Abstract
Objective: To determine the diagnostic value of multimodal ultrasound combined with tumor markers in breast cancer (BC). Methods: A retrospective analysis was conducted on 198 patients with breast lesions treated at the Affiliated Wuxi People’s Hospital of Nanjing Medical University between May 2020 and May 2023. All patients underwent multimodal ultrasound and tumor marker testing. Among the 198 patients, 88 patients were pathologically diagnosed with benign disease (benign group) and 110 patients were pathologically diagnosed with malignant disease (malignant group). With the pathological results as the gold standard, the benign and malignant results from different diagnostic methods were compared, focusing on specificity, sensitivity and accuracy. Results: The areas under the curves (AUCs) of carbohydrate antigen 153 (CA153), CA125, and carcinoembryonic antigen (CEA) for diagnosing BC were 0.810, 0.812, and 0.790, respectively. When these tumor markers were used in combination for diagnosing BC, the AUC increased to 0.928. The AUC of multimodal ultrasound alone in diagnosing BC was 0.845. Additionally, the AUC of multimodal ultrasound combined with tumor markers in diagnosing BC reached 0.971, with the corresponding specificity, sensitivity and accuracy of 90.00%, 94.43% and 91.92%, respectively. Conclusion: In patients with early BC, the combination of multimodal ultrasound and tumor marker detection significantly improves the accuracy of diagnosing benign and malignant breast lesions compared to using either modality alone.
Keywords: Multimodal ultrasound, tumor markers, breast cancer, benign and malignant, diagnostic value
Introduction
Breast cancer (BC) is a prevalent malignant tumor globally and remains a leading cause of cancer-related mortality [1]. The incidence of BC continues to rise, likely influenced by changes in lifestyle. Notably, there is a growing trend of BC affecting younger individuals [2]. Early-stage BC typically refers to cancer detected before it has spread beyond the breast or nearby lymph nodes, without evidence of distant metastasis [2]. At this stage, BC does not have obvious clinical features, with patients typically experiencing only mild symptoms such as local discomfort or breast masses, which are frequently overlooked. As a result, many patients are diagnosed at a more advanced stage, having missed the optimal window for treatment. This delay can lead to significant psychological distress and an increased risk of mortality [2,3]. Thus, it is of profound significance to improve the early diagnosis rate of BC.
At the current stage, significant progress has been made in the diagnosis and treatment of BC, shifting from a generalized approach to personalized medicine [4]. Among the various diagnostic tools available, ultrasound has emerged as a crucial adjunctive method for early screening and diagnosis of BC in clinical practice, offering real-time imaging and convenience [5,6]. Nevertheless, malignant breast tumors exhibit diverse ultrasonographic features, often requiring additional information for accurate early diagnosis. The utilization of multimodal ultrasound, which provides various types of ultrasonic imaging information, has gained significant popularity in clinical practice [7]. This approach combines two or more ultrasound examination methods for a multi-angle analysis, thereby enhancing diagnostic accuracy [8]. Techniques involved in multimodal ultrasound involve two-dimensional ultrasound, color Doppler flow imaging, contrast-enhanced ultrasound (CEUS), ultrasound elastography (UE), three-dimensional ultrasound, automated breast volume scanning (ABVS) and so on [9]. In addition to ultrasound, serum tumor markers also play a crucial role in the diagnosis of BC. Highly sensitive and specific tumor markers are conducive to improve the survival rate of BC patients and are also helpful to detect the disease, evaluate the efficacy and judge the prognosis of patients in treatment [10]. Serum tumor markers commonly adopted in clinical practice include serum carbohydrate antigen (CA) 153, CA125 and carcinoembryonic antigen (CEA) [11]. Tumor markers, which are present in the bloodstream, provide a specific response to tumor-related parameters and can be detected via biochemical or immunohistochemical methods [11]. CA125, a glycoprotein antigen, is used to diagnose and monitor breast and ovarian cancers, showing elevated expression in affected patients [12]. CEA is an important marker for determining malignant tumors [13]. Studies have found that CA153, a variant of a glycoprotein present on the surface of breast cell membranes, is an effective tumor marker for BC. Targeted therapies against CA153 can further improve the treatment outcomes of BC and extend patients’ survival [14]. Early diagnosis of BC can be achieved through a combination of ultrasound examination and serum tumor markers. Ultrasound technology provides information about the morphology and blood flow of breast masses, and multimodal ultrasound imaging techniques can further improve diagnostic accuracy. Meanwhile, serum tumor marker testing can assist in screening and monitoring early-stage BC. The integrated application of these methods can improve the early detection rate of BC, thereby better guiding patient treatment and management. However, while there have been many studies on the use of ultrasound or serum tumor markers in the diagnosis of BC, there is limited research on the value of multimodal ultrasound combined with tumor markers for BC diagnosis.
