Abstract
Objective: To investigate the regulatory effect of glycyrrhizin (GL) on the release of neutrophil extracellular traps (NETs) from neutrophils in sepsis. Methods: HL-60 cells were induced to differentiate into neutrophil-like dHL-60 cells to establish a neutrophil-like sepsis model. Expression levels of high-mobility group box 1 (HMGB1), citrullinated histone H3 (Cit-H3), and Toll-like receptor 9 (TLR9) were assessed by Western blotting. Free DNA, a component of NETs, was quantified using a fluorescence microplate reader. Cellular immunofluorescence analysis was used to detect the expression of the key NETs protein, Cit-H3. Results: dHL-60 cells stimulated with 200 ng/ml LPS exhibited the highest expression of Cit-H3. The neutrophil-like sepsis model showed significantly increased levels of Cit-H3 and HMGB1. GL intervention significantly reduced the expression levels of HMGB1 and Cit-H3 and decreased the free DNA level. These findings suggest that GL decreases HMGB1 expression and NET release in the neutrophil-like sepsis model. TLR9 expression was significantly elevated in the sepsis model. Exogenous recombinant human HMGB1 protein further increased TLR9 expression, while GL inhibited this increase. Conclusion: GL may inhibit NET release in sepsis through the HMGB1/TLR9 pathway.
Keywords: Neutrophil extracellular trap, high-mobility group box 1, sepsis, Glycyrrhizin, Toll-like receptor 9
Introduction
Neutrophils play a critical role as a frontline defense against invading pathogens [1]. Neutrophils clear pathogens through phagocytosis, degranulation, and the production of large amounts of reactive oxygen species [2]. Recent studies have shown that neutrophils can also form neutrophil extracellular traps (NETs) by releasing their own nucleic acids as a backbone, anchoring numerous antibacterial proteins outside the cell, mediating the capture and clearance of pathogens [3-5]. However, growing evidence suggests that excessive NETs released by neutrophils can damage surrounding cells and tissues [6], contributing to the pathological processes of certain diseases [7], including transfusion-related acute lung injury [8], septic liver injury [9], and autoimmune diseases such as systemic lupus erythematosus [10]. The human promyelocytic leukemia cell line (HL-60) can be induced to differentiate into neutrophil-like cells (dHL-60), making it a valuable model for studying NET formation.
Lipopolysaccharide (LPS), a major component of the cell wall in Gram-negative bacteria, plays a crucial role in the pathogenesis of sepsis. LPS activates immune cells by binding to pattern recognition receptors in the immune system, triggering pro-inflammatory mechanisms [11]. This excessive inflammatory response results in widespread tissue damage, increased vascular permeability, and subsequent fluid exudation and tissue edema. These changes provide a biological basis for extensive organ damage, a critical aspect of the pathological process in sepsis. Research has demonstrated that LPS can induce neutrophils to produce NETs in the context of sepsis [12]. Various NETs components can trigger intravascular coagulation, eventually leading to disseminated intravascular coagulation [13-15]. Therefore, exploring interventions to suppress NETs overproduction in sepsis is crucial.
Licorice has been used for thousands of years to treat various infectious and inflammatory diseases. Glycyrrhizin (GL), an active ingredient extracted from licorice, is known for its high sweetness, low calorie content, safety, and non-toxicity. GL has demonstrated anti-inflammatory, antiviral, antiallergic, and immunomodulatory effects [16,17]. High-mobility group box 1 (HMGB1) is a nonhistone chromatin-associated protein that, under pathological conditions, is released extracellularly and activates inflammatory responses by binding to Toll-like receptors (TLRs) or advanced glycation end product receptors. For example, damaged hepatocytes can release HMGB1 and stimulate NET formation through the TLR4 and TLR9-MyD88 signaling pathways [18]. As an HMGB1 inhibitor, GL has protective effects against ischemia-reperfusion injury in organs and can mitigate HMGB1-mediated cell death and inflammation following renal ischemia-reperfusion injury [19]. Therefore, as a commonly used anti-inflammatory Chinese herbal medicine, GL is a promising candidate for the clinical treatment of sepsis, with potential application in both prevention and therapy. However, the effects of GL on NETs in sepsis and the underlying mechanisms remain unclear. In this study, LPS was used to establish a neutrophil-like sepsis cell model to observe the effects of GL on LPS-induced neutrophil-like NET release and to explore the underlying mechanisms, hoping to provide a theoretical basis for the clinical application of GL in treating sepsis.
