Abstract
Objective: To investigate the correlation between serum levels of Heart-type fatty acid binding protein (H-FABP), Soluble Triggering Receptor Expressed on Myeloid Cells 1 (sTREM-1), and High mobility group box 1 protein (HMGB1) with disease severity, and their prognostic value in sepsis. Methods: A retrospective analysis was conducted using the clinical data from 86 sepsis patients admitted to West China Hospital of Sichuan University between June 2021 and December 2023, and these cases constituted the observation group. In addition, clinical data from 80 healthy individuals who underwent medical examinations at our hospital during the same period served as the control group. Serum levels of H-FABP, sTREM-1, and HMGB1 were measured in both groups. Based on disease severity, the patients were categorized into mild (n=42), severe (n=28), and shock (n=16) groups. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was used to assess the patients’ condition. Follow-up evaluations showed that 60 patients survived and 26 died. Results: Serum levels of H-FABP, sTREM-1, and HMGB1 were significantly higher in the observation group compared to the control group (all P<0.05). Among the sepsis patients, the severe and shock groups exhibited significantly elevated levels of H-FABP, sTREM-1, and HMGB1 compared to the mild group, with the shock group showing the highest levels (all P<0.05). The levels of H-FABP, sTREM-1, and HMGB1 were positively correlated with APACHE II scores (r=0.760, r=0.715, r=0.709, all P<0.001). Furthermore, the levels of these biomarkers were significantly higher in patients who died than in survivors (all P<0.05). The AUCs of H-FABP, sTREM-1, and HMGB1 for predicting prognosis were 0.786, 0.790, and 0.781, respectively. Their combined prediction yielded an AUC of 0.834. Log-rank test showed that the survival time of patients with different expression levels of sTREM-1 (<856.50 pg/ml, ≥856.50 pg/ml) and HMGB1 (<395.80 ng/ml, ≥395.80 ng/ml) were significantly different (P<0.05). Conclusion: Serum levels of H-FABP, sTREM-1, and HMGB1 are elevated in sepsis patients and closely associated with the disease severity, making them valuable biomarkers for monitoring the severity of sepsis. Combined detection of serum H-FABP, sTREM-1, and HMGB1 shows promising prognostic value in sepsis, with lower levels of sTREM-1 and HMGB1 linked to improved survival.
Keywords: Sepsis, H-FABP, sTREM-1, HMGB1, prognostic value
Introduction
Sepsis is a systemic inflammatory response syndrome caused by infection and is one of the common critical conditions, with high morbidity and mortality rates [1,2]. Despite significant advancements in understanding the pathophysiology of sepsis and improvements in treatment strategies, the mortality rate of sepsis remains high [3,4]. Early identification of high-risk groups is crucial for the early assessment and treatment of sepsis, improving patient outcomes and increasing treatment success rates.
Heart-type fatty acid binding protein (H-FABP), a soluble protein, has a similar function to myoglobin but with lower molecular weight, and is mainly found in cytoplasm. H-FABP was initially used in coronary heart disease and acute myocardial infarction (AMI). In recent years, it has also been used as a marker for myocardial damage in sepsis [5,6]. When myocardial cells are damaged, H-FABP is quickly released from the myocardial layer into the circulatory system, and its presence in serum can detect even minor myocardial injury, making it a highly sensitive indicator for AMI [7]. It has been shown that H-FABP level is related to the severity of sepsis, reflecting the cardiac function of sepsis patients and helping to evaluate disease progression [8].
Soluble triggering receptor expressed on myeloid cells 1 (sTREM-1) is mainly produced by the proteolytic cleavage of triggering receptor expressed on myeloid cells 1 (TREM-1), which mainly exists in blood and urine. During bacterial or fungal infections, sTREM-1 is upregulated in neutrophils and mature monocytes and released into biological fluids. In sepsis, an increase in sTREM-1 level is associated with the severity of infection and the extent of the inflammatory response, making it a useful biomarker reflecting disease activity [9,10].
High mobility group box 1 protein (HMGB1), commonly present in the nuclei of various immune cells, endothelial cells, and epithelial cells, is a member of the integrin family and a representative damage-related molecular pattern (DAMP) [11,12]. HMGB1 can be actively or passively released from activated monocytes/macrophages and necrotic cells. In a physiological state, HMGB1 binds nuclear chromosomes to regulate biologic gene transcription, participate in cell replication, differentiation, and maturation, and can activate the innate immune system independently of foreign pathogens. In sepsis and septic shock, HMGB1 plays a dual role: On the one hand, it triggers inflammation by inducing the production and release of other inflammatory mediators and lead to vascular endothelial cell damage. On the other hand, HMGB1 responds to early inflammatory signals, initiating a cascade reaction that exacerbates multi-organ damage. HMGB1 levels are typically elevated in sepsis, reflecting the severity of the systemic inflammatory response. This makes HMGB1 an important biomarker for assessing the severity of sepsis and disease activity [13,14].
This study conducted a retrospective analysis of clinical data from sepsis patients treated at our institution. The aim of this study was to investigate the serum levels of H-FABP, sTREM-1, and HMGB1, explore their correlations with disease severity, and assess their prognostic value in sepsis patients.