Therefore, this study enrolled a total of 198 patients with breast lesions who underwent multimodal ultrasound examination and tumor marker testing (CA153, CA125, and CEA). The objective was to investigate the diagnostic value of combining multimodal ultrasound with tumor markers in detecting BC and to provide valuable insights for improving the clinical diagnosis of BC.
Materials and methods
Patient data
The data of 198 patients with breast lesions treated at the Affiliated Wuxi People’s Hospital of Nanjing Medical University between May 2020 and May 2023 were retrospectively analyzed in this study. All patients underwent multimodal ultrasound (two-dimensional ultrasound, CDFI, UE, and ABVS) and tumor marker testing (CA153, CA125 and CEA). With pathological diagnosis as the gold standard, 88 patients were confirmed as having benign disease (benign group), and 110 patients were pathologically diagnosed with malignant disease (malignant group). This study was conducted with permission of the Medical Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University.
Inclusion criteria: (1) Patients who had not received any breast-associated therapy before the examination; (2) Patients with detailed multimodal ultrasound results; (3) All patients included in the study underwent either puncture biopsy or surgical resection for all identified breast lesions. Tissue samples were collected during these procedures and subjected to pathological examination for definitive diagnosis. With pathological diagnosis results as the gold standard, the lesions were accurately diagnosed as benign or malignant nodules; and (4) Patients with complete medical data, including medical records, past medical history, laboratory and tumor marker test results.
Exclusion criteria: (1) Patients comorbid with other malignant tumors; (2) Patients with severe dysfunction of vital organs; (3) Patients unable to cooperate with the study or those who dropped out midway; and (4) Patients during pregnancy or lactation.
Methods
Multimodal ultrasound
All patients underwent multimodal ultrasound examinations (two-dimensional ultrasound, CDFI, UE, and ABVS) by the same group of medical staff: (1) Two-dimensional ultrasound: Patients were positioned supine with the affected breast exposed. A Super Sonic Imaging Aix Plorer-SWE color Doppler ultrasonic diagnostic apparatus, with a probe frequency of 4-15 MHz, was utilized. The entire breast surface was scanned, focusing on the four quadrants to assess the shape, size, margins, presence of calcifications, and axillary lymph nodes associated with the breast lesions. (2) Color Doppler Flow Imaging (CDFI): CDFI was used to detect blood flow inside and around the mass, providing insight into vascularization. (3) Ultrasound Elastography (UE): Under the conventional ultrasound mode, nodular lesions with well-defined section were selected, and the image was converted to the elastography mode. The imaging field included the pectoralis major muscle in the background and subcutaneous fat in the foreground, with both sides of the image exceeding beyond 5 mm of the lesion. The probe was adjusted in time to apply gentle pressure and decompression, with color distribution displayed on the two-dimensional gray scale and elastography images according to the pressure index of grade 3-4. (4) Automated Breast Volume Scanning (ABVS): The ABVS examination was performed using Siemens ACUSONABVS ultrasound system. The patients were positioned supine with their hands raised above their heads. Firstly, two-dimensional ultrasound was adopted to locate the mass and then the mode was switched to the ABVS mode. According to the size and location of the breast, the appropriate imaging preset was selected, and the probe was adjusted to the appropriate angle. Appropriate pressure was applied to ensure optimal contact with the breast surface. The ultrasound probe was secured, and the ABVS scan was initiated, lasting 65 seconds. The scan captured a maximum breast volume of 15.40 cm × 16.80 cm × 6.00 cm. Subsequently, the acquired data was transferred to a dedicated workstation for reconstruction and detailed analysis.
Multimodal ultrasound was interpreted by two experienced deputy chief radiologists using a double-blind method. Any discrepancies or disagreements in the interpretations were resolved through mutual discussion, and consensus was eventually reached. The final diagnosis was determined collaboratively, relying on the combined expertise and agreement of the radiologists.
Detection of tumor markers
Fasting venous blood (3-5 mL) was acquired from each patient in the morning. After centrifugation, the upper serum was taken. The serum levels of CA153, CA125 and CEA were detected through chemiluminescence microparticle enzyme-linked immunosorbent assay (CMIA) on an Abbott i2000 automatic analyzer with corresponding kits. The interval between blood sample collection and the multimodal ultrasound examination was kept within one week.
