Abstract
Objective: To investigate the correlation between residual Syntax score (rSS) and long-term prognosis in diabetic patients with renal insufficiency undergoing percutaneous coronary intervention (PCI). Methods: In this retrospective study, we included 510 patients with coronary heart disease, diabetes, and renal insufficiency who received PCI at the Third People’s Hospital of Chengdu from July 2018 to December 2020. Patients were divided into three groups based on their eGFR levels: 113 patients with eGFR ≥ 60 mL/min/1.73 m2, 256 patients with eGFR between 30 and 60 mL/min/1.73 m2, and 141 patients with eGFR < 30 mL/min/1.73 m2. Revascularization was quantified using the residual SYNTAX score (rSS), with an rSS > 8 indicating incomplete revascularization. We collected baseline data on cardiovascular adverse events and followed up with patients for 12 months, analyzing the correlations between rSS and biochemical markers such as blood glucose, uric acid, urea, serum creatinine, and eGFR, as well as the relationship between major adverse cardiovascular events (MACE) and rSS. Results: Univariate analysis identified myocardial infarction (MI), β-blocker use, and follow-up duration as factors significantly associated with the long-term prognosis of diabetic patients with renal insufficiency after PCI (P < 0.05). MI (OR=3.053, P=0.009), β-blocker use (OR=3.134, P=0.009), and follow-up duration (OR=0.998, P=0.05) were independent risk factors for long-term prognosis in these patients. rSS was positively correlated with blood glucose (r=0.973, P=0.000), uric acid (r=0.933, P=0.000), urea (r=0.907, P=0.000), serum creatinine (r=0.588, P=0.000), and eGFR (r=0.623, P=0.000). Syntax score was also positively correlated with long-term prognosis (OR=0.138, P=0.001). Conclusion: The rSS is a valuable tool for evaluating independent risk factors such as incomplete revascularization, MI, β-blocker use, and follow-up duration, all of which are positively correlated with the long-term prognosis of diabetic patients with renal insufficiency after PCI.
Keywords: Residual Syntax score, percutaneous coronary intervention, diabetes, renal insufficiency, long-term prognosis
Introduction
With the continuous improvement in living standards, coronary heart disease and diabetes have become increasingly prevalent in clinical practice. Both conditions have a chronic course and can significantly affect individuals’ physical and mental well-being [1]. Although the mechanisms of coronary heart disease in diabetic patients differ, there is a clear correlation between the two conditions [2]. Diabetes can lead to kidney damage, clinically referred to as diabetic nephropathy [3]. According to relevant data, approximately 25% of diabetic patients in China concurrently experience varying degrees of kidney disease [4]. As the population ages, the prevalence of these diseases is rising, posing a significant threat to public health and increasing the burden on families and society.
Diabetes and coronary heart disease are metabolic disorders with shared risk factors that mutually influence each other [5]. Moreover, patients with coronary heart disease complicated by diabetes are at an increased risk of developing renal insufficiency, which adversely affects their health and quality of life. Coronary heart disease, also known as ischemic heart disease, has a high incidence [6]. Current treatment options include conventional drug therapy, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG) [7]. Among these, PCI is the most commonly utilized treatment. Advances in medical technology, particularly the widespread use of new eluting stents, have improved patient outcomes from PCI [8].
The Syntax score, a novel scoring method, predicts blood flow from the coronary arteries to the left ventricle [9]. It provides a semi-quantitative analysis of the anatomic characteristics of coronary heart disease and offers a reliable basis for deciding between PCI and CABG in patients with left main artery or multi-vessel disease. Additionally, it serves as a dependable predictor of long-term prognosis in patients with coronary artery disease [10]. Research [11] indicates that lower Syntax scores are associated with better cardiovascular outcomes compared to moderate or high Syntax scores. However, as complete revascularization has become the focus of PCI treatment, there is a lack of large-scale studies examining the impact of incomplete revascularization on patient outcomes [11]. The residual Syntax score (rSS) can be used to assess incomplete revascularization and has been shown to be a valuable prognostic factor in patients undergoing PCI. However, there is still a lack of relevant clinical research on the impact of rSS on the long-term prognosis of patients with chronic total occlusion [12]. Therefore, the rSS was used for related research analysis in this retrospective study.
Materials and methods
Study population
In our study, we strategically examined a cohort of 510 patients with coronary heart disease, diabetes, and renal insufficiency, admitted to the Third People’s Hospital of Chengdu from July 2018 to December 2020. The patients were categorized into three groups based on their estimated glomerular filtration rate (eGFR) levels: 113 patients with eGFR ≥ 60 mL/min per 1.73 m2, 256 patients with eGFR between 30 and 60 mL/min per 1.73 m2, and 141 patients with eGFR < 30 mL/min per 1.73 m2. These groups correspond to the higher eGFR group, the moderate eGFR group, and the lower eGFR group, respectively. The study utilized a convenience sampling method, and the patient selection process is detailed in Figure 1.
Figure 1.
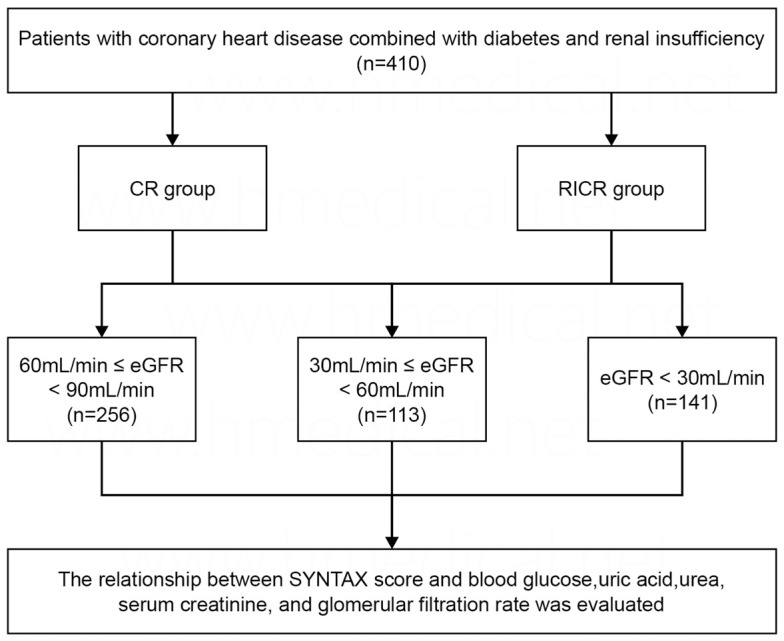
Flow chart. eGFR, estimated glomerular filtration rate; CR, complete revascularization; RICR, reasonable incomplete revascularization.
Inclusion criteria
① Age between 18 and 80 years; ② The diagnosis of type 2 diabetes mellitus (T2DM) is based on a fasting blood glucose level ≥ 7.0 mmol/L, a 2-hour blood glucose level v 11.1 mmol/L after an oral glucose tolerance test, and a random blood glucose level v 11.1 mmol/L, with classic symptoms of hyperglycemia or hyperglycemic crisis; ③ Diagnosis of coronary heart disease [13]; ④ Diagnosis of renal insufficiency (estimated glomerular filtration rate (eGFR) < 90 ml/min/1.73 m2, CKD-EPI creatinine method) undergoing PCI; ⑤ Availability of complete medical records, interventional surgery reports, and relevant imaging data [14].