Materials and methods
The human promyelocytic leukemia cell line HL-60 was obtained from the laboratory at the School of Basic Science, Army Military Medical University. CD-11b-GFP fluorescent antibody and SYTOX Green nucleic acid stain were purchased from Thermo Fisher (USA). HMGB1 antibody, citrullinated Histone H3 (Cit-H3) antibody, TLR9 antibody, and β-actin were purchased from Abcam (UK). DAPI, the anti-fluorescence quencher, RIPA lysis buffer, anhydrous methanol, the prestained protein marker, the protease inhibitor, and the BCA protein concentration determination kit were purchased from Biyuntian (China). LPS (Escherichia coli, O111:B4), and dimethyl sulfoxide (DMSO) were purchased from Sigma (USA). IMDM medium and fetal bovine serum were obtained from Gibco (USA).
HMGB1, purchased from Sigma (USA), was prepared as a 300 ng/ml solution according to storage instructions. GL, purchased from Selleck Chemicals (USA), was prepared as a 100 µg/ml solution for experiments based on prior research, following the provided instructions.
Cell culture and the induction of differentiation
The human promyelocytic leukemia cell line HL-60 was cultured in IMDM medium supplemented with 20% fetal bovine serum in an incubator with 5% carbon dioxide at 37°C. Upon reaching the logarithmic growth phase, 1.25% DMSO was added to induce differentiation for varying durations, and the cells were subsequently harvested. Successful differentiation into neutrophil-like cells was confirmed by flow cytometry.
Flow cytometric analysis of CD11b expression in neutrophil-like (dHL-60) cells
dHL-60 cells were obtained after inducing differentiation with 1.25% DMSO for 2 and 5 days, with the required cell count ranging from 1 × 10^4-1 × 10^6. Then, 2 µl of CD11b fluorescent antibody was added to each tube of cells, mixed, protected from light, and incubated at 4°C for 30 minutes. The samples were centrifuged at 4°C and 1000 rpm for 5 minutes, the supernatant was discarded, and the samples were resuspended in precooled PBS. This process was repeated three times. The samples were analyzed using the instrument and protected from light.
Cellular immunofluorescence analysis
Cell samples were fixed with 4% paraformaldehyde at room temperature for 20 minutes. Slides were prepared using adhesion slides, labeled, and air-dried. The cells were washed with PBS three times, 5 minutes for each, followed by permeabilization with 0.3% Triton X-100 at 37°C for 30 minutes and washed again with PBS three times, 5 minutes for each. The cells were then incubated with goat serum at 37°C for 1 hour. Next, diluted primary antibody (Cit-H3, 1:1000) was added and incubated overnight at 4°C. Cells were then washed with PBS three times, 5 minutes each. Fluorescent secondary antibody was introduced and incubated in the dark at 37°C for 1 hour. Again, cells were washed with PBS three times, 5 minutes each. Cells were stained with DAPI at 37°C for 10 minutes and washed with PBS three times, 5 minutes each. Finally cells were mounted on the slides with an anti-fade mounting medium, stored in the dark, and observed under a fluorescence microscope.
Quantification of NETs
dHL-60 cells were seeded in black 96-well plates at a density of 1 × 10^5 cells/well, treated with LPS or LPS+GL, and incubated at 37°C with 5% CO2 for 12 hours. Untreated dHL-60 cells were used as the control. After treatment, the supernatant was collected, and SYTOX Green nucleic acid stain was added to a final concentration of 1 µM. The cells were covered and incubated in the dark for 15-30 minutes, then mixed well and measured using a fluorescence microplate reader (excitation wavelength: 485 nm, emission wavelength: 535 nm). The fluorescence value of each sample was measured in the center of the well, with a higher fluorescence value indicating a higher level of extracellular free DNA.