Diagnostic criteria
Evaluation criteria of two-dimensional ultrasound results [15]: Typical BC manifestations include a breast mass without capsule, an aspect ratio >1, irregular shape, fuzzy or jagged edges resembling crab claws, and posterior wall attenuation of the hypoechoic area.
Evaluation criteria of CDFI [16]: Abundant blood flow within and around the mass was considered indicative of malignancy, with blood flow graded as follows: Grade 3: rich blood flow with more than 4 small vessel signals; grade 2: moderate blood flows, with one main vessel’s length exceeding 1/4 of the sum of the long and short diameters of lymph nodes; grade 1: minimal blood flow, with 1-2 spot signals; grade 0: no detectable blood flow signal.
Scoring criteria of UE [17]: 1 point: The entire mass was deformed, and the color map was predominantly light green; 2 points: Most of the mass was deformed, or the center remained unchanged, with the color chart primarily light green and some blue parts; 3 points: Most of the mass was not deformed, with only peripheral deformation, and the color map was mainly blue with some green areas; 4 points: The entire mass was not deformed, and the color map was fully blue; 5 points: Both the mass and its periphery were undeformed, with the lesion larger than that seen on two-dimensional ultrasound. Lesions scoring 3 or below were initially classified as benign, while those scoring 4 or higher were categorized as malignant.
Evaluation criteria of ABVS results [18]: The size, shape, growth pattern, boundary, edge, internal echo, posterior echo, calcification and “convergence sign” of the lesion were evaluated. Lesions with blurred boundary, irregular shape, spiculated or angular edges, small lobulations, longitudinal growth, internal calcification, posterior acoustic shadowing were highly suspected to be malignant. A “convergence sign” observed in the coronal plane of ABVS is also indicative of malignant tumor.
The screening and grouping process is shown in Figure 1.
Figure 1.
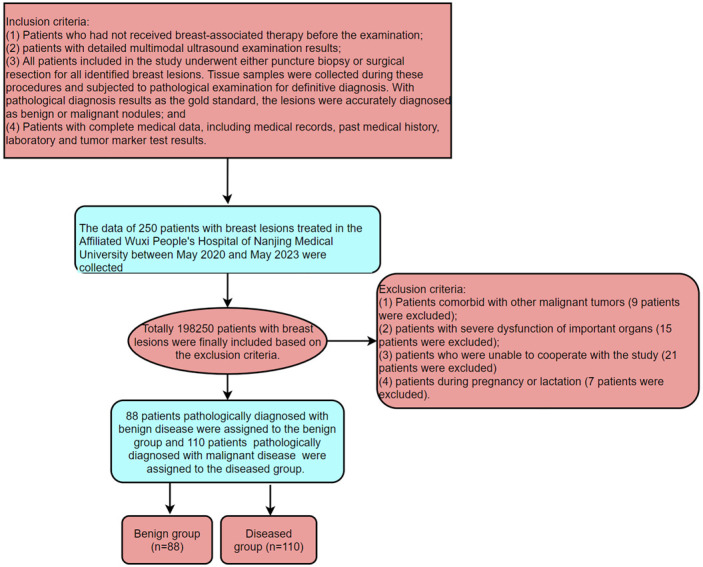
Screening and grouping process.
Outcome measures
The serum levels of CA153, CA125, and CEA, and two-dimensional ultrasound, CDFI, UE, and ABVS results of all patients were recorded. The final pathological diagnosis was used as the gold standard for comparison with the diagnostic findings. The diagnostic outcomes for benign and malignant tumors, obtained through individual and combined diagnostic methods were assessed. Receiver Operating Characteristic (ROC) curves were constructed to calculate and compare the specificity, sensitivity, and accuracy of each diagnostic method, aiming to evaluate their diagnostic efficacy.
Statistical analyses
SPSS 20.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. The chi-square test was adopted for comparison of the count data (n, %). The ROC curves were drawn to assess the diagnostic value of various diagnostic methods for distinguishing between benign and malignant tumors, and the figures were drawn with GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA, USA). The AUCs of each indicator were compared using the DeLong test. A Nomogram was created using the online tool at https://shiny.medsta.cn/coxpre1/ to visualize the diagnostic value in BC. P<0.05 was considered statistically significant.
Results
Pathological results
A total of 198 patients with breast lesions were included in the study. Pathological diagnosis confirmed 88 benign patients and 110 malignant patients. The postoperative pathological results are summarized in Table 1.
