.441 × severity - 0.654 × LVEF - 0.778 × LVFS - 0.541 × CD4 was used to perform ROC curve analysis, as shown in Figure 1. The area under the ROC curve for predicting poor prognosis in neonatal sepsis using this Logit value was 0.878, with sensitivity and specificity of 85.30% and 84.10%, respectively.
Table 5.
Variable assignment table
| Variable | Symbol | Assignment |
|---|---|---|
| Prognosis | Y | Good prognosis = 0, Poor prognosis = 1 |
| LVEF | X1 | Measured value |
| LVFS | X2 | Measured value |
| CD3+ | X3 | Measured value |
| CD4+ | X4 | Measured value |
| CD64 | X5 | Measured value |
| Severity | X6 | Non-critically ill = 0, Critically ill = 1 |
Notes: LVEF: left ventricular ejection fraction. LVFS: left ventricular fractional shortening.
Table 6.
Logistic regression analysis results
| Factor | b | SE | Walds | P | OR (95% CI) |
|---|---|---|---|---|---|
| LVEF | -0.654 | 0.213 | 9.427 | 0 | 0.520 (0.343-0.789) |
| LVFS | -0.778 | 0.22 | 12.506 | 0 | 0.459 (0.298-0.707) |
| CD3+ | -0.226 | 0.431 | 0.275 | 0.665 | 0.798 (0.343-1.857) |
| CD4+ | -0.541 | 0.187 | 8.37 | 0 | 0.582 (0.404-0.840) |
| CD64 | 0.922 | 0.243 | 14.396 | 0 | 2.514 (1.562-4.048) |
| Severity | 1.441 | 0.398 | 13.109 | 0 | 4.225 (1.937-9.217) |
| Constant | 5.541 | 1.032 | 28.828 | 0 | - |
Figure 1.
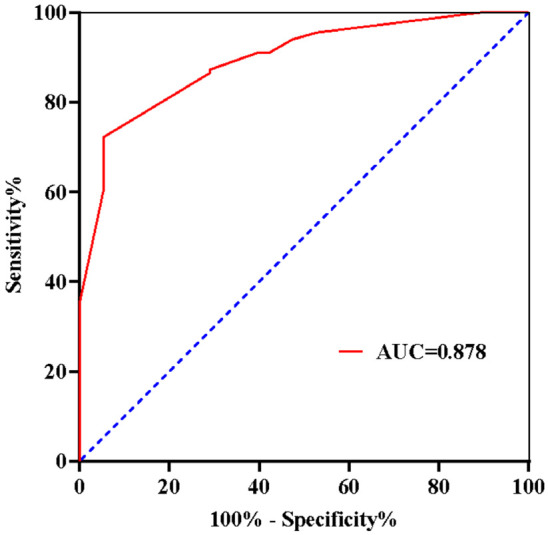
ROC curve for the equation predicting neonatal sepsis mortality.
Discussion
Neonatal sepsis is a severe infectious condition that occurs within the first 28 days after birth, primarily caused by bacterial, viral, or fungal infections [11,12]. It is more prevalent in preterm infants and low-birth-weight infants and is one of the leading causes of neonatal mortality [13]. The pathogenesis of neonatal sepsis is not yet fully understood, but it is generally believed to be associated with multiple factors. The underdeveloped immune system of newborns, particularly in the early postnatal stages, results in a weaker immune function, making neonates more susceptible to infections [14,15]. When pathogens invade the body, the immune system initiates an inflammatory response to combat the infection. However, in neonates, the inflammatory response is often inadequate, leading to uncontrolled infection, exacerbating inflammation and infection severity, and ultimately leading to sepsis [15,16]. This is one of the reasons why infectious diseases such as neonatal sepsis are particularly severe in newborns. Therefore, early diagnosis and prognosis of neonatal sepsis can guide clinical treatment decisions and prevent decompensation.
Our study showed that critically ill neonates had significantly lower LVEF, LVFS, CD3+, and CD4+ T lymphocyte levels and significantly higher NF-κB and CD64 levels than non-critically ill neonates. This indicates that echocardiographic parameters, T lymphocyte subsets, NF-κB, and CD64 levels can reflect the severity of neonatal sepsis [17,18].