Exclusion criteria
① Patients lacking essential baseline information [15]; ② Patients who died during early hospitalization; ③ Patients with a history of CABG; ④ Patients without effective follow-up information; ⑤ Patients with severe mechanical complications, valve dysfunction, or cardiomyopathy; ⑥ Patients with severe chronic lung disease, liver disease, hematologic disorders, malignant tumors, or a life expectancy of less than one year.
Data
Data collection
Data collection was designed by the researchers after consulting relevant literature and experts. The collected data included general patient information (e.g., age, gender, height, weight, body mass index [BMI], and smoking history), medical history, and comorbidities (e.g., PCI, stroke, chronic obstructive pulmonary disease, hypertension, and atrial fibrillation). Clinical data related to coronary heart disease include the types of coronary heart disease (e.g., stable angina pectoris, unstable angina pectoris, non-ST elevation myocardial infarction (MI), and ST elevation MI). Laboratory tests include hemoglobin, brain natriuretic peptide (BNP), eGFR, glycated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). Auxiliary examinations include color Doppler echocardiography to assess left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVED), and left ventricular end-systolic diameter (LVSD). Coronary angiography results include the number, severity, location, and pathologic features of diseased vessels, as well as PCI surgical data such as interventional therapy details, including the number, diameter, and length of implanted stents.
Scoring methods
The baseline SYNTAX score (bSS) and residual rSS were assessed based on pre- and post-coronary angiography results using the SYNTAX score calculator (Version 2.28) from www.syntaxscore.com. The scoring was conducted by two coronary intervention specialists. A lower rSS indicates more favorable outcomes. For patients scheduled for and undergoing short-term revascularization, the relative oversaturation after the final revascularization procedure is considered. A coronary artery diameter ≥ 1.5 mm with stenosis a 50% is defined as a diseased vessel, and the presence of one lesion is considered a positive result in coronary angiography.
Data collection method
The primary data source for this study was electronic medical and nursing records from the hospital information system. A retrospective collection of records for all hospitalized patients from July 2018 to December 2020 was conducted. Research subjects were selected by the researchers based on the inclusion and exclusion criteria, with data extraction completed independently. In case of conflicting opinions during data extraction, the research team resolved them. After completing data extraction, further verification of the research materials was conducted by the researchers.
Follow-up
The endpoint clinical follow-up is scheduled at 1, 3, 6, and 12 months, followed by annual clinical visits or telephone contacts. The primary endpoints include all-cause mortality and cardiac mortality, where any death not attributable to non-cardiac causes is classified as cardiac death. Secondary endpoints include MI, stroke, unplanned revascularization, readmission rates (with a review of inpatient medical records to identify any adverse events for readmitted patients), and major adverse cardiovascular events (MACCE), defined as a composite endpoint comprising all-cause death, MI, stroke, and unplanned revascularization.
All endpoints are evaluated by two independent cardiologists, with discrepancies resolved through consensus.
Main observation indicators and endpoint events
Main observation indicators
The primary endpoint is defined as a composite event of major adverse cardiovascular events (MACE), including all-cause mortality, recurrent MI, and unplanned revascularization during the follow-up period.
Secondary observation indicators
Secondary endpoints include all-cause mortality, death from cardiac causes, recurrent MI, and unplanned revascularization.
Relevant definitions
MI
Type 1: Characterized by the rupture, ulceration, or erosion of atherosclerotic plaque, leading to intracoronary thrombosis in one or more coronary arteries, resulting in decreased myocardial blood flow and/or distal embolism, ultimately causing MI. Type 2: Myocardial necrosis caused by an imbalance of myocardial oxygen supply and demand due to reasons other than unstable coronary plaque. Type 3: MI where no elevation of myocardial markers is detected, but the cause of death is confirmed as a heart attack. Type 4: MI related to PCI for treating coronary artery disease [16].
Diabetes
Glycosylated hemoglobin A1c 1 6.5%. Fasting blood glucose (FPG) c 7.0 mmol/L (fasting is defined as no calorie intake for at least 8 hours). 2-hour blood glucose d as nommol/L during an oral glucose tolerance test. In patients with typical hyperglycemia or hyperglycemic crisis, random blood glucose m><remommol/L [13].
Renal insufficiency
Defined as an estimated glomerular filtration rate (eGFR) of less than 90 ml/min/1.73 m2, calculated using the CKD-EPI creatinine method.
Cardiogenic death
Refers to death resulting from MI, exacerbation of heart failure, malignant arrhythmia events, or sudden unexplained death [9].
Unplanned revascularization
Refers to the need for ischemia-driven revascularization (PCI or CABG) during the follow-up period, excluding second-stage revascularization that is part of the short-term treatment plan.
New-onset stroke
Defined as the occurrence of an ischemic or hemorrhagic stroke during the follow-up period, confirmed by imaging and diagnosed by a neurologist [17].
Statistical methods
The study employed SPSS 23.0 and GraphPad Prism for data analysis and visualization. Continuous variables were assessed for normality and compared among groups using analysis of variance, while categorical variables were analyzed with the chi-square test. Kendall’s tau correlation was applied to examine the relationship between the Syntax score and MACCE. Logistic regression identified independent risk factors for prognosis. The ROC curve’s AUC assessed the predictive accuracy of the Syntax score, with significance set at P < 0.05.
Results
Univariate analysis of long-term prognosis in diabetic patients with renal insufficiency after PCI
Univariate analysis revealed that MI, β-blocker usage, and follow-up duration were significantly associated with the long-term prognosis of diabetic patients with renal insufficiency after treatment (both P < 0.05) (Figure 2). No significant differences were observed between other baseline data and long-term prognosis (all P > 0.05), (Table 1).
Figure 2.