Table 1.
Pathological results
| Benign group (n=88) | Malignant group (n=110) | ||
|---|---|---|---|
| Fibroadenoma | 30 (34.09) | Invasive ductal carcinoma | 41 (37.27) |
| Mastopathy | 26 (29.55) | Ductal carcinoma in situ | 32 (29.09) |
| Intraductal papilloma | 14 (15.91) | Intraductal papillary carcinoma | 20 (18.18) |
| Breast inflammatory disease | 10 (11.36) | Invasive lobular carcinoma | 17 (15.46) |
| Benign phyllodes tumors | 8 (9.09) | ||
Analysis of multimodal ultrasound diagnosis results
Two-dimensional ultrasound, CDFI, UE and ABVS confirmed 81, 87, 91 and 91 cases of malignant lesions in the malignant group, respectively, and revealed 62, 66, 70 and 72 cases of benign lesions in the benign group, respectively. The multimodal approach revealed 96 cases of malignant lesions in the malignant group and 72 cases of benign lesions in benign group (Table 2).
Table 2.
The results of multimodal ultrasound diagnosis
| Benign group (n=88) | Malignant group (n=110) | |||
|---|---|---|---|---|
|
|
|
|||
| Malignant | Benign | Malignant | Benign | |
| Two-dimensional ultrasound | 26 | 62 | 81 | 29 |
| Color Doppler flow imaging | 22 | 66 | 87 | 23 |
| Ultrasound elastography | 18 | 70 | 91 | 19 |
| ABVS | 16 | 72 | 91 | 19 |
| Multimodal ultrasound | 16 | 72 | 96 | 14 |
ABVS, automated breast volume scanning.
Comparison of serum tumor markers between the two groups
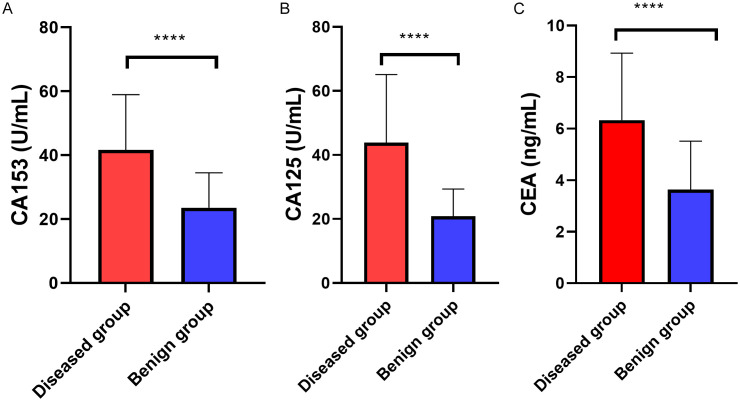
The serum levels of CA153, CA125 and CEA in the malignant group and the benign group were compared and analyzed. The malignant group showed significantly higher serum levels of CA153, CA125 and CEA than the benign group (all P<0.001, Figure 2).
Figure 2.
Comparison of serum tumor markers between the two groups. A: The malignant group showed a notably higher CA153 level than the benign group. B: The malignant group showed a notably higher CA125 level than the benign group. C: The malignant group showed a notably higher CEA level than the benign group. ****P<0.001. CA, carbohydrate antigen; CEA, carcinoembryonic antigen.
Comparison of diagnostic efficacy of tumor markers and their combination in diagnosing early BC
ROC curves were generated for CA153, CA125, CEA, and their combination in diagnosing BC. The results showed that the AUC values for CA153, CA125, and CEA in diagnosing BC were 0.810, 0.812, and 0.790, respectively. When combined, the AUC increased to 0.928. The accuracy of the combined diagnosis was significantly higher than that of the individual tumor markers (Figure 3; Tables 3 and 4).
Figure 3.
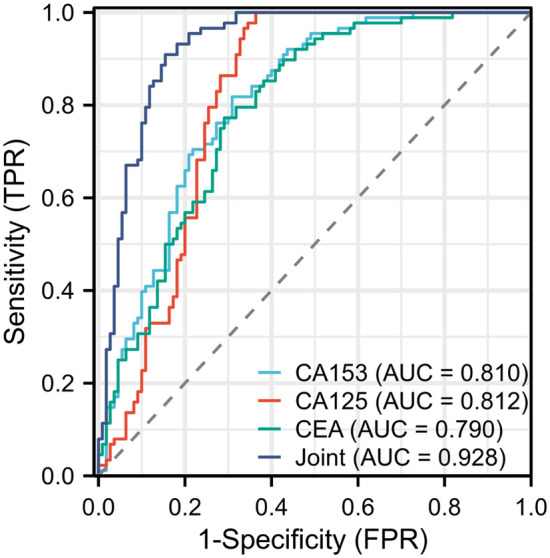
ROC curves of each tumor markers and their combination in diagnosing BC. BC, breast cancer.