Critically ill neonatal sepsis is a severe form of neonatal sepsis, characterized by significant pathophysiologic changes, including circulatory dysfunction and organ damage. In such cases, pathogen invasion triggers an inflammatory response by activation of the NF-κB pathway, leading to increased release of inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), causing myocardial inflammation and injury [19,20]. This myocardial damage results in decreased cardiac contractility, reflected by significantly reduced LVEF and LVFS and elevated NF-κB levels. CD3+ and CD4+ T lymphocytes play critical roles in the immune system, reflecting the body’s immune function [21,22]. In critically ill neonatal sepsis, immune cells such as macrophages and lymphocytes are excessively activated. This overactivation triggers apoptotic signaling pathways, leading to immune cell apoptosis, thus suppressing immune function and reducing resistance to infection. CD64 is a cell surface marker primarily expressed on monocytes and neutrophils [23]. In critically ill neonatal sepsis, bacterial infection activates inflammatory cells such as monocytes and neutrophils, increasing CD64 expression.
Furthermore, our study shows that neonates with poor prognosis had significantly lower LVEF, LVFS, CD3+, and CD4+ T lymphocyte levels and significantly higher CD64 levels as well as a greater proportion of critically ill cases compared to those with good prognosis [24]. The significant decrease in CD4+ T lymphocyte levels observed in our study is consistent with the findings from a previous study [25], which reported that a weakened immune response in neonates, characterized by lower CD4+ levels, hinders the clearance of pathogens, thereby worsening the clinical course of sepsis. This suggests a correlation between poor prognosis in neonatal sepsis and these indicators. Multivariate analysis further revealed that LVEF, LVFS, CD4+, CD64, and disease severity are significant factors influencing the prognosis of neonatal sepsis. Decreased LVEF and LVFS indicate reduced cardiac function and output, leading to insufficient blood circulation, impaired organ perfusion, and decreased oxygen supply, which can aggravate sepsis severity and worsens prognosis. Reduced CD4+ T lymphocyte levels reflect decreased immune function, making it difficult for neonates to eliminate pathogens, worsening sepsis and contributing to poor prognosis. Elevated CD64 levels reflect the heightened inflammatory response, likely associated with immune cell activation and release of inflammatory mediators. Excessive inflammatory responses cause tissue and organ damage, possibly leading to systemic inflammatory response syndrome and multiple organ dysfunction syndrome, significantly increasing treatment difficulty and causing poor prognosis [26,27].
The ROC curve analysis of the logistic regression equation incorporating the above indicators showed a strong predictive value for poor prognosis in neonatal sepsis, with an AUC of 0.878, a sensitivity of 85.30%, and a specificity 84.10%. Therefore, monitoring T lymphocyte subsets, NF-κB, and CD64 levels in clinical practice can help assess the risk of poor prognosis in neonates. The innovation of this study lies in combining echocardiographic parameters with immunological markers like T lymphocyte subsets, NF-κB, and CD64, offering a novel approach for more accurate prognosis prediction in neonatal sepsis.
However, there are several limitations to this study. First, as a retrospective study, the data collection and analysis may be subject to selection bias, and the findings may not be generalizable due to the single-center nature of the study. Second, although the biomarkers investigated show potential for predicting neonatal sepsis prognosis, their clinical utility has yet to be validated in larger, multicenter studies. Moreover, the study did not explore the underlying mechanisms, particularly the roles of NF-κB and CD64 in the pathophysiology of neonatal sepsis. Future research should expand the sample size, employ prospective study designs to confirm the clinical applicability of these biomarkers, and enhance the mechanistic understanding of their involvement in neonatal sepsis.
In conclusion, echocardiographic parameters, T lymphocyte subsets, NF-κB, and CD64 levels are associated with the severity of neonatal sepsis. Additionally, LVEF, LVFS, CD4+ T lymphocytes, CD64, and disease severity are significant indicators of the prognosis.
Acknowledgements
This work was supported by Beijing Clinical Key Specialty Project (2-1-2-18-300).
Disclosure of conflict of interest
None.