Western blot analysis of the expression of Cit-H3, HMGB1 and TLR9
Western blotting was used to detect the expression of HMGB1, TLR9, and Cit-H3. Each sample was lysed with 100 µL of RIPA buffer and 1 µL of PMSF, followed by centrifugation at 12,000 rpm for 15 minutes at 4°C. The supernatant was collected, and protein concentrations were determined using a BCA protein assay kit. Protein samples were separated by SDS-PAGE and transferred onto a PVDF membrane. The membrane was blocked with 5% non-fat milk for 1 hour. Primary antibodies against HMGB1 (1:10,000), Cit-H3 (1:1,000), and TLR9 (1:200) were added, and then incubated with a secondary antibody at room temperature for 1 hour. Protein bands were visualized using the Odyssey CLx near-infrared imaging system, and the results were visualized using a densitometry system. Relative protein levels were normalized to β-actin.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 9.0 software. Data were presented as means ± standard deviations (x̅ ± s) for normally distributed continuous variables. Differences between two groups were compared using an independent samples t-test. For comparisons among multiple groups, one-way analysis of variance (ANOVA) was employed followed with post-hoc Tukey’s test or Dunnett’s T3 test. A p-value less than 0.05 was considered statistically significant.
Results
HL-60 cells were successfully differentiated into dHL-60 cells after treatment with 1.25% DMSO
HL-60 cells were treated with 1.25% DMSO and collected on days 2 and 5. CD11b expression in these cells was measured by flow cytometry, showing that CD11b expression was 32.3% on day 2 and peaked at over 95% on day 5. This indicates that after 5 days of induction, dHL-60 cells exhibited neutrophil characteristics based on CD11b expression (Figure 1), making them suitable for further analyses of neutrophil nuclear morphology and viability. These findings confirm that HL-60 cells successfully differentiated into dHL-60 cells, which can be used for subsequent experiments. Based on these results, dHL-60 cells induced with 1.25% DMSO for 5 days were selected for further studies.
Figure 1.
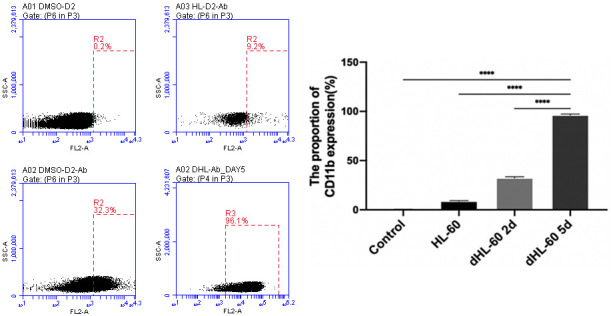
Flow cytometry analysis of human promyelocytic leukemia cells (HL-60) treated with 1.25% DMSO for CD11b expression. On day 2 post-induction, CD11b expression in differentiated HL-60 cells (dHL-60) increased. By day 5 post-induction, CD11b expression increased over 95%, indicating that dHL-60 cells exhibit characteristics of neutrophil differentiation after 5 days of induction. ****P<0.0001.
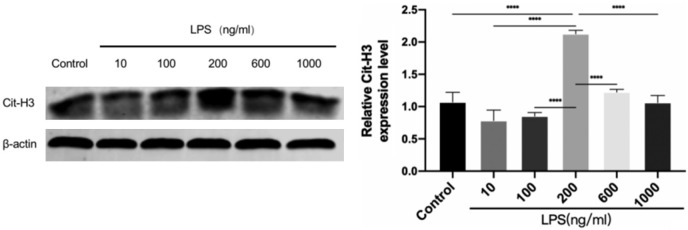
200 ng/ml was identified as the optimal concentration for LPS stimulation in the neutrophil-like sepsis model
To determine the optimal concentration of LPS for stimulating dHL-60 cells, the cells were treated with 10, 100, 200, 600, and 1000 ng/ml LPS for 12 hours. Western blotting was used to measure the expression of the key NETs protein Cit-H3. The results indicated that Cit-H3 expression was highest with 200 ng/ml LPS stimulation and was significantly greater than that in the control group (Figure 2). Based on these results, 200 ng/ml was selected as the optimal LPS concentration for subsequent experiments.
Figure 2.
Expression of citrullinated histone H3 (Cit-H3) induced by different concentrations of Lipopolysaccharide (LPS). 200 ng/ml was the optimal concentration for LPS stimulation in the neutrophil-like sepsis model. Data are presented as mean ± SD, n=3. ****P<0.0001.