Patients and methods
Ethical approval statement
This study was approved by the Ethics Committee of West China Hospital of Sichuan University. As it is a retrospective observational study, the clinical trial registration was exempted.
Patients
A retrospective analysis was conducted on the clinical data from 86 sepsis patients admitted to West China Hospital of Sichuan University between June 2021 and December 2023, and these cases constituted the observation group. Inclusion criteria: confirmed diagnosis of sep-sis [15], Sequential Organ Failure Assessment (SOFA) score ≥2.0; and complete clinical documentation. Exclusion criteria: a history of organ transplantation; glucocorticoids use; presence of malignancy; or recent onset of acute infection. Additionally, 80 healthy individuals who underwent medical examinations at our hospital during the same period served as the control group. There were no significant differences in general demographic characteristics between the two groups (all P>0.05, Table 1).
Table 1.
Comparison of general information between two groups
| Group | Age (years) | Gender | BMI (kg/m2) | |
|---|---|---|---|---|
|
| ||||
| Male | Female | |||
| Control group (n=80) | 50.49±8.98 | 46 | 34 | 25.46±2.98 |
| Observation group (n=86) | 51.07±9.20 | 45 | 41 | 25.93±3.15 |
| t/χ2 | 0.411 | 0.448 | 0.986 | |
| P | 0.682 | 0.503 | 0.326 | |
Note: BMI: body mass index.
Research methods
Serum levels of H-FABP, sTREM-1, and HMGB1 were measured as follows: A fasting venous blood sample of 10 ml was collected from each participant in the morning. The supernatant was carefully collected and stored in 2 ml clean, dry serum storage tubes, which were then frozen at -70°C until analysis. Enzyme-linked immunosorbent assays were used to quantify serum levels of H-FABP, sTREM-1, and HMGB1. H-FABP detection kit was purchased from Shanghai Baiyi Biotechnology Co., Ltd. (product number: by-B11179); STREM-1 test kit was purchased from Shanghai Bangjing Industrial Co., Ltd. (product number: BJ-E987883); HMGB1 detection kit was purchased from Shanghai Siger Biotechnology Co., Ltd. (product number: XG-E99197).
The severity of sepsis was categorized based on disease progression in accordance with established guidelines [16]. Mild sepsis: defined as systemic inflammatory response syndrome caused by confirmed or suspected infection; Severe sepsis: characterized by organ dysfunction and/or tissue hypoperfusion in the presence of sepsis; Septic shock: severe sepsis accompanied by irreversible hypotension that is unresponsive to treatment. According to these criteria, patients were categorized into mild group (n=42), severe group (n=28), and shock group (n=16). Patients were assessed using the Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring system, with higher scores indicating more severe disease conditions.
Patients were followed up starting from admission on days 1, 3, and 5, and then weekly until day 28. Follow-up was conducted through outpatient visit and telephone contact. Based on these follow-up results, patients were categorized into survivors (n=60) and those who died (n=26).
Statistical methods
All data were analyzed using SPSS 22.0 statistical software, and GraphPad Prism 8 was used for graphical representation. Counted data were expressed as rates and compared using a chi-square test. Continuous variables conforming to a normal distribution were expressed as mean ± standard deviation and compared between groups using a T-test. Pearson correlation analysis was conducted to assess relationships between variables. ROC curve analysis was employed to evaluate the prognostic significance of serum levels of H-FABP, sTREM-1, and HMGB1 in sepsis patients. The Kaplan-Meier method was used to plot the survival curve, and a log-rank test was used to compare the survival time of sepsis patients with different levels of H-FABP, sTREM-1, and HMGB1. A P-value of less than 0.05 was considered significant.
Results
Comparison of serum levels of H-FABP, sTREM-1, and HMGB1 between the two groups
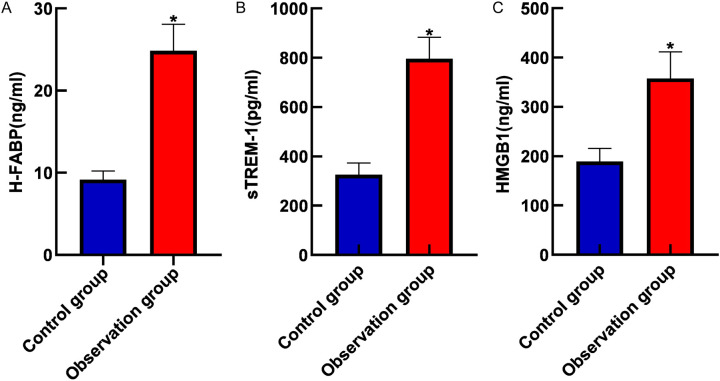
Compared to the control group, the observation group showed significantly elevated serum levels of H-FABP, sTREM-1, and HMGB1 (all P<0.05), as shown in Figure 1.
Figure 1.
Comparison of serum H-FABP (A), sTREM-1 (B) and HMGB1 (C) levels between the two groups. Note: H-FABP: Heart-type fatty acid binding protein; sTREM-1: Soluble triggering receptor expressed on myeloid cells 1; HMGB1: High mobility group box 1 protein. *, P<0.05.