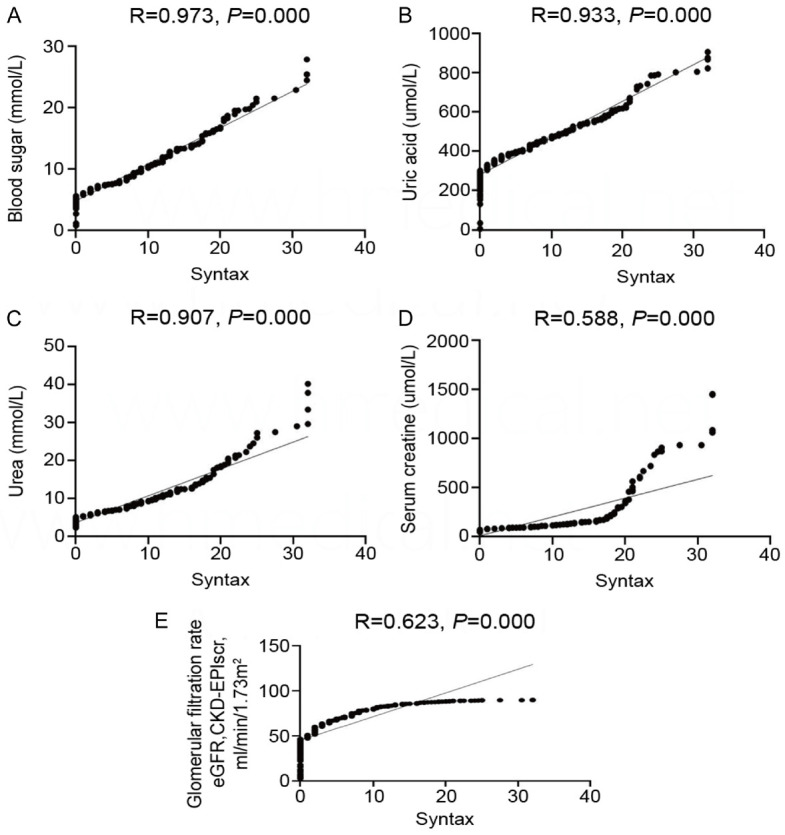
Correlation between residual Syntax score and blood sugar, uric acid, urea, serum creatinine and glomerular filtration rate. A. Correlation between Residual Syntax Score and Blood Glucose; B. Correlation between residual Syntax score and uric acid; C. Correlation between residual Syntax score and urea; D. Correlation between residual Syntax score and serum creatinine; E. Correlation between residual Syntax score and estimated glomerular filtration rate.
Table 1.
Univariate analysis of long-term prognosis in diabetic patients with renal insufficiency after PCI
| eGFR grouping | N | Mean value | Standard deviation | F | p | |
|---|---|---|---|---|---|---|
| Gender (0= male; 1= female) | Higher | 36 | 0.44 | 0.504 | 1.566 | 0.21 |
| Moderate | 105 | 0.46 | 0.501 | |||
| lower | 248 | 0.36 | 0.482 | |||
| age | Higher | 36 | 71.72 | 9.617 | 0.252 | 0.777 |
| Moderate | 106 | 72.44 | 9.881 | |||
| Lower | 248 | 72.79 | 8.299 | |||
| Nationality (0= Han; 1= ethnic minorities) | Higher | 21 | 0 | 0 | 0.221 | 0.802 |
| Moderate | 60 | 0.02 | 0.129 | |||
| Lower | 147 | 0.02 | 0.142 | |||
| BMI | Higher | 34 | 24.699 | 2.714 | 0.008 | 0.992 |
| Moderate | 102 | 24.680 | 4.171 | |||
| Lower | 239 | 24.735 | 3.561 | |||
| Diabetes mellitus (0= none, 1= yes) | Higher | 36 | 1 | 0 | - | - |
| Moderate | 106 | 1 | 0 | |||
| Lower | 248 | 1 | 0 | |||
| Hypertension (0= none, 1= yes) | Higher | 35 | 0.94 | 0.236 | 5.194 | 0.006 |
| Moderate | 104 | 0.9 | 0.296 | |||
| Lower | 242 | 0.79 | 0.409 | |||
| Myocardial infarction (0, 1) | Higher | 36 | 0.67 | 0.478 | 5.156 | 0.006 |
| Moderate | 106 | 0.49 | 0.502 | |||
| Lower | 248 | 0.4 | 0.491 | |||
| Diagnosis (0= angina; 1-NSTEMI, 2= STEMI) | Higher | 36 | 1.61 | 0.838 | 0.83 | 0.437 |
| Moderate | 106 | 1.55 | 0.947 | |||
| Lower | 248 | 1.44 | 1.008 | |||
| Length of stay | Higher | 35 | 12.57 | 7.559 | 3.749 | 0.024 |
| Moderate | 104 | 11.35 | 6.743 | |||
| Lower | 246 | 10.22 | 4.354 | |||
| Smoking (1= no; 2= quit smoking; 3= active smoking) | Higher | 35 | 1.57 | 0.778 | 0.005 | 0.995 |
| Moderate | 105 | 1.58 | 0.852 | |||
| Lower | 246 | 1.59 | 0.827 | |||
| Previous PCI history (0= no; 1= Yes) | Higher | 35 | 0.11 | 0.323 | 0.235 | 0.791 |
| Moderate | 105 | 0.14 | 0.352 | |||
| Lower | 247 | 0.12 | 0.323 | |||
| History of CABG surgery (0= no; 1= Yes) | Higher | 35 | 0 | 0 | - | - |
| Moderate | 105 | 0 | 0 | |||
| Lower | 247 | 0 | 0 | |||
| History of COPD (0= no; 1= Yes) | Higher | 35 | 0.03 | 0.169 | 0.36 | 0.698 |
| Moderate | 104 | 0.06 | 0.234 | |||
| Lower | 246 | 0.04 | 0.198 | |||
| History of cardiac insufficiency (0= no; 1= Yes) | Higher | 35 | 0.23 | 0.426 | 8.975 | 0 |
| Moderate | 105 | 0.1 | 0.295 | |||
| Lower | 247 | 0.04 | 0.197 | |||
| History of atrial fibrillation (0= no; 1= Yes) | Higher | 35 | 0.06 | 0.236 | 0.429 | 0.652 |
| Moderate | 105 | 0.03 | 0.167 | |||
| Lower | 247 | 0.05 | 0.215 | |||
| History of hypertension (0= no, 1= yes) | Higher | 35 | 0.89 | 0.323 | 6.958 | 0.001 |
| Moderate | 105 | 0.84 | 0.37 | |||
| Lower | 245 | 0.68 | 0.467 | |||
| History of diabetes (0= no, 1= yes) | Higher | 35 | 0.89 | 0.323 | 0.105 | 0.901 |
| Moderate | 105 | 0.87 | 0.342 | |||
| Lower | 247 | 0.86 | 0.349 | |||
| History of stroke (0= no; 1= Yes) | Higher | 35 | 0.06 | 0.236 | 1.042 | 0.354 |
| Moderate | 105 | 0.05 | 0.214 | |||
| Lower | 244 | 0.09 | 0.287 | |||
| History of abnormal renal function (0= no; 1= Yes) | Higher | 35 | 0.63 | 0.49 | 124.883 | 0 |
| Moderate | 103 | 0.1 | 0.298 | |||
| Lower | 245 | 0 | 0.064 | |||
| Chronic kidney disease (0= no; 1= Yes) | Higher | 34 | 0.59 | 0.5 | 116.24 | 0 |
| Moderate | 103 | 0.08 | 0.269 | |||
| Lower | 245 | 0 | 0.064 | |||
| History of renal dialysis (0= no, 1= yes) | Higher | 35 | 0.371 | 0.4902 | 102.013 | 0 |
| Moderate | 103 | 0 | 0 | |||
| Lower | 245 | 0 | 0 | |||
| Systolic blood pressure | Higher | 35 | 132.54 | 30.666 | 1.079 | 0.341 |
| Moderate | 105 | 131.26 | 22.853 | |||
| Lower | 247 | 135.09 | 21.877 | |||
| Diastolic blood pressure | Higher | 35 | 70.69 | 16.128 | 3.209 | 0.041 |
| Moderate | 105 | 73.63 | 14.556 | |||
| Lower | 247 | 76.12 | 12.502 | |||
| Heart rate | Higher | 35 | 79.51 | 21.07 | 0.195 | 0.823 |
| Moderate | 105 | 78.55 | 15.761 | |||
| Lower | 247 | 77.91 | 14.568 | |||
| Cardiogenic shock (0= no; 1= Yes) | Higher | 35 | 0.23 | 0.731 | 7.363 | 0.001 |