References
- 1.Procianoy RS, Silveira RC. The challenges of neonatal sepsis management. J Pediatr (Rio J) 2020;96(Suppl 1):80–86. doi: 10.1016/j.jped.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celik IH, Hanna M, Canpolat FE, Mohan Pammi. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res. 2022;91:337–350. doi: 10.1038/s41390-021-01696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bethou A, Bhat BV. Neonatal sepsis-newer insights. Indian J Pediatr. 2022;89:267–273. doi: 10.1007/s12098-021-03852-z. [DOI] [PubMed] [Google Scholar]
- 4.Sands K, Spiller OB, Thomson K, Portal EAR, Iregbu KC, Walsh TR. Early-onset neonatal sepsis in low- and middle-income countries: current challenges and future opportunities. Infect Drug Resist. 2022;15:933–946. doi: 10.2147/IDR.S294156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molloy EJ, Bearer CF. Paediatric and neonatal sepsis and inflammation. Pediatr Res. 2022;91:267–269. doi: 10.1038/s41390-021-01918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Basmaci R, Titomanlio L, Sun B, Mercier JC. Neonatal sepsis: within and beyond China. Chin Med J (Engl) 2020;133:2219–2228. doi: 10.1097/CM9.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindner JR. Contrast echocardiography: current status and future directions. Heart. 2021;107:18–24. doi: 10.1136/heartjnl-2020-316662. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Zhou H, Xu J, Lu Y, Ji X, Yao Y, Chao H, Zhang J, Zhang X, Yao S, Wu Y, Wan J. Different T-cell subsets in glioblastoma multiforme and targeted immunotherapy. Cancer Lett. 2021;496:134–143. doi: 10.1016/j.canlet.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5:209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cong S, Ma T, Di X, Tian C, Zhao M, Wang K. Diagnostic value of neutrophil CD64, procalcitonin, and interleukin-6 in sepsis: a meta-analysis. BMC Infect Dis. 2021;21:384. doi: 10.1186/s12879-021-06064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis AL, Carcillo JA, Aneja RK, Deymann AJ, Lin JC, Nguyen TC, Okhuysen-Cawley RS, Relvas MS, Rozenfeld RA, Skippen PW, Stojadinovic BJ, Williams EA, Yeh TS, Balamuth F, Brierley J, de Caen AR, Cheifetz IM, Choong K, Conway E Jr, Cornell T, Doctor A, Dugas MA, Feldman JD, Fitzgerald JC, Flori HR, Fortenberry JD, Graciano AL, Greenwald BM, Hall MW, Han YY, Hernan LJ, Irazuzta JE, Iselin E, van der Jagt EW, Jeffries HE, Kache S, Katyal C, Kissoon N, Kon AA, Kutko MC, MacLaren G, Maul T, Mehta R, Odetola F, Parbuoni K, Paul R, Peters MJ, Ranjit S, Reuter-Rice KE, Schnitzler EJ, Scott HF, Torres A Jr, Weingarten-Abrams J, Weiss SL, Zimmerman JJ, Zuckerberg AL. The American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: executive summary. Pediatr Crit Care Med. 2017;18:884–890. doi: 10.1097/PCC.0000000000001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wattal C, Kler N, Oberoi JK, Fursule A, Kumar A, Thakur A. Neonatal sepsis: mortality and morbidity in neonatal sepsis due to multidrug-resistant (MDR) organisms: part 1. Indian J Pediatr. 2020;87:117–121. doi: 10.1007/s12098-019-03106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rueda ZV, Aguilar Y, Maya MA, López L, Restrepo A, Garcés C, Morales O, Roya-Pabón C, Trujillo M, Arango C, Copete ÁR, Vera C, Giraldo MR, Herrera M, Vélez LA. Etiology and the challenge of diagnostic testing of community-acquired pneumonia in children and adolescents. BMC Pediatr. 2022;22:169. doi: 10.1186/s12887-022-03235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milton R, Gillespie D, Dyer C, Taiyari K, Carvalho MJ, Thomson K, Sands K, Portal EAR, Hood K, Ferreira A, Hender T, Kirby N, Mathias J, Nieto M, Watkins WJ, Bekele D, Abayneh M, Solomon S, Basu S, Nandy RK, Saha B, Iregbu K, Modibbo FZ, Uwaezuoke S, Zahra R, Shirazi H, Najeeb SU, Mazarati JB, Rucogoza A, Gaju L, Mehtar S, Bulabula ANH, Whitelaw AC, Walsh TR BARNARDS Group. Chan GJ. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Glob Health. 