Comparison of serum levels of H-FABP, sTREM-1, and HMGB1 among sepsis patients of varying disease severity
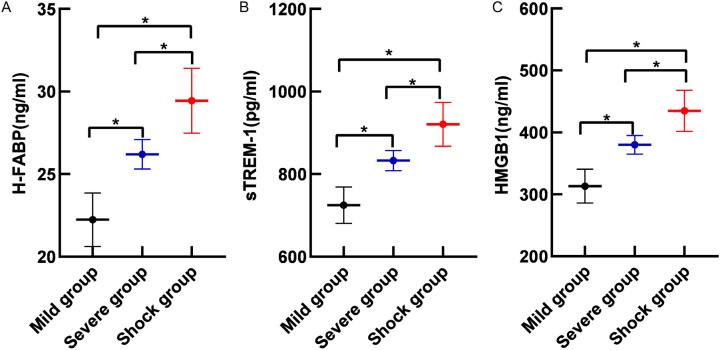
The serum levels of H-FABP, sTREM-1, and HMGB1 were significantly higher in the severe and shock groups than those of the mild group, with the septic shock group exhibiting the highest levels (all P<0.05), as shown in Figure 2.
Figure 2.
Comparison of serum H-FABP (A), sTREM-1 (B) and HMGB1 (C) levels in sepsis patients with different disease severity. Note: H-FABP: Heart-type fatty acid binding protein; sTREM-1: Soluble triggering receptor expressed on myeloid cells 1; HMGB1: High mobility group box 1 protein. *, P<0.05.
Correlation between serum H-FABP, sTREM-1, and HMGB1 levels and severity of sepsis
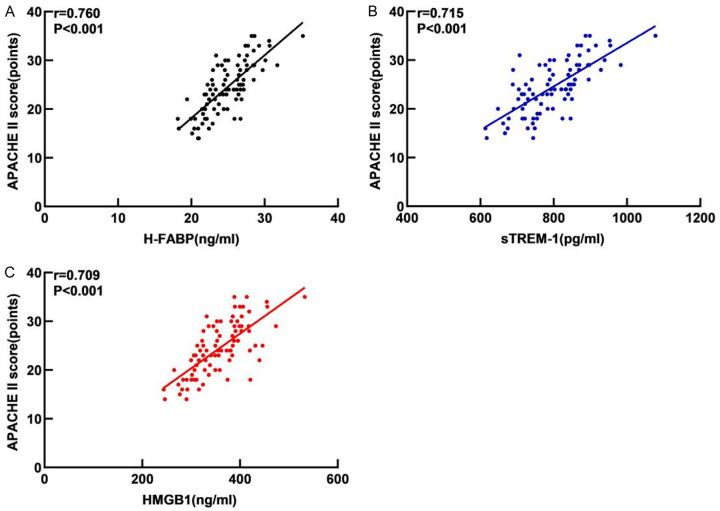
As shown in Figure 3, the serum levels of H-FABP, sTREM-1, and HMGB1 in sepsis patients were positively correlated with APACHE II scores (r=0.760, r=0.715, r=0.709, all P<0.001).
Figure 3.
Correlation between serum H-FABP (A), sTREM-1 (B) and HMGB1 (C) levels and disease severity in sepsis patients. Note: H-FABP: Heart-type fatty acid binding protein; sTREM-1: Soluble triggering receptor expressed on myeloid cells 1; HMGB1: High mobility group box 1 protein.
Comparison of serum levels of H-FABP, sTREM-1, and HMGB1 between sepsis patients with different outcomes
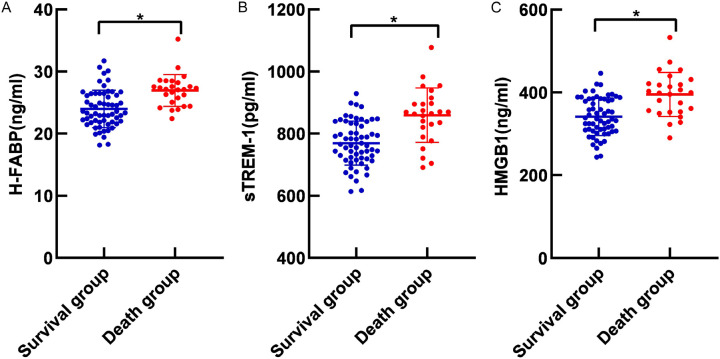
Compared to the sruvivors, patients who died exhibited significantly elevated serum levels of H-FABP, sTREM-1, and HMGB1 (all P<0.05, Figure 4).
Figure 4.
Serum H-FABP (A), sTREM-1 (B) and HMGB1 (C) levels in sepsis patients with different prognosis. Note: H-FABP: Heart-type fatty acid binding protein; sTREM-1: Soluble triggering receptor expressed on myeloid cells 1; HMGB1: High mobility group box 1 protein. *, P<0.05.