| Moderate | 105 | 0.05 | 0.214 | |||
| Lower | 245 | 0.03 | 0.178 | |||
| Cardiac arrest (0= no; 1= Yes) | Higher | 35 | 0 | 0 | 1.34 | 0.263 |
| Moderate | 105 | 0.01 | 0.098 | |||
| Lower | 246 | 0 | 0 | |||
| Mechanical complications (0= no; 1= Yes) | Higher | 35 | 0 | 0 | - | - |
| Moderate | 105 | 0 | 0 | |||
| Lower | 246 | 0 | 0 | |||
| Serum creatinine (umol/L) | Higher | 36 | 508.725 | 384.11467 | 211.94 | 0 |
| Moderate | 106 | 121.1264 | 24.31825 | |||
| Lower | 248 | 80.6041 | 13.91188 | |||
| Glomerular filtration rate eGFR, CKD-EPIscr method, ml/min/1.73 m2 | Higher | 36 | 13.88194 | 9.542039 | 1103.085 | 0 |
| Moderate | 106 | 47.15961 | 8.036214 | |||
| Lower | 248 | 76.86808 | 8.607524 | |||
| CystatinC (mg/L) | Higher | 33 | 4.5255 | 2.39087 | 1.203 | 0.301 |
| Moderate | 101 | 2.777 | 9.09857 | |||
| Lower | 231 | 1.938 | 10.11731 | |||
| Blood sugar (mmol/L) | Higher | 36 | 8.9928 | 4.52914 | 0.829 | 0.437 |
| Moderate | 104 | 9.0252 | 4.64898 | |||
| Lower | 245 | 8.466 | 3.67347 | |||
| Triglycerides (mmol/L) | Higher | 34 | 2.0071 | 0.98164 | 0.375 | 0.688 |
| Moderate | 104 | 1.9387 | 1.41477 | |||
| Lower | 236 | 1.8499 | 1.13331 | |||
| Total cholesterol (mmol/L) | Higher | 34 | 4.27429 | 1.197683 | 0.582 | 0.559 |
| Moderate | 104 | 4.41788 | 1.259396 | |||
| Lower | 236 | 4.25797 | 1.284429 | |||
| HDL-C (mmol/L) | Higher | 34 | 1.0656 | 0.29516 | 2.086 | 0.126 |
| Moderate | 104 | 1.1907 | 0.32945 | |||
| Lower | 236 | 1.153 | 0.30572 | |||
| Lp (a) (mg/L) | Higher | 32 | 335.6344 | 344.0388 | 1.179 | 0.309 |
| Moderate | 94 | 243.9319 | 304.1704 | |||
| Lower | 219 | 241.42 | 335.71296 | |||
| Homocysteine (µmol/L) | Higher | 29 | 24.1207 | 10.76241 | 20.315 | 0 |
| Moderate | 92 | 19.3413 | 7.37967 | |||
| Lower | 216 | 15.0507 | 8.29136 | |||
| Myocardial infarction (0= no, 1= yes) | Higher | 23 | 0.65 | 0.487 | 0.194 | 0.823 |
| Moderate | 68 | 0.54 | 0.502 | |||
| Lower | 159 | 0.55 | 0.862 | |||
| Multibranch disease | Higher | 35 | 0.86 | 0.355 | 0.85 | 0.428 |
| Moderate | 102 | 0.78 | 0.413 | |||
| Lower | 242 | 0.76 | 0.428 | |||
| calcification | Higher | 35 | 0.29 | 0.458 | 1.236 | 0.292 |
| Moderate | 102 | 0.27 | 0.448 | |||
| Lower | 242 | 0.21 | 0.406 | |||
| thrombus | Higher | 35 | 0.03 | 0.169 | 1.148 | 0.318 |
| Moderate | 102 | 0.06 | 0.236 | |||
| Lower | 242 | 0.09 | 0.288 | |||
| Chronic total occlusion | Higher | 35 | 0.37 | 0.49 | 2.228 | 0.109 |
| Moderate | 102 | 0.2 | 0.399 | |||
| Lower | 242 | 0.24 | 0.425 | |||
| Diffuse long lesion | Higher | 10 | 1 | 0 | - | - |
| Moderate | 28 | 1 | 0 | |||
| Lower | 52 | 1 | 0 | |||
| Diffuse long lesion | Higher | 34 | 1.147 | 0.3595 | 2.04 | 0.132 |
| Moderate | 97 | 1.103 | 0.3057 | |||
| Lower | 221 | 1.057 | 0.2571 | |||
| Number of implanted stents (1=1, 2=2, 3=≥ 3) | Higher | 17 | 1.176 | 0.7276 | 0.904 | 0.407 |
| Moderate | 45 | 1.022 | 0.2601 | |||
| Lower | 104 | 1.087 | 0.4029 | |||
| Number of implanted stents (1=1, 2=2, 3=≥ 3) | Higher | 5 | 0.8 | 0.447 | 1.246 | 0.297 |
| Moderate | 13 | 1.15 | 0.555 | |||
| Lower | 31 | 1.06 | 0.359 | |||
| Total length of stent implantation (mm) | Higher | 4 | 27.75 | 8.057 | 1.425 | 0.251 |
| Moderate | 13 | 32.46 | 10.952 | |||
| Lower | 31 | 27.29 | 8.757 | |||
| Number of implanted stents (1=1, 2=2, 3=≥ 3) | Higher | 2 | 1 | 0 | 0.242 | 0.79 |
| Moderate | 2 | 1 | 0 | |||
| Lower | 7 | 1.29 | 0.756 | |||
| IABP (0= no, 1= yes) | Higher | 14 | 0.14 | 0.363 | 1.648 | 0.197 |
| Moderate | 28 | 0.04 | 0.189 | |||
| Lower | 87 | 0.03 | 0.184 | |||
| Temporary pacemaker (0= no, 1= yes) | Higher | 12 | 0 | 0 | 0.209 | 0.812 |
| Moderate | 28 | 0.04 | 0.189 | |||
| Lower | 89 | 0.03 | 0.181 | |||
| Spinning mill (0= no, 1= yes) | Higher | 13 | 0.08 | 0.277 | 0.008 | 0.992 |
| Moderate | 28 | 0.07 | 0.262 | |||
| Lower | 89 | 0.08 | 0.271 | |||
| IVUS | Higher | 16 | 0.19 | 0.403 | 1.261 | 0.287 |
| Moderate | 30 | 0.1 | 0.305 | |||
| Lower | 90 | 0.07 | 0.251 | |||
| Thrombus aspiration (0= no, 1= yes) | Higher | 13 | 0.08 | 0.277 | 0.031 | 0.97 |
| Moderate | 30 | 0.1 | 0.305 | |||
| Lower | 92 | 0.1 | 0.299 | |||
| Preoperative syntax | Higher | 36 | 20.083 | 11.6512 | 2.218 | 0.11 |
| Moderate | 106 | 17 | 9.473 | |||
| Lower | 247 | 16.549 | 9.0124 | |||
| Preoperative grouping of syntax | Higher | 36 | 2.86 | 0.351 | 0.515 | 0.598 |