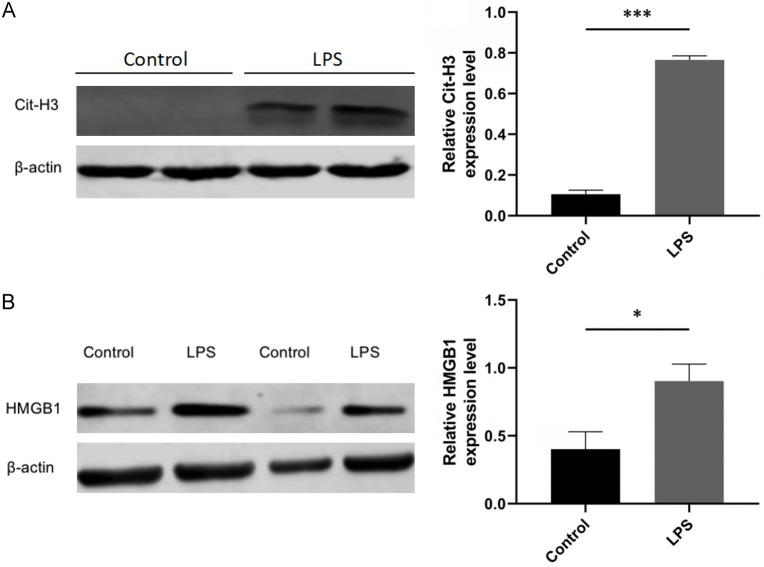
LPS significantly increased the expression levels of Cit-H3 and HMGB1 in the neutrophil-like sepsis model
The cells were divided into two groups: the control group and the LPS group. The control group was not treated with LPS, while the LPS group was treated with 200 ng/ml LPS. After 12 hours, the cells were collected, and HMGB1 and Cit-H3 expression levels were measured by Western blotting. The results showed that Cit-H3 and HMGB1 expression was significantly higher in the LPS group compared to the control group (Figure 3A, 3B).
Figure 3.
Expression levels of Cit-H3 and high-mobility group box 1 (HMGB1) in a neutrophil-like sepsis model. A. Expression levels of Cit-H3, a key neutrophil extracellular trap (NET) protein, were significantly elevated in the LPS group compared to the control group in the neutrophil-like sepsis model. B. Expression levels of HMGB1 were also increased in the LPS group in the neutrophil-like sepsis model. Data are presented as mean ± standard deviation, n=3, *P<0.05, ***P<0.001.
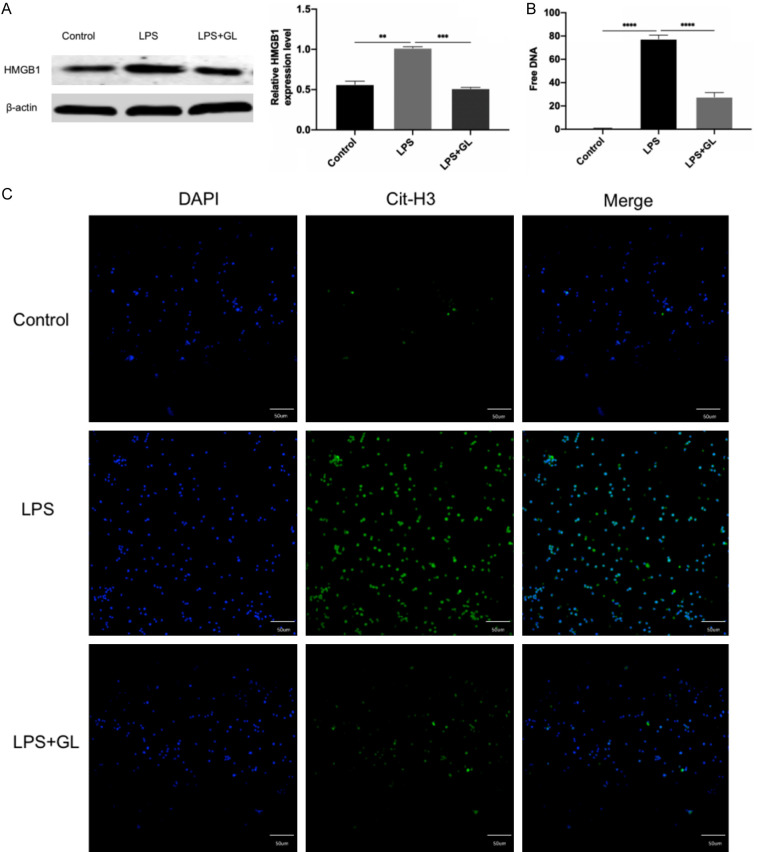
GL reduced both HMGB1 expression and NETs release in the neutrophil-like sepsis model
dHL-60 cells were divided into three groups based on treatment: (1) control group; (2) LPS group; and (3) LPS+GL group. The cells in control and LPS groups were treated as described above. The LPS+GL group was treated with LPS at the 200 ng/ml and GL 100 at μg/ml. HMGB1 expression was measured by Western blotting. The results showed that HMGB1 expression in the LPS group was significantly higher than that in the control group; however, its expression was significantly reduced after GL treatment (Figure 4A).