Prognostic values of serum levels of H-FABP, sTREM-1, and HMGB1 in sepsis patients
ROC curve analysis revealed that serum H-FABP had an AUC of 0.786 (95% CI: 0.689-0.883), with an optimal cutoff value of 23.67 ng/ml and a Youden index of 0.512, yielding a sensitivity of 96.20% and specificity of 55.00% for predicting sepsis patient outcomes. Serum sTREM-1 showed an AUC of 0.790 (95% CI: 0.678-0.903), with an optimal cutoff value of 856.50 pg/ml and a Youden index of 0.532, achieving a sensitivity of 61.50% and specificity of 91.70%. Serum HMGB1 had an AUC of 0.781 (95% CI: 0.674-0.888), with an optimal cutoff value of 395.80 ng/ml and a Youden index of 0.494, showing a sensitivity of 57.70% and specificity of 91.70% for prognostic evaluation in sepsis patients. When combined, the three biomarkers yielded an AUC of 0.834 (95% CI: 0.739-0.930), with a Youden index of 0.549, sensitivity of 61.50%, and specificity of 93.40% for predicting patient outcome in sepsis. See Table 2 and Figure 5.
Table 2.
Prognostic values of serum H-FABP, sTREM-1 and HMGB1 levels in sepsis patients
| Predictive index | AUC | 95% CI | Youden index | Sensitivity (%) | Specificity (%) | Cut-off value |
|---|---|---|---|---|---|---|
| H-FABP | 0.786 | 0.689-0.883 | 0.512 | 96.20 | 55.00 | 23.67 ng/ml |
| sTREM-1 | 0.790 | 0.678-0.903 | 0.532 | 61.50 | 91.70 | 856.50 pg/ml |
| HMGB1 | 0.781 | 0.674-0.888 | 0.494 | 57.70 | 91.70 | 395.80 ng/ml |
| Combined prediction | 0.834 | 0.739-0.930 | 0.549 | 61.50 | 93.40 | - |
Note: H-FABP: Heart-type fatty acid binding protein; sTREM-1: Soluble triggering receptor expressed on myeloid cells 1; HMGB1: High mobility group box 1 protein.
Figure 5.
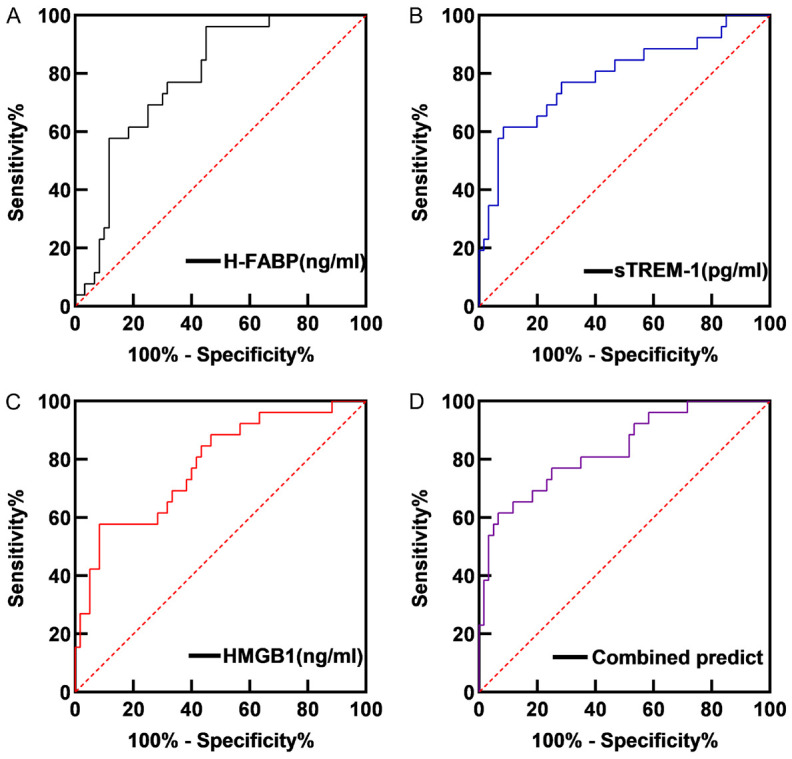
ROC curve of serum levels of H-FABP (A), sTREM-1 (B), HMGB1 (C) and Combined (D) in predicting the prognosis of sepsis patients. Note: H-FABP: Heart-type fatty acid binding protein; sTREM-1: Soluble triggering receptor expressed on myeloid cells 1; HMGB1: High mobility group box 1 protein.
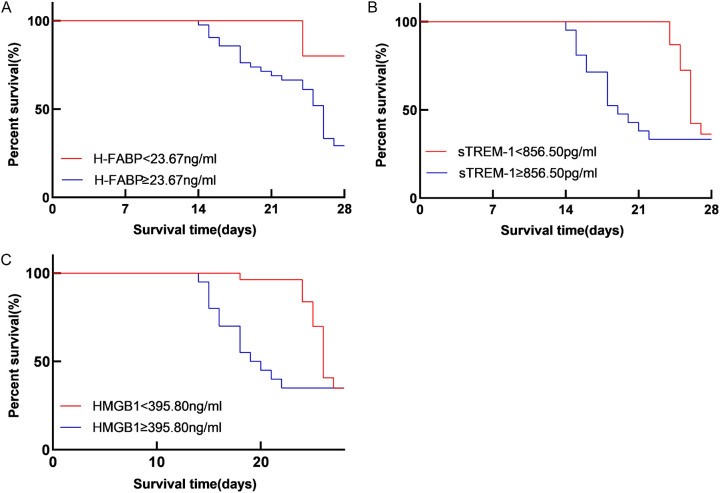
Comparison of survival time of sepsis patients with different levels of H-FABP, sTREM-1, and HMGB1
Log-rank test showed that the survival time of patients with high and low expression levels of sTREM-1 (cut-off value at 856.50 pg/ml) and HMGB1 (cut-off value at 395.80 ng/ml) differed significantly (χ2=5.576, 4.132, all P<0.05). However, no significant difference was observed in the survival time of patients with high and low H-FABP expression levels (cut-off value at 23.67 ng/ml) (χ2=3.234, P>0.05), as shown in Figure 6.