Table 3.
Diagnostic efficacy of tumor markers and their combination in diagnosing BC
| Cutoff | Specificity | Sensitivity | Accuracy | AUC | |
|---|---|---|---|---|---|
| CA153 | 32.948 | 69.09% | 81.82% | 74.75% | 0.810 |
| CA125 | 39.578 | 63.36% | 100.00% | 79.80% | 0.812 |
| CEA | 5.0004 | 70.91% | 77.73% | 73.74% | 0.790 |
| Joint | 0.6075 | 84.55% | 90.91% | 87.37% | 0.928 |
CA, carbohydrate antigen; CEA, carcinoembryonic antigen.
Table 4.
Pairwise comparison of ROC curves for tumor markers and their combinationa
| Difference between areas | 95% Confidence Interval | Significance level | |
|---|---|---|---|
| CA125/CA153 | 0.002 | -0.077 to 0.080 | P=0.961 |
| CA125/CEA | 0.022 | -0.065 to 0.109 | P=0.618 |
| CA125/Joint | 0.116 | 0.063 to 0.168 | P<0.001 |
| CA153/CEA | 0.020 | -0.057 to 0.097 | P=0.611 |
| CA153/Joint | 0.118 | 0.067 to 0.168 | P<0.001 |
| CEA/Joint | 0.138 | 0.083 to 0.192 | P<0.001 |
DeLong et al. [19], 1988.
CA, carbohydrate antigen; CEA, carcinoembryonic antigen.
Comparison of diagnostic efficacy among multimodal ultrasound, tumor markers and their combination in diagnosing BC
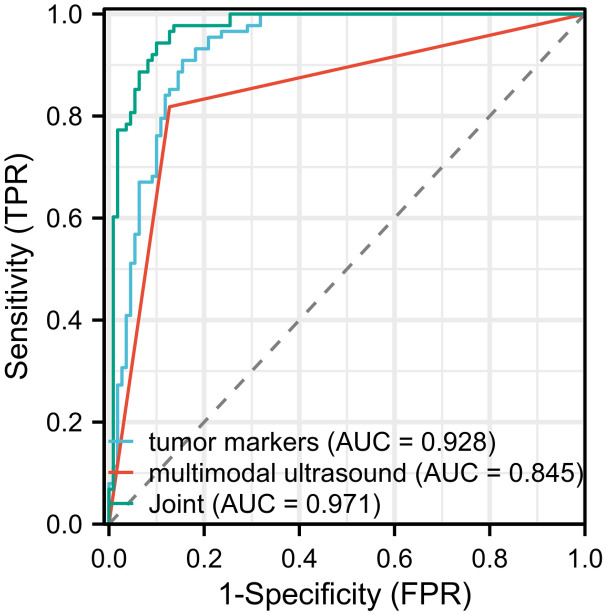
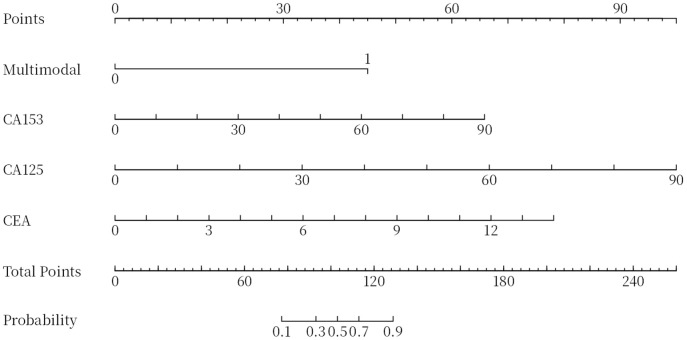
The ROC curves of multimodal ultrasound, combined tumor markers and their combination in diagnosing BC were generated. According to the results, the AUCs of multimodal ultrasound and tumor markers (CA153, CA125 and CEA) in the diagnosis of BC were 0.845 and 0.928, respectively. When these two approached were combined, the AUC increased to 0.971, indicating significantly higher diagnostic accuracy compared to using either multimodal ultrasound or tumor markers alone (Figure 4; Tables 5 and 6). In addition, a nomogram was used to visualize the diagnostic performance of tumor markers and multimodal ultrasound in diagnosing BC (Figure 5).