2022;10:e661–e672. doi: 10.1016/S2214-109X(22)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Xiao Y, Zhang G, Li H, Zhao J, Chen M, Chen F, Liu L, Li Y, Peng L, Zhao F, Yang D, Wen Z, Wu L, Wu S, Sun Y, Wang Y, Chen L, Wang X, Wang L, Li W, Qiu H, Chen Y, Gao Z, Ren L, Wang J. Identification of priority pathogens for aetiological diagnosis in adults with community-acquired pneumonia in China: a multicentre prospective study. BMC Infect Dis. 2023;23:231. doi: 10.1186/s12879-023-08166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beudeker CR, Vijlbrief DC, van Montfrans JM, Rooijakkers SHM, van der Flier M. Neonatal sepsis and transient immunodeficiency: potential for novel immunoglobulin therapies? Front Immunol. 2022;13:1016877. doi: 10.3389/fimmu.2022.1016877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glaser MA, Hughes LM, Jnah A, Newberry D. Neonatal sepsis: a review of pathophysiology and current management strategies. Adv Neonatal Care. 2021;21:49–60. doi: 10.1097/ANC.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 18.Barnabei L, Laplantine E, Mbongo W, Rieux-Laucat F, Weil R. NF-κB: at the borders of autoimmunity and inflammation. Front Immunol. 2021;12:716469. doi: 10.3389/fimmu.2021.716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen B, Hanauer SB, Gibson PR, Turner JR, Hart J, Rubin DT. Segmental histological normalisation occurs in ulcerative colitis but does not improve clinical outcomes. J Crohns Colitis. 2020;14:1345–1353. doi: 10.1093/ecco-jcc/jjaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Zheng Y, Sheng J, Han Y, Yang Y, Pan H, Yao J. CD3+CD4-CD8- (double-negative) T cells in inflammation, immune disorders and cancer. Front Immunol. 2022;13:816005. doi: 10.3389/fimmu.2022.816005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel Khalik H, Humphries B, Zoratti M, Axelrod D, Kruse C, Ristevski B, Rajaratnam K, Gardner M, Tarride JÉ, Johal H. Reverse total shoulder arthroplasty is the most cost-effective treatment strategy for proximal humerus fractures in older adults: a cost-utility analysis. Clin Orthop Relat Res. 2022;480:2013–2026. doi: 10.1097/CORR.0000000000002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haug T, Aigner M, Peuser MM, Strobl CD, Hildner K, Mougiakakos D, Bruns H, Mackensen A, Völkl S. Human double-negative regulatory T-cells induce a metabolic and functional switch in effector T-cells by suppressing mTOR activity. Front Immunol. 2019;10:883. doi: 10.3389/fimmu.2019.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berastegui-Cabrera J, Aguilar-Guisado M, Crespo-Rivas JC, López-Verdugo M, Merino L, Escoresca-Ortega A, Calero-Acuña C, Carrasco-Hernández L, Toral-Marín JI, Abad-Arranz M, Ramírez-Duque N, Barón-Franco B, Pachón J, Álvarez-Marín R, Sánchez-Céspedes J. Prepandemic viral community-acquired pneumonia: diagnostic sensitivity and specificity of nasopharyngeal swabs and performance of clinical severity scores. J Med Virol. 2023;95:e28317. doi: 10.1002/jmv.28317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rostamzadeh D, Yousefi M, Haghshenas MR, Ahmadi M, Dolati S, Babaloo Z. mTOR signaling pathway as a master regulator of memory CD8+ T-cells, Th17, and NK cells development and their functional properties. J Cell Physiol. 2019;234:12353–12368. doi: 10.1002/jcp.28042. [DOI] [PubMed] [Google Scholar]
- 25.Boix-Palop L, Vergara A, Padilla E, Martínez D, Blanco A, Pérez J, Calbo E, Vila J, Casals-Pascual C. Evaluation of plasma lipocalin-2 as a predictor of etiology and severity in adult patients with community-acquired pneumonia. Microorganisms. 2023;11:1160. doi: 10.3390/microorganisms11051160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venge P, Douhan-Håkansson L, Garwicz D, Peterson C, Xu S, Pauksen K. Human neutrophil lipocalin as a superior diagnostic means to distinguish between acute bacterial and viral infections. Clin Vaccine Immunol. 2015;22:1025–1032. doi: 10.1128/CVI.00347-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