Figure 6.
Comparison of survival time of sepsis patients with different levels of H-FABP (A), sTREM-1 (B), and HMGB1 (C). Note: H-FABP: Heart-type fatty acid binding protein; sTREM-1: Soluble triggering receptor expressed on myeloid cells 1; HMGB1: High mobility group box 1 protein.
Discussion
Sepsis is characterized by acute onset, rapid disease progression, and high mortality rate. Its pathogenesis is complex, involving an imbalance in immune responses triggered by invading pathogens, leading to a pathologic syndrome marked by sustained excessive inflammation and immune suppression. Sepsis exhibits complex changes in clinical progression, necessitating further investigation into its pathophysiologic mechanisms. Studies [17] show that during infection, the immune system is activated and imbalance in immune system regulation plays a key role in the occurrence, development, and prognosis of sepsis. Disruption of physiological homeostasis triggers the release of numerous pro-inflammatory cytokines, leading to a cascade of inflammatory responses [18]. This can result in severe dysfunction of multiple organ systems, such as cardiovascular, pulmonary, coagulation, and digestive systems, posing life-threatening risks. Early immune dysregulation in sepsis may contribute to its challenging management and high clinical mortality rate [19,20]. At present, most of the clinical diagnostic methods for sepsis patients have low specificity and show obvious shortcomings in assessing disease severity and treatment efficacy, failing to meet the current clinical needs [21,22]. Early identification and diagnosis can reduce the likelihood of sepsis progressing to septic shock. Therefore, finding a diagnostic method with high sensitivity and specificity is crucial for improving the prognosis of sepsis. Commonly used indicators in clinical practice to assess the prognosis of sepsis patients include APACHE II score and SOFA score [23,24]. Thus, this study utilized the APACHE II score to evaluate the severity of illness in sepsis patients.
H-FABP is a cytoplasmic protein abundant in myocardial cells, with specific immunological characteristics and molecular structure. It functions as a carrier for long-chain fatty acids in the cytoplasm, facilitating their entry into mitochondria for oxidation. When myocardial cells are damaged, H-FABP levels increase, as the body mobilizes fatty acids to supply energy, which then enter circulation through endothelial channels. Shortly after cellular damage, it is rapidly released from myocardial cells into circulation, leading to a rapid increase of H-FABP in the blood [25,26]. Previous studies [27] have confirmed that most sepsis patients are accompanied by symptoms of myocardial injury, though there has been limited research specifically evaluating H-FABP levels to assess the severity of sepsis. The findings of this study demonstrate a significant elevation in serum H-FABP levels in sepsis patients, with H-FABP levels showing a significant positive correlation with APACHE II scores. This suggests that higher levels of H-FABP are associated with increased disease severity. Zhang et al. [28] reported that H-FABP, as a biomarker of myocardial injury and systemic inflammatory reaction, was significantly increased in sepsis patients, positively correlated with the severity of the disease. The analysis suggests that the extensive presence of H-FABP across multiple organs and tissues contributes to its role as a serum biomarker for assessing the severity of sepsis. Following myocardial cell damage, H-FABP is released into the bloodstream, resulting in an increased serum concentration of H-FABP. This underscores the utility of H-FABP as an indicator of sepsis severity.
TREM-1 is primarily expressed on the surface of mature neutrophils, monocytes, and macrophages, and plays a key role in promoting inflammation. TREM-1 is an important factor in triggering and inducing an inflammatory cascade reaction by promoting the production and release of inflammatory mediators [29,30]. STREM-1 is one of the secretory subtypes of TREM-1, which is likely formed by hydrolyzing the extracellular segment of TREM-1 on the cell membrane by matrix metalloproteinases [31-33]. sTREM-1 exists as a soluble form of TREM-1. It has a deletion of three extracellular potential N-linked glycosylation sites, the transmembrane segment, and the intracellular segment, and is a splice variant of TREM-1 that is released into blood and body fluids during infection [34,35]. Bellos et al. [36] found that the level of sTREM-1 was significantly increased in sepsis patients and closely related to the disease severity, which is consistent with the results in our study that an elevated sTREM-1 level in septic patients was positively correlated with APACHE II scores, suggesting that higher sTREM-1 levels are associated with greater disease severity. The analysis suggests that infection-induced upregulation of TREM-1 on cell surfaces, in synergy with Toll-like receptors, triggers and amplifies the production of inflammatory factors, consequently elevating sTREM-1 levels. Therefore, sTREM-1 serves as a useful serum biomarker for monitoring the severity of sepsis.