Figure 4.
ROC curve of multimodal ultrasound, tumor markers and their combination in diagnosing BC. BC, breast cancer.
Table 5.
Diagnostic efficacy of multimodal ultrasound, combined tumor markers and their combination in diagnosing BC
| Specificity | Sensitivity | Accuracy | AUC | |
|---|---|---|---|---|
| Multimodal ultrasound | 87.27% | 81.82% | 84.85% | 0.845 |
| Combined tumor markers | 84.55% | 90.91% | 87.37% | 0.928 |
| Multimodal ultrasound combined with tumor markers | 90.00% | 94.43% | 91.92% | 0.971 |
Table 6.
Pairwise comparison of ROC curves for multimodal ultrasound, combined tumor markers and their combinationa
| Difference between areas | 95% Confidence Interval | Significance level | |
|---|---|---|---|
| Joint/multimodal ultrasound | 0.125 | 0.078 to 0.172 | P<0.001 |
| Joint/tumor markers | 0.043 | 0.016 to 0.070 | P=0.002 |
| Multimodal ultrasound/tumor markers | 0.082 | 0.019 to 0.146 | P=0.011 |
DeLong et al. [19], 1988.
Figure 5.
The nomogram of tumor markers and multimodal ultrasound for diagnosing breast cancer.
Discussion
BC is the most common cancer affecting women worldwide. In 2018, the incidence and mortality rates of BC in women were reported as 46.3 per 100,000 and 13.0 per 100,000, respectively, indicating a rising trend [20]. The incidence of BC is expected to continue rising in the long term due to changes in lifestyle, fertility patterns, environmental factors, as well as the aging and growth of the population [3,21]. In China, many BC patients seek medical attention at a late stage, resulting in late diagnosis. These patients often exhibit characteristics such as lower TNM grade, lower positive rate of hormone receptors, higher likelihood for recurrence, and poor prognosis. This situation poses significant challenges and burdens on both the society and the families of affected patients [22,23]. Therefore, it is of great importance to effectively improve the diagnostic effect of patients with early BC.
Ultrasound is frequently employed as an auxiliary examination technique for the detection and diagnosis of BC due to its non-invasive nature, painlessness, high repeatability, and ease of operation [18,24]. Ultrasonic technology includes UE, CEUS, three-dimensional ultrasound and ABVS etc. Guo et al. [5] have revealed that new ultrasound imaging techniques, ultrasound-guided biopsies and the combination of ultrasound and other methods can provide important tools for the management of breast disease. While breast ultrasonography is painless and simple to perform, it can has limitations in detecting subtle masses and accurately displaying parasternal lymph nodes [25]. In recent years, CEA, CA199 and CA153 are frequently adopted as clinical tumor markers [26]. Wang et al. [27] have revealed that serum CEA, CA199, CA125, CA15-3 and TPS can be adopted in the diagnosis of metastatic BC, with different combinations of tumor markers offering varying diagnostic value. According to a study by Song et al. [28], color Doppler ultrasound combined with joint detection of serum CA153, CEA and TSGF improves diagnostic accuracy, facilitates early diagnosis, and aids in clinical intervention of BC. In this study, patients with breast lesions who had received multimodal ultrasound and detection of tumor markers were enrolled to explore the diagnostic value of combining these methods in detecting BC.
With pathological diagnosis as the gold standard, the diagnostic effectiveness of multimodal ultrasound (two-dimensional ultrasound, CDFI, UE and ABVS) were evaluated. The results showed that two-dimensional ultrasound, CDFI, UE and ABVS identified 81, 87, 91 and 91 cases of malignant lesions in malignant group, and identified 62, 66, 70 and 72 cases of benign lesions in the benign group, respectively. The multimodal approach identified 96 malignant cases in the malignant group and 72 cases of benign lesions in benign group. The results indicate better diagnostic effect of multimodal ultrasound in early-stage BC than the each of the four ultrasound modalities alone.
Li et al. [29] have revealed that preoperative serum CEA can serve as an independent predictor of unfavorable prognosis in young BC patients. To further explore the diagnostic value of tumor markers in early BC, serum levels of tumor markers (CA153, CA125 and CEA) in both groups were determined. According to the results, the malignant group showed notably higher levels of serum A153, CA125 and CEA than the benign group, highlighting their diagnostic value in early BC. This study found that the levels of CA125, CA153, and CEA were correlated with disease status, and the combined detection of these three markers significantly improved specificity and accuracy compared to individual markers.