| Moderate | 106 | 2.86 | 0.35 | |||
| Lower | 248 | 2.82 | 0.397 | |||
| Syntactic operation | Higher | 36 | 6.986 | 6.4282 | 0.262 | 0.769 |
| Moderate | 106 | 6.642 | 6.7198 | |||
| Lower | 247 | 6.259 | 6.6225 | |||
| Classification of revascularization degree (1= CR, 2= rIR (0-8), 3= IR) | Higher | 35 | 2.29 | 0.75 | 0.915 | 0.401 |
| Moderate | 102 | 2.12 | 0.722 | |||
| Lower | 242 | 2.1 | 0.758 | |||
| aspirin | Higher | 35 | 0.91 | 0.284 | 0.979 | 0.377 |
| Moderate | 104 | 0.97 | 0.168 | |||
| Lower | 246 | 0.95 | 0.216 | |||
| Clopidogrel/Ticagrelor | Higher | 35 | 0.97 | 0.169 | 1.232 | 0.293 |
| Moderate | 104 | 0.97 | 0.168 | |||
| Lower | 246 | 0.99 | 0.09 | |||
| Lipid-lowering drugs (0, 1) | Higher | 35 | 0.94 | 0.236 | 2.614 | 0.075 |
| Moderate | 104 | 0.95 | 0.215 | |||
| Lower | 246 | 0.99 | 0.11 | |||
| β-Blocker (None, metoprolol, Bisoprolol, others --) | Higher | 34 | 0.74 | 0.448 | 0.444 | 0.642 |
| Moderate | 103 | 0.7 | 0.461 | |||
| Lower | 245 | 0.67 | 0.473 | |||
| Diuretics (none, spirolactone, furosemide, azine chlorothiazide, others --) | Higher | 33 | 0.45 | 0.506 | 7.756 | 0.001 |
| Moderate | 103 | 0.39 | 0.49 | |||
| Lower | 241 | 0.22 | 0.415 | |||
| ACEI/ARB (none) | Higher | 34 | 0.32 | 0.475 | 3.683 | 0.026 |
| Moderate | 104 | 0.59 | 0.495 | |||
| Lower | 245 | 0.54 | 0.499 | |||
| CCB (none) | Higher | 35 | 0.6 | 0.497 | 5.345 | 0.005 |
| Moderate | 102 | 0.44 | 0.499 | |||
| Lower | 246 | 0.34 | 0.474 | |||
| Aldosterone receptor antagonists (none, spironolactone, others) | Higher | 35 | 0.17 | 0.382 | 0.344 | 0.709 |
| Moderate | 101 | 0.15 | 0.357 | |||
| Lower | 245 | 0.13 | 0.333 | |||
| Insulin (No) | Higher | 35 | 0.51 | 0.507 | 6.542 | 0.002 |
| Moderate | 103 | 0.34 | 0.476 | |||
| Lower | 246 | 0.24 | 0.428 | |||
| Hypoglycemic agents (none, metformin, dagliazine, acarbose, others) | Higher | 34 | 0.24 | 0.431 | 20.141 | 0 |
| Moderate | 101 | 0.74 | 0.439 | |||
| Lower | 242 | 0.74 | 0.442 | |||
| Follow-up duration (days) | Higher | 35 | 550.06 | 228.034 | 0.846 | 0.43 |
| Moderate | 102 | 545.81 | 238.145 | |||
| Lower | 242 | 579.54 | 235.063 | |||
| Follow-up duration (month) | Higher | 35 | 18.335 | 7.601 | 0.846 | 0.43 |
| Moderate | 102 | 18.194 | 7.938 | |||
| Lower | 242 | 19.318 | 7.836 | |||
| Follow-up duration (month) | Higher | 36 | 0.17 | 0.447 | 1.919 | 0.148 |
| Moderate | 106 | 0.16 | 0.439 | |||
| Lower | 248 | 0.08 | 0.332 | |||
| cardiogenic | Higher | 22 | 0.18 | 0.395 | 2.31 | 0.102 |
| Moderate | 69 | 0.1 | 0.304 | |||
| Lower | 143 | 0.06 | 0.231 | |||
| Stroke or not | Higher | 35 | 0 | 0 | 1.143 | 0.32 |
| Moderate | 97 | 0.03 | 0.174 | |||
| Lower | 239 | 0.05 | 0.219 | |||
| Recurrent myocardial infarction (Yes/No) | Higher | 35 | 0.09 | 0.284 | 1.47 | 0.231 |
| Moderate | 97 | 0.03 | 0.174 | |||
| Lower | 239 | 0.03 | 0.169 | |||
| Time to repeat myocardial infarction | Higher | 1 | 43719 | - | 0.213 | 0.838 |
| Moderate | 2 | 43765 | 72.1249 | |||
| Lower | 1 | 43717 | - | |||
| Neostenosis (0= no, 1= yes) | Higher | 1 | 1 | - | 9 | 0.007 |
| Moderate | 4 | 0.25 | 0.5 | |||
| Lower | 7 | 1 | 0 | |||
| Intrastent restenosis (0= no, 1= yes) | Higher | 1 | 1 | - | 0.333 | 0.667 |
| Moderate | 2 | 0.5 | 0.707 | |||
| Lower | 0 | - | - | |||
| Smoking status (Yes/quit/No) | Higher | 0 | - | - | 1.875 | 0.22 |
| Moderate | 3 | 0.33 | 0.577 | |||
| Lower | 5 | 0 | 0 | |||
| MACCE (All-cause death + stroke + unplanned revascularization + myocardial infarction) | Higher | 35 | 0.34 | 0.482 | 2.541 | 0.08 |
| Moderate | 103 | 0.27 | 0.447 | |||
| Lower | 244 | 0.2 | 0.398 |
Note: EGFR: Estimated Glomerular Filtration Rate; BMI: Body Mass Index; T2DM: Type 2 Diabetes Mellitus; MI: Myocardial Infarction; NSTEMI: Non-ST Elevation Myocardial Infarction; STEMI: ST Elevation Myocardial Infarction; COPD: Chronic Obstructive Pulmonary Disease; LVEF: Left Ventricular Ejection Fraction; LVED: Left Ventricular End-Diastolic Diameter; LVSD: Left Ventricular End-Systolic Diameter; PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Graft; BS: Blood Sugar; TC: Total Cholesterol; TG: Triglycerides; HDL-C: High-Density Lipoprotein Cholesterol; Lp(a): Lipoprotein(a); IVUS: Intravascular Ultrasound; MACCE: Major Adverse Cardiovascular and Cerebrovascular Events, a composite of all-cause death, stroke, unplanned revascularization, and myocardial infarction.