HMGB1 is a DNA-binding protein primarily existing in the nucleus and plays a role in maintaining nucleosome integrity and promoting gene transcription. Beyond its role in regulating gene expression and cellular motility, HMGB1 also participates in modulating inflammatory and immune responses. It can be released into the extracellular matrix by granulocytes or necrotic cells and act as a chemotactic cytokine during infection, hypoxia, and ischemia-reperfusion, thereby promoting inflammatory response, aggravating septic shock, and increasing the risk of poor prognosis in affected patients [37]. HMGB1 can also polarize Th2 cells by regulating T cell activation, causing inflammatory factors to escape immune system monitoring [38]. Previous literature has shown that HMGB1 is associated with the development of encephalitis, arthritis, and sepsis, where it acts as a late inflammatory mediator [39]. HMGB1 exhibits potent pro-inflammatory activity and plays a crucial role as an inflammatory cytokine and important mediator in the process of inflammatory immune responses, closely linked to the occurrence of sepsis [40]. The pro-inflammatory function and characteristics of HMGB1 hold greater potential for clinical application than some early inflammatory mediators. Research indicates that HMGB1 is closely associated with inflammatory responses, mediating the release of various inflammatory mediators and cytokines [41]. Moreover, studies suggest that inhibiting HMGB1-induced inflammation can protect intestinal barrier integrity and alleviate gastrointestinal dysfunction in septic animals [42]. These findings propose HMGB1 as a novel therapeutic target for treating intestinal mucosal barrier damage in sepsis, positioning it as a potential therapeutic target in sepsis research models. In recent years, targeting HMGB1 has been recognized for its significant role in modulating the inflammatory cytokine profile and its correlation with sepsis prognosis. Qiang et al. [43] reported that increased HMGB1 level was closely related to the severity and prognosis of sepsis patients. HMGB1 can aggravate sepsis by promoting the release of inflammatory mediators, a mechanism also verified in our study, suggesting its use as a powerful biomarker for prognosis evaluation of sepsis. The findings of this study reveal significantly elevated serum levels of HMGB1 in septic patients, which correlate positively with APACHE II scores. This can be explained by HMGB1’s dual role as a pro-inflammatory mediator that activates inflammation and promotes tissue repair. Following inflammation, injury, or stress, HMGB1 can be passively released from necrotic cells or actively secreted by activated inflammatory cells.
The ROC curve analysis in this study revealed that the AUCs of serum H-FABP, sTREM-1, and HMGB1 for predicting prognosis were 0.786, 0.790, and 0.781, respectively. When combined, these three biomarkers achieved an AUC of 0.834. These findings indicate that the combined assessment of these biomarkers significantly improves the prognostic evaluation compared to each single marker, highlighting their enhanced diagnostic value. The survival time of patients with sTREM-1≥856.50 pg/ml and HMGB1≥395.80 ng/ml was notably shorter than that of patients with sTREM-1<856.50 pg/ml and HMGB1<395.80 ng/ml. This indicates that patients with low levels of sTREM-1 and HMGB1 have better survival.
The limitations of this study are as follows: First, this was a single-center study, and the results may be influenced by regional factors and local healthcare conditions, which inevitably introduces limitations. Therefore, multi-center and multi-regional clinical studies are needed to further validate the findings. Second, as a retrospective study, the collected data may have inherent limitations. Prospective studies or randomized controlled trials are needed to better control confounding factors. Additionally, this study did not assess the dynamic changes in the observed indicators, so further large-scale experiments and dynamic observational studies are required. Lastly, the sample size of this study was relatively small,soexpanding the sample size is necessary to improve the accuracy of the research.
Conclusion
Serum levels of H-FABP, sTREM-1, and HMGB1 are consistently elevated in patients with sepsis, and these levels exhibit a close correlation with disease severity. Higher serum levels of H-FABP, sTREM-1, and HMGB1 are indicative of more severe disease, establishing them as critical serum biomarkers for monitoring the severity of sepsis. Finally, lower levels of sTREM-1 and HMGB1 predict longer survival of septic patients.
Disclosure of conflict of interest
None.