Finally, the diagnostic value of combining multimodal ultrasound with these tumor markers was explored. The results showed that the AUCs of multimodal ultrasound and combined tumor markers (CA153, CA125 and CEA) in diagnosing BC were 0.845 and 0.928 respectively, while the AUC for the combination of both was 0.971. This joint diagnostic approach demonstrated higher accuracy and specificity than multimodal ultrasound and combined tumor markers alone, indicating that joint examination is of higher value in the diagnosis of breast tumors. Similarly, Kong et al. [30] have revealed a crucial role of the combination of ultrasound, enhanced CT, and tumor markers (AFP and CA199) in the early diagnosis of liver cancer, which is consistent with the results of this study.
Despite the promising results obtained in this study, certain limitations should be acknowledged. First, the sample size was relatively small, which may limit the generalizability of our findings. Furthermore, the retrospective nature of this analysis inevitably introduces inherent biases and limitations typical of such designs. Future studies with larger sample sizes and prospective designs could help provide more robust and reliable evidence regarding the diagnostic value of multimodal ultrasound and tumor markers in the diagnosis of BC. Future studies could specifically focus on comparing different imaging modalities and using multivariable regression analysis to account for potential confounding factors. By addressing these limitations, we can further enhance our understanding of the diagnostic capabilities and clinical utility of multimodal ultrasound in BC diagnosis.
In conclusion, for patients with early BC, the combination of multimodal ultrasound and tumor marker detection offers greater accuracy in the diagnosis of benign and malignant early breast lesions compared to either method alone, making it a valuable approach worth promoting in clinical practice.
Disclosure of conflict of interest
None.
References
- 1.Katsura C, Ogunmwonyi I, Kankam HK, Saha S. Breast cancer: presentation, investigation and management. Br J Hosp Med (Lond) 2022;83:1–7. doi: 10.12968/hmed.2021.0459. [DOI] [PubMed] [Google Scholar]
- 2.Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, Zaguia A, Koundal S, Belay A. Global increase in breast cancer incidence: risk factors and preventive measures. Biomed Res Int. 2022;2022:9605439. doi: 10.1155/2022/9605439. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17:43–46. doi: 10.7314/apjcp.2016.17.s3.43. [DOI] [PubMed] [Google Scholar]
- 4.Jafari SH, Saadatpour Z, Salmaninejad A, Momeni F, Mokhtari M, Nahand JS, Rahmati M, Mirzaei H, Kianmehr M. Breast cancer diagnosis: imaging techniques and biochemical markers. J Cell Physiol. 2018;233:5200–5213. doi: 10.1002/jcp.26379. [DOI] [PubMed] [Google Scholar]
- 5.Guo R, Lu G, Qin B, Fei B. Ultrasound imaging technologies for breast cancer detection and management: a review. Ultrasound Med Biol. 2018;44:37–70. doi: 10.1016/j.ultrasmedbio.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisel J, Raghu M, Hooley R. The role of ultrasound in breast cancer screening: the case for and against ultrasound. Semin Ultrasound CT MR. 2018;39:25–34. doi: 10.1053/j.sult.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Zhuang Z, Yang Z, Raj ANJ, Wei C, Jin P, Zhuang S. Breast ultrasound tumor image classification using image decomposition and fusion based on adaptive multi-model spatial feature fusion. Comput Methods Programs Biomed. 2021;208:106221. doi: 10.1016/j.cmpb.2021.106221. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Zhou ZY, Ran HL, Yuan XC, Zeng X, Zhang ZY. Diagnosis of liver tumors by multimodal ultrasound imaging. Medicine (Baltimore) 2020;99:e21652. doi: 10.1097/MD.0000000000021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloth C, Kratzer W, Schmidberger J, Beer M, Clevert DA, Graeter T. Ultrasound 2020 - diagnostics & therapy: on the way to multimodal ultrasound: contrast-enhanced ultrasound (CEUS), microvascular doppler techniques, fusion imaging, sonoelastography, interventional sonography. Rofo. 2021;193:23–32. doi: 10.1055/a-1217-7400. [DOI] [PubMed] [Google Scholar]