Multivariate regression analysis of long-term prognosis in diabetic patients with renal insufficiency after PCI
Logistic regression analysis included significant factors identified from the univariate analysis as covariates to determine their association with the likelihood of the outcome variable. The analysis revealed that MI (OR=3.053, P=0.009), β-blocker usage (OR=3.134, P=0.009), and follow-up duration were significantly associated with long-term outcome (Table 2).
Table 2.
Multivariate logistic regression analysis of long-term prognosis of diabetic patients with renal insufficiency after percutaneous coronary intervention
| factor | β | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Myocardial infarction | 1.116 | 0.428 | 6.799 | 3.053 | 1.319~7.063 | 0.009 |
| β-Blocker | 1.142 | 0.439 | 6.765 | 3.134 | 1.325~7.414 | 0.009 |
| Follow-up duration | -0.002 | 0.001 | 3.857 | 0.998 | 0.996~1.000 | 0.05 |
Correlation between rSS and blood sugar, uric acid, urea, serum creatinine, and eGFR
Linear regression analysis was conducted to examine the correlation between rSS and blood sugar, uric acid, urea, serum creatinine, and glomerular filtration rate in diabetic patients with renal insufficiency following PCI. The analysis revealed significant positive correlations between rSS and blood sugar (r=0.973; P=0.000), uric acid (r=0.933; P=0.000), urea (r=0.907; P=0.000), serum creatinine (r=0.588; P=0.000), and glomerular filtration rate (r=0.623; P=0.000). These findings are depicted in Figure 3. The AUC for the Syntax score was 0.604, with a sensitivity of 46.6% and a specificity of 72.5%. Notably, the specificity outperformed other evaluation factors, including blood sugar (54.1%), uric acid (68.2%), urea (49.6%), serum creatinine (72.0%), and eGFR (65.5%) (Table 3).
Figure 3.
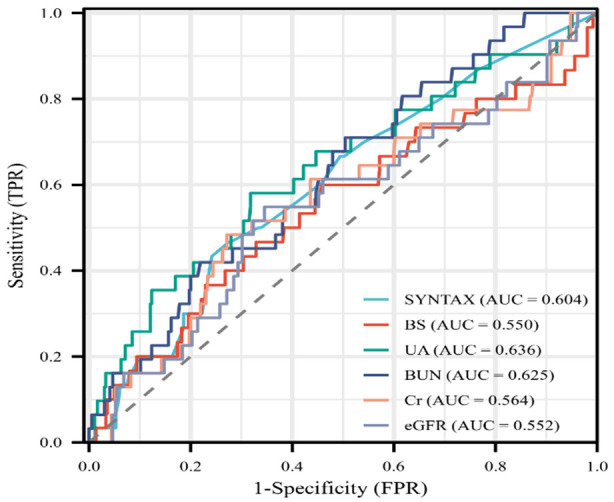
ROC Curve Analysis of residual Syntax score and blood sugar, uric acid, urea, serum creatinine and glomerular filtration rate. BS, blood glucose; UA, uric acid; BUN, blood urea nitrogen; Cr, Creatinine; eGFR, estimated Glomerular filtration rate.
Table 3.
The SYNTAX score was analyzed in relation to blood sugar, uric acid, urea, creatinine, and glomerular filtration rate using ROC curve analysis
| AUC | 95% (CI) | Cut-ff | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Lower Limit | Upper Limit | |||||
| SYNTAX | 0.604 | 0.499 | 0.709 | 8.75 | 46.6% | 72.5% |
| BS | 0.550 | 0.430 | 0.670 | 7.68 | 60% | 54.1% |
| UA | 0.636 | 0.525 | 0.747 | 436.6 | 58.1% | 68.2% |
| BUN | 0.625 | 0.527 | 0.723 | 6.625 | 70.9% | 49.6% |
| Cr | 0.564 | 0.449 | 0.679 | 107.45 | 48.4% | 72.0% |
| eGFR | 0.552 | 0.439 | 0.666 | 59.805 | 54.8% | 65.5% |
Note: P < 0.05 was considered significant. Abbreviations: BS, blood glucose; UA, uric acid; BUN, blood urea nitrogen; Cr, Creatinine; eGFR, estimated glomerular filtration rate.
Correlation analysis of long-term prognosis in diabetic patients with renal insufficiency after PCI
Kendall’s analysis was used to examine the association between the Syntax score and the long-term prognosis of diabetic patients with renal insufficiency following PCI. The study revealed a significant positive correlation between Syntax score and long-term prognosis (OR=0.138; P=0.001), as shown in Table 4 and Figure 4.
Table 4.
Correlation analysis of long-term prognosis of diabetic patients with renal insufficiency after percutaneous coronary intervention
| Index | Kendall correlation | Significance (bilateral) | N | Standard error | 95% Confidence interval | |
|---|---|---|---|---|---|---|
|
| ||||||
| lower limit | upper limit | |||||
| Syntax score | 0.138** | 0.001 | 382 | 0.04 | 0.061 | 0.214 |
refers to P < 0.001 and shows a significant difference.
Figure 4.
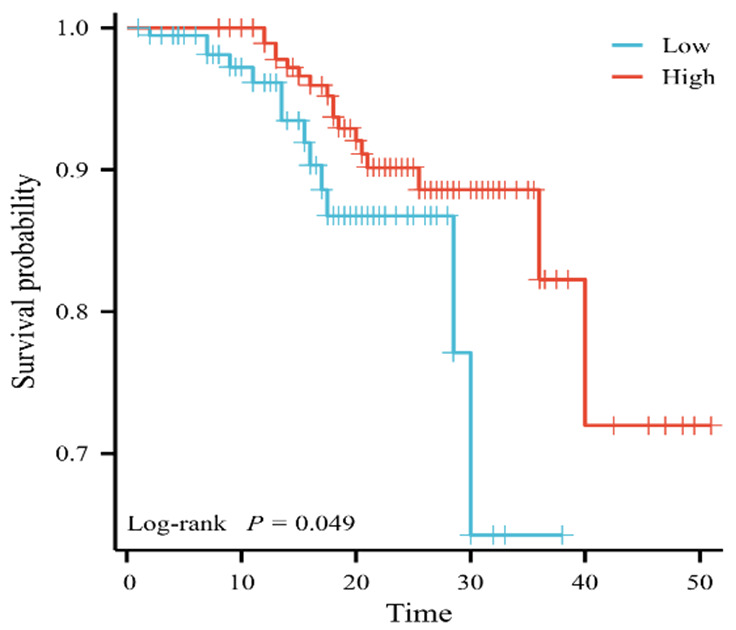
Prognosis of the Syntax score.
Discussion
Coronary heart disease, atherosclerotic heart disease of the coronary arteries, is one of the most prevalent and deadly non-communicable chronic diseases globally [18]. According to the “2020 China Cardiovascular Health and Disease Report”, there are 11.39 million coronary heart disease patients in China, and this number continues to rise [19]. High blood sugar in diabetic patients can lead to inflammation and endothelial dysfunction under oxidative stress, altering lipids and other nutrients, promoting the progression of atherosclerosis, and accelerating the deterioration of coronary heart disease [20].