References
- 1.Weiss SL, Nicolson SC, Naim MY. Clinical update in pediatric sepsis: focus on children with pre-existing heart disease. J Cardiothorac Vasc Anesth. 2020;34:1324–1332. doi: 10.1053/j.jvca.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Carey MG, Valcin EK, Lent D, White M. Nursing care for the initial resuscitation of severe sepsis patients. Crit Care Nurs Clin North Am. 2021;33:263–274. doi: 10.1016/j.cnc.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Sanyaolu A, Patidar R, Ayodele O, Marinkovic A, Desai P. Pediatric sepsis: the importance of understanding criteria for diagnosis. Pediatr Ann. 2022;51:e405–e408. doi: 10.3928/19382359-20220803-07. [DOI] [PubMed] [Google Scholar]
- 4.Stephen AH, Montoya RL, Aluisio AR. Sepsis and septic shock in low- and middle-income countries. Surg Infect (Larchmt) 2020;21:571–578. doi: 10.1089/sur.2020.047. [DOI] [PubMed] [Google Scholar]
- 5.Chen FC, Xu YC, Zhang ZC. Multi-biomarker strategy for prediction of myocardial dysfunction and mortality in sepsis. J Zhejiang Univ Sci B. 2020;21:537–548. doi: 10.1631/jzus.B2000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng G, Tian P, Yan X, Cheng Q. Altered function of the left ventricle and clinical significance of heart-type fatty acid-binding protein in cardiac dysfunction among patients with sepsis. Exp Ther Med. 2020;20:58. doi: 10.3892/etm.2020.9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo YH, Kim K, Lee JH, Rhee JE, Lee JH, Kang KW, Rim KP, Hwang SS, Park HM. Heart-type fatty acid-binding protein as a prognostic factor in patients with severe sepsis and septic shock. Am J Emerg Med. 2012;30:1749–1755. doi: 10.1016/j.ajem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Yang D, Liu G, Guo S, Ren H, Lu H, Zhou L, Bao L. Prognostic value of serum heart-type fatty acid-binding protein in patients with sepsis: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e24715. doi: 10.1097/MD.0000000000024715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dholariya S, Parchwani DN, Singh R, Radadiya M, Katoch CDS. Utility of P-SEP, sTREM-1 and suPAR as novel sepsis biomarkers in SARS-CoV-2 infection. Indian J Clin Biochem. 2022;37:131–138. doi: 10.1007/s12291-021-01008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y, Xiao P, Wu B, Chen F, Shi X. Significance of sTREM-1 and sST2 combined diagnosis for sepsis detection and prognosis prediction. Open Life Sci. 2023;18:20220639. doi: 10.1515/biol-2022-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng M, Scott MJ, Fan J, Billiar TR. Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J Leukoc Biol. 2019;106:161–169. doi: 10.1002/JLB.3MIR1218-497R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Zhu CS, He L, Qiang X, Chen W, Wang H. A two-decade journey in identifying high mobility group box 1 (HMGB1) and procathepsin L (pCTS-L) as potential therapeutic targets for sepsis. Expert Opin Ther Targets. 2023;27:575–591. doi: 10.1080/14728222.2023.2239495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Ye Z, Tang Y. Serum HMGB1 and soluble urokinase plasminogen activator receptor levels aid diagnosis and prognosis prediction of sepsis with acute respiratory distress syndrome. Biomark Med. 2023;17:231–239. doi: 10.2217/bmm-2022-0899. [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Zhang X. A potential new pathway for heparin treatment of sepsis-induced lung injury: inhibition of pulmonary endothelial cell pyroptosis by blocking hMGB1-LPS-induced caspase-11 activation. Front Cell Infect Microbiol. 2022;12:984835. doi: 10.3389/fcimb.2022.984835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Hylander Møller M, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 16.Ding R, Wang Z, Lin Y, Liu B, Zhang Z, Ma X. Comparison of a new criteria for sepsis-induced coagulopathy and International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul Fibrinolysis. 2018;29:551–558. doi: 10.1097/MBC.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter BL, Underwood J. Sepsis and the brain: a review for acute and general physicians. Clin Med (Lond) 2022;22:392–395. doi: 10.7861/clinmed.2022-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramoni D, Tirandi A, Montecucco F, Liberale L. Sepsis in elderly patients: the role of neutrophils in pathophysiology and therapy. Intern Emerg Med. 2024;19:901–917. doi: 10.1007/s11739-023-03515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu AC, Patel K, Vunikili RD, Johnson KW, Abdu F, Belman SK, Glicksberg BS, Tandale P, Fontanez R, Mathew OK, Kasarskis A, Mukherjee P, Subramanian L, Dudley JT, Shameer K. Sepsis in the era of data-driven medicine: personalizing risks, diagnoses, treatments and prognoses. Brief Bioinform. 2020;21:1182–1195. doi: 10.1093/bib/bbz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobi J. The pathophysiology of sepsis - 2021 update: part 2, organ dysfunction and assessment. Am J Health Syst Pharm. 2022;79:424–436. doi: 10.1093/ajhp/zxab393. [DOI] [PubMed] [Google Scholar]