- 10.Bayo J, Castano MA, Rivera F, Navarro F. Analysis of blood markers for early breast cancer diagnosis. Clin Transl Oncol. 2018;20:467–475. doi: 10.1007/s12094-017-1731-1. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Zhang H, Zhang L, Liu H, Li S, Wang Y, Deng X. Serum markers CA125, CA153, and CEA along with inflammatory cytokines in the early detection of lung cancer in high-risk populations. Biomed Res Int. 2022;2022:1394042. doi: 10.1155/2022/1394042. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Lu L, Ma W, Johnson CH, Khan SA, Irwin ML, Pusztai L. In silico designed mRNA vaccines targeting CA-125 neoantigen in breast and ovarian cancer. Vaccine. 2023;41:2073–2083. doi: 10.1016/j.vaccine.2023.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi J, Zhang H, Cao W, Yuan C. FTO, PIK3CB serve as potential markers to complement CEA and CA15-3 for the diagnosis of breast cancer. Medicine (Baltimore) 2023;102:e35361. doi: 10.1097/MD.0000000000035361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Z, Yang J, Liang J, Xu Y, Lu G, Han Y, Zhu J, Tan H, Xu T, Ren M. Monitoring of postoperative neutrophil-to-lymphocyte ratio, D-dimer, and CA153 in: diagnostic value for recurrent and metastatic breast cancer. Front Surg. 2023;9:927491. doi: 10.3389/fsurg.2022.927491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evain E, Raynaud C, Ciofolo-Veit C, Popoff A, Caramella T, Kbaier P, Balleyguier C, Harguem-Zayani S, Dapvril H, Ceugnart L, Monroc M, Chamming’s F, Doutriaux-Dumoulin I, Thomassin-Naggara I, Haquin A, Charlot M, Orabona J, Fourquet T, Bousaid I, Lassau N, Olivier A. Breast nodule classification with two-dimensional ultrasound using Mask-RCNN ensemble aggregation. Diagn Interv Imaging. 2021;102:653–658. doi: 10.1016/j.diii.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Cheng HD, Huang JH, Zhang YT, Tang XL, Tian JW, Wang Y. Computer aided diagnosis system for breast cancer based on color Doppler flow imaging. J Med Syst. 2012;36:3975–3982. doi: 10.1007/s10916-012-9869-4. [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Zhang C, Pang J, Wang S, Zhang L, Li K, Song B. Qualitative diagnosis of benign breast lesions and breast carcinoma with elastographic ultrasonic imaging. J BUON. 2018;23:919–924. [PubMed] [Google Scholar]
- 18.Du YR, Wu Y, Chen M, Gu XG. Application of contrast-enhanced ultrasound in the diagnosis of small breast lesions. Clin Hemorheol Microcirc. 2018;70:291–300. doi: 10.3233/CH-170368. [DOI] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 20.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol. 2022;95:20211033. doi: 10.1259/bjr.20211033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE. Breast cancer in China. Lancet Oncol. 2014;15:e279–289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Wang C, Guan J, Chen B, Xu L, Chen C. Progress of breast cancer basic research in China. Int J Biol Sci. 2021;17:2069–2079. doi: 10.7150/ijbs.60631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C, Li Y, Du M, Chen Z. Recent advances in ultrasound-triggered therapy. J Drug Target. 2019;27:33–50. doi: 10.1080/1061186X.2018.1464012. [DOI] [PubMed] [Google Scholar]
- 25.Rahman WT, Helvie MA. Breast cancer screening in average and high-risk women. Best Pract Res Clin Obstet Gynaecol. 2022;83:3–14. doi: 10.1016/j.bpobgyn.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Lertkhachonsuk AA, Buranawongtrakoon S, Lekskul N, Rermluk N, Wee-Stekly WW, Charakorn C. Serum CA19-9, CA-125 and CEA as tumor markers for mucinous ovarian tumors. J Obstet Gynaecol Res. 2020;46:2287–2291. doi: 10.1111/jog.14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–55. doi: 10.1016/j.cca.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Song X, Liang B, Wang C, Shi S. Clinical value of color Doppler ultrasound combined with serum CA153, CEA and TSGF detection in the diagnosis of breast cancer. Exp Ther Med. 2020;20:1822–1828. doi: 10.3892/etm.2020.8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Dai D, Chen B, Tang H, Xie X, Wei W. Determination of the prognostic value of preoperative CA15-3 and CEA in predicting the prognosis of young patients with breast cancer. Oncol Lett. 2018;16:4679–4688. doi: 10.3892/ol.2018.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong Y, Jing Y, Sun H, Zhou S. The diagnostic value of contrast-enhanced ultrasound and enhanced CT combined with tumor markers AFP and CA199 in liver cancer. J Healthc Eng. 2022;2022:5074571. doi: 10.1155/2022/5074571. [DOI] [PMC free article] [PubMed] [Google Scholar]