The incidence and mortality rates of diabetes and coronary heart disease are steadily rising in our country, contributing to an increasingly severe burden of these combined conditions. Research indicates [10] that the prevalence of diabetes has reached 11.2%, affecting 110 million individuals, with 43.2% of diabetic patients dying from cardiovascular diseases and 18.7% specifically from ischemic heart disease. Renal insufficiency is frequently observed in diabetic patients and is the most common cause of renal failure [19]. Cardiovascular disease remains the leading cause of morbidity and mortality in diabetic patients, with renal insufficiency further elevating the risk of cardiovascular complications [21]. As a result, diabetic patients with renal insufficiency are at an increased risk of developing cardiovascular disease [22].
PCI is a minimally invasive diagnostic and therapeutic procedure that plays a crucial role in managing coronary heart disease. PCI effectively addresses vascular diseases, enhances the quality of life in patients with coronary heart disease, and significantly reduces mortality rates. It is widely used globally, with over 900,000 cases treated annually in China alone [23]. While PCI presents challenges in treating complex anatomical structures, such as left main lesions, multivessel disease, calcifications, and chronic total occlusion, ample clinical evidence supports the feasibility of PCI in these complex cases [24]. Advances in postoperative antithrombotic therapy and the extensive use of drug-eluting stents have significantly mitigated the long-term adverse effects of blood flow reconstruction in patients undergoing PCI [25]. Consequently, PCI is widely recommended as a treatment option globally, with its efficacy considered equivalent to that of CABG in many cases.
The SYNTAX score (http://www.syntaxscore.com) is an anatomically based tool used to objectively determine the complexity of coronary artery disease [26]. This study represents one of the first clinical investigations comparing the efficacy of drug-eluting stents and CABG in patients with three-vessel or left main coronary artery disease. The SYNTAX score has become a widely used tool for stratifying patients who may benefit from PCI or CABG, potentially identifying those most suited for standalone PCI. The analysis demonstrates the potential advantages of the SYNTAX score and its applications in interventional cardiology [27]. Current findings suggest that lower SYNTAX scores are significantly associated with reduced adverse cardiovascular outcome compared to moderate or higher SYNTAX scores. Additionally, research indicates that lower SYNTAX scores predict a lower incidence of MACE [28].
This study stratifies patient risk using the SYNTAX score, providing a foundation for vascular reconstruction planning by physicians. The rSS has been widely recognized and utilized by current researchers, offering valuable guidance in formulating vascular reconstruction strategies and assessing prognosis. The rSS allows for accurate and quantitative evaluation of the characteristics of residual coronary artery disease and myocardial ischemic burden [29]. Additionally, research by Song et al. found a significant correlation between rSS and exercise tolerance in patients with coronary heart disease following PCI [28].
Our research indicates that β-blockers (OR=3.053, P=0.009), β-receptor blockers (OR=3.134, P=0.009), and follow-up duration (OR=0.998, P=0.05) are independent risk factors affecting the long-term prognosis of diabetic patients with renal insufficiency after PCI. The study also found that rSS was positively correlated with blood glucose (r=0.973; P=0.000), uric acid (r=0.933; P=0.000), urea (r=0.907; P=0.000), serum creatinine (r=0.588; P=0.000), and glomerular filtration rate (r=0.623; P=0.000). Furthermore, the study indicated that a higher rSS was associated with a worse long-term prognosis for diabetic patients with renal insufficiency after PCI, which aligns with the findings of Lee et al. [29].
The introduction of the SYNTAX score has renewed interest in the risk stratification of patients undergoing PCI. Including clinically significant variables in the SYNTAX score allows for an individualized assessment of mortality risk associated with different revascularization strategies [30].
However, the SYNTAX score has inherent limitations, including low intra- and inter-observer reproducibility. First, its retrospective nature prevents establishing a causal relationship between diabetes with renal insufficiency and the SYNTAX score. Second, variables such as dietary habits, medication therapy, and exercise patterns may not have been fully accounted for. Last, our study results are limited to a specific local population with a relatively small sample size, which restricts their generalizability. Therefore, further large-scale studies are needed to confirm the clinical value of the SYNTAX score in predicting the long-term prognosis of patients with diabetes and concomitant renal insufficiency undergoing PCI.
In conclusion, rSS can be used to evaluate incomplete revascularization. This study suggests that MI, β-blocker use, and follow-up duration are independent factors influencing the long-term prognosis of diabetic patients with renal insufficiency. Therefore, dynamic monitoring of these factors during PCI in diabetic patients with renal insufficiency can provide clinicians with evidence to guide treatment and holds significant clinical application and promotion value.
Acknowledgements
Thanks to the Affiliated Hospital of Southwest Medical University for its support.
Disclosure of conflict of interest
None.
Abbreviations
- rSS
Residual Syntax Score
- MACE
correlation between cardiovascular adverse events
- PCI
percutaneous coronary intervention
- CTO
chronic total occlusion
- CABG
coronary artery bypass grafting
- MI
myocardial infarction
- MACCE
Major Adverse Cardiovascular and Cerebrovascular Events
- eGFR
estimated glomerular filtration rate
- T2DM
type 2 diabetes mellitus
- BMI
body mass index
- BNP
brain natriuretic peptide
- HbA1c
glycated hemoglobin
- TC
total cholesterol
- TG
triglycerides
- LDL-C
low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- LVEF
left ventricular ejection fraction
- LVED
left ventricular end-diastolic diameter
- LVSD
left ventricular end-systolic diameter
- bSS
baseline SYNTAX score
- FPG
fasting blood glucose
- ROC
Receiver Operating Characteristic
- AUC
area under the curve
References
- 1.Goodarzi MO, Rotter JI. Genetics insights in the relationship between type 2 diabetes and coronary heart disease. Circ Res. 2020;126:1526–1548. doi: 10.1161/CIRCRESAHA.119.316065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katta N, Loethen T, Lavie CJ, Alpert MA. Obesity and coronary heart disease: epidemiology, pathology, and coronary artery imaging. Curr Probl Cardiol. 2021;46:100655. doi: 10.1016/j.cpcardiol.2020.100655. [DOI] [PubMed] [Google Scholar]