- 21.Esposito S, De Simone G, Boccia G, De Caro F, Pagliano P. Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist. 2017;10:204–212. doi: 10.1016/j.jgar.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Russell N, Barday M, Okomo U, Dramowski A, Sharland M, Bekker A. Early-versus late-onset sepsis in neonates - time to shift the paradigm? Clin Microbiol Infect. 2024;30:38–43. doi: 10.1016/j.cmi.2023.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149:252–261. doi: 10.1378/chest.15-1703. [DOI] [PubMed] [Google Scholar]
- 24.Paternoster G, Guarracino F. Sepsis after cardiac surgery: from pathophysiology to management. J Cardiothorac Vasc Anesth. 2016;30:773–780. doi: 10.1053/j.jvca.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 25.He S, Leng W, Du X, He Y, Zhao Y, Wang Y, Yu S. Diagnostic significance of heart-type fatty acid-binding protein as a potential biomarker to predict the mortality rate of patients with sepsis: a systematic review and meta-analysis. Expert Rev Mol Diagn. 2022;22:379–386. doi: 10.1080/14737159.2022.2046464. [DOI] [PubMed] [Google Scholar]
- 26.Chen YX, Li CS. The prognostic and risk-stratified value of heart-type fatty acid-binding protein in septic patients in the emergency department. J Crit Care. 2014;29:512–516. doi: 10.1016/j.jcrc.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Ding R, Cheng Q, Tian P, Guli B, Xu C. Diagnostic value of heart-type fatty acid-binding protein combined with echocardiography in sepsis with cardiac insufficiency. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:478–482. doi: 10.3760/cma.j.cn121430-20191111-00064. [DOI] [PubMed] [Google Scholar]
- 28.Zhang ZC, Dai HW, Yu YH, Yang JD, Hu CB. Usefulness of heart-type fatty acid-binding protein in patients with severe sepsis. J Crit Care. 2012;27:415, e13–8. doi: 10.1016/j.jcrc.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Qin Q, Liang L, Xia Y. Diagnostic and prognostic predictive values of circulating sTREM-1 in sepsis: a meta-analysis. Infect Genet Evol. 2021;96:105074. doi: 10.1016/j.meegid.2021.105074. [DOI] [PubMed] [Google Scholar]
- 30.Smok B, Domagalski K, Pawłowska M. Diagnostic and prognostic value of IL-6 and sTREM-1 in SIRS and sepsis in children. Mediators Inflamm. 2020;2020:8201585. doi: 10.1155/2020/8201585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng Y, Wu D, Zhuo X, Song J. Effects of continuous blood purification without heparin on strem-1, NSE, and IL-10 levels in patients with sepsis. Cell Mol Biol (Noisy-le-grand) 2022;68:178–187. doi: 10.14715/cmb/2022.68.4.21. [DOI] [PubMed] [Google Scholar]
- 32.Aksaray S, Alagoz P, Inan A, Cevan S, Ozgultekin A. Diagnostic value of sTREM-1 and procalcitonin levels in the early diagnosis of sepsis. North Clin Istanb. 2016;3:175–182. doi: 10.14744/nci.2016.26023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su L, Xie L, Liu D. Urine sTREM-1 may be a valuable biomarker in diagnosis and prognosis of sepsis-associated acute kidney injury. Crit Care. 2015;19:281. doi: 10.1186/s13054-015-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang W, Peng F, Meng SS, Xu JY, Yang Y. Diagnostic value of serum soluble triggering expressed receptor on myeloid cells 1 (sTREM-1) in suspected sepsis: a meta-analysis. BMC Immunol. 2020;21:2. doi: 10.1186/s12865-020-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tranca S, Oever JT, Ciuce C, Netea M, Slavcovici A, Petrișor C, Hagau N. sTREM-1, sIL-2Rα, and IL-6, but not sCD163, might predict sepsis in polytrauma patients: a prospective cohort study. Eur J Trauma Emerg Surg. 2017;43:363–370. doi: 10.1007/s00068-016-0678-1. [DOI] [PubMed] [Google Scholar]
- 36.Bellos I, Fitrou G, Daskalakis G, Thomakos N, Papantoniou N, Pergialiotis V. Soluble TREM-1 as a predictive factor of neonatal sepsis: a meta-analysis. Inflamm Res. 2018;67:571–578. doi: 10.1007/s00011-018-1149-4. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Choo S, Sim H, Baek MC, Bae JS. Biapenem reduces sepsis mortality via barrier protective pathways against HMGB1-mediated septic responses. Pharmacol Rep. 2021;73:786–795. doi: 10.1007/s43440-020-00212-0. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Wei S, Gao Y, Dai X, Fu W, Cai S, Fang H, Zeng Z, Chen Z. SIRT1-mediated HMGB1 deacetylation suppresses sepsis-associated acute kidney injury. Am J Physiol Renal Physiol. 2019;316:F20–F31. doi: 10.1152/ajprenal.00119.2018. [DOI] [PubMed] [Google Scholar]
- 40.Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F, Gill PS, Ha T, Liu L, Williams DL, Li C. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022;29:133–146. doi: 10.1038/s41418-021-00841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin XY, Tang XH, Wang SX, Zhao YC, Jia M, Yang JJ, Ji MH, Shen JC. HMGB1 mediates synaptic loss and cognitive impairment in an animal model of sepsis-associated encephalopathy. J Neuroinflammation. 2023;20:69. doi: 10.1186/s12974-023-02756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyer S, Kennedy JN, Powell R, Brant E, Martin-Gill C, Seymour CW. The association between prehospital HMGB1 and sepsis in emergency care. Eur J Emerg Med. 2023;30:52–54. doi: 10.1097/MEJ.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiang X, Peng Y, Wang Z, Fu W, Li W, Zhao Q, He D. Synthesis of glycyrrhizin analogues as HMGB1 inhibitors and their activity against sepsis in acute kidney injury. Eur J Med Chem. 2023;259:115696. doi: 10.1016/j.ejmech.2023.115696. [DOI] [PubMed] [Google Scholar]