- 3.Giralt-López A, Molina-Van den Bosch M, Vergara A, García-Carro C, Seron D, Jacobs-Cachá C, Soler MJ. Revisiting experimental models of diabetic nephropathy. Int J Mol Sci. 2020;21:3587. doi: 10.3390/ijms21103587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zhang J, Ren Y, Sun R, Zhai X. Unveiling the pathogenesis and therapeutic approaches for diabetic nephropathy: insights from panvascular diseases. Front Endocrinol (Lausanne) 2024;15:1368481. doi: 10.3389/fendo.2024.1368481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kourtidou C, Stangou M, Marinaki S, Tziomalos K. Novel cardiovascular risk factors in patients with diabetic kidney disease. Int J Mol Sci. 2021;22:11196. doi: 10.3390/ijms222011196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palazzuoli A, Iacoviello M. Diabetes leading to heart failure and heart failure leading to diabetes: epidemiological and clinical evidence. Heart Fail Rev. 2023;28:585–596. doi: 10.1007/s10741-022-10238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9:53–62. doi: 10.1038/nrcardio.2011.132. [DOI] [PubMed] [Google Scholar]
- 8.Premkumar S, Ramamoorthy L, Pillai AA. Impact of nurse-led cardiac rehabilitation on patient’s behavioral and physiological parameters after a coronary intervention: a pilot randomized controlled trial. J Fam Community Med. 2022;29:17–23. doi: 10.4103/jfcm.jfcm_315_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Battrawy I, Lang S, Ansari U, Tülümen E, Schramm K, Fastner C, Zhou X, Hoffmann U, Borggrefe M, Akin I. Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. Europace. 2018;20:843–850. doi: 10.1093/europace/eux073. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Serruys PW, Fuster V, Farkouh ME, Spertus JA, Cohen DJ, Park SJ, Park DW, Ahn JM, Kappetein AP, Head SJ, Thuijs DJ, Onuma Y, Kent DM, Steyerberg EW, van Klaveren D SYNTAXES, FREEDOM, BEST, and PRECOMBAT trial investigators. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: secondary analysis of the multicentre randomised controlled SYNTAXES trial with external cohort validation. Lancet. 2020;396:1399–1412. doi: 10.1016/S0140-6736(20)32114-0. [DOI] [PubMed] [Google Scholar]
- 11.Mo Y, Xing B. Correlation between coronary CTA-SYNTAX score and invasive coronary angiography-SYNTAX score. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46:884–888. doi: 10.11817/j.issn.1672-7347.2021.200837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13–S22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 14.Barker JM, Yater WM. Diagnostic criteria for coronary arteriosclerotic heart disease. J Lancet. 1952;72:364–9. passim. [PubMed] [Google Scholar]
- 15.Gregorich M, Kammer M, Heinzel A, Böger C, Eckardt KU, Heerspink HL, Jung B, Mayer G, Meiselbach H, Schmid M, Schultheiss UT, Heinze G, Oberbauer R BEAt-DKD Consortium. Development and validation of a prediction model for future estimated glomerular filtration rate in people with type 2 diabetes and chronic kidney disease. JAMA Netw Open. 2023;6:e231870. doi: 10.1001/jamanetworkopen.2023.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindahl B, Mills NL. A new clinical classification of acute myocardial infarction. Nat Med. 2023;29:2200–2205. doi: 10.1038/s41591-023-02513-2. [DOI] [PubMed] [Google Scholar]
- 17.Potter TBH, Tannous J, Vahidy FS. A contemporary review of epidemiology, risk factors, etiology, and outcomes of premature strok. Curr Atheroscler Rep. 2022;24:939–948. doi: 10.1007/s11883-022-01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Lu FH, Zhao Q. Impact of specialized nursing outpatient case management on post-coronary artery bypass grafting patients. World J Clin Cases. 2024;12:3035–3044. doi: 10.12998/wjcc.v12.i17.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The WCOTROCHADIC. Report on cardiovascular health and diseases in China 2022: an updated summary. Biomed Environ Sci. 2023;36:669–701. doi: 10.3967/bes2023.106. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty S, Verma A, Garg R, Singh J, Verma H. Cardiometabolic risk factors associated with type 2 diabetes mellitus: a mechanistic insight. Clin Med Insights Endocrinol Diabetes. 2023;16:11795514231220780. doi: 10.1177/11795514231220780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng X, Yu H, Qiu X, Chair SY, Wong EM, Wang Q. The effects of a nurse-led lifestyle intervention program on cardiovascular risk, self-efficacy and health promoting behaviours among patients with metabolic syndrome: Randomized controlled trial. Int J Nurs Stud. 2020;109:103638. doi: 10.1016/j.ijnurstu.2020.103638. [DOI] [PubMed] [Google Scholar]
- 22.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 23.In China TWCOTROCHAD. Hu SS. Report on cardiovascular health and diseases in China 2021: an updated summary. J Geriatr Cardiol. 2023;20:399–430. doi: 10.26599/1671-5411.2023.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biscaglia S, Uretsky B, Barbato E, Collet C, Onuma Y, Jeremias A, Tebaldi M, Hakeem A, Kogame N, Sonck J, Escaned J, Serruys PW, Stone GW, Campo G. Invasive coronary physiology after stent implantation: another step toward precision medicine. JACC Cardiovasc Interv. 2021;14:237–246. doi: 10.1016/j.jcin.2020.10.055. [DOI] [PubMed] [Google Scholar]
- 25.Zhuo Q, Liang H, Bai Y, Hu Q, Hanum AL, Yang M, Wang Y, Wei W, Ding L, Ma F. Perceptions of patients undergoing percutaneous coronary intervention on pre-operative education in China: a qualitative study. Health Expect. 2021;24:121–130. doi: 10.1111/hex.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farooq V, Head SJ, Kappetein AP, Serruys PW. Widening clinical applications of the SYNTAX score. Heart. 2014;100:276–287. doi: 10.1136/heartjnl-2013-304273. [DOI] [PubMed] [Google Scholar]
- 27.Park TK, Hahn JY, Yang JH, Song YB, Choi SH, Choi JH, Lee SH, Ahn J, Carriere KC, Gwon HC. Modified residual SYNTAX score and clinical outcomes in patients with multivessel disease undergoing percutaneous coronary intervention. EuroIntervention. 2017;13:87–96. doi: 10.4244/EIJ-D-16-00685. [DOI] [PubMed] [Google Scholar]
- 28.Song Y, Gao Z, Tang X, Jiang P, Xu J, Yao Y, Li J, Zhao X, Qiao S, Yang Y, Gao R, Xu B, Yuan J. Impact of residual SYNTAX score on clinical outcomes after incomplete revascularisation percutaneous coronary intervention: a large single-centre study. EuroIntervention. 2017;13:1185–1193. doi: 10.4244/EIJ-D-17-00132. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Choi KH, Lee JM, Shin D, Hwang D, Kim HK, Doh JH, Nam CW, Shin ES, Jang MJ, Im SY, Park TK, Yang JH, Song YB, Hahn JY, Choi SH, Koo BK, Gwon HC. Residual functional SYNTAX score by quantitative flow ratio and improvement of exercise capacity after revascularization. Catheter Cardiovasc Interv. 2021;97:E454–E466. doi: 10.1002/ccd.29118. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Lønborg J, Jong A, Nishi T, De Bruyne B, Høfsten DE, Kelbæk H, Layland J, Nam CW, Pijls NHJ, Tonino PAL, Warnøe J, Oldroyd KG, Berry C, Engstrøm T, Fearon WF DANAMI-3-PRIMULTI, FAME, and FAMOUS-NSTEMI Study Investigators. Prognostic value of the residual SYNTAX score after functionally complete revascularization in ACS. J Am Coll Cardiol. 2018;72:1321–1329. doi: 10.1016/j.jacc.2018.06.069. [DOI] [PubMed] [Google Scholar]