Figure 4.
Effects of GL on HMGB1 expression and NET release in a neutrophil-like sepsis model. A. Effect of GL on HMGB1 expression. In the LPS group, HMGB1 protein levels were elevated, while GL treatment inhibited this increase. B. Effect of GL on the release of free DNA, a key component of NETs. Free DNA release, indicative of NET formation, was reduced by GL treatment under LPS-induced conditions, suggesting that GL can suppress NET release in the neutrophil-like sepsis model. C. Effect of GL on Cit-H3 expression in the neutrophil-like sepsis model. Compared to the control group, Cit-H3 expression was significantly increased in the LPS group. GL treatment suppressed Cit-H3 expression, indicating that GL can inhibit NETs release. (DAPI: blue; Cit-H3: green; bar =50 µm, 200× magnification). Data are presented as mean ± standard deviation, n=3, **P<0.01, ***P<0.001, ****P<0.0001.
For NETs assessment, cell supernatants were added to a black 96-well microtiter plate, and 5 μM SYTOX Green nucleic acid stain was used to measure the free DNA in the NETs fraction. Free DNA was quantified by measuring green fluorescence intensity with a fluorescence microplate reader (excitation: 485 nm; emission: 535 nm). Fluorescence intensity was close to 0 in the control group, and significantly decreased in the LPS+GL group compared to the LPS group (Figure 4B).
To assess the effect of GL on Cit-H3 expression, cellular immunofluorescence analysis was performed on the three groups. Immunofluorescence results showed that the green fluorescence intensity was significantly higher in the LPS group compared to the control group, indicating increased Cit-H3 expression. This intensity was significantly reduced in the LPS+GL group, suggesting decreased Cit-H3 expression in the LPS+GL group (Figure 4C).
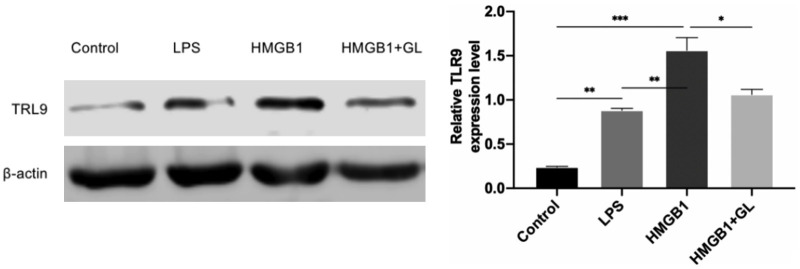
GL inhibited the TLR9 expression induced by exogenous recombinant human HMGB1 protein
The cells were divided into four groups: control, LPS, HMGB1, and HMGB1+GL groups. The control and LPS groups were treated as described above. Based on previous studies, the HMGB1 group was stimulated with LPS and 300 ng/ml recombinant human HMGB1 protein. The HMGB1+GL group was treated with LPS, 300 ng/ml recombinant human HMGB1 protein, and 100 μg/ml GL. After 12 hours, the cells were collected, and TLR9 expression was measured by Western blotting. The results showed that TLR9 expression was significantly higher in the LPS and HMGB1 groups compared to the control group, while TLR9 expression in the HMGB1+GL group was significantly lower than that in the HMGB1 group (Figure 5).
Figure 5.
Effects of glycyrrhizin (GL) and exogenous recombinant human HMGB1 protein on Toll-like receptor 9 (TLR9) levels in a neutrophil-like sepsis model. The TLR9 expression levels were significantly higher in the LPS and HMGB1 groups compared to the control group. However, TLR9 expression was notably reduced in the HMGB1+GL group compared to the HMGB1 group, indicating that GL inhibits the increased TLR9 expression induced by exogenous recombinant human HMGB1 protein. *P<0.05, **P<0.01, ***P<0.001.
Discussion
Sepsis is defined as a dysregulated host response to infection and/or infectious factors that leads to life-threatening organ dysfunction [20]. Recent studies have shown that NETs are involved in coagulation activation and thrombosis during sepsis [13,15] and are closely related to coagulation dysfunction and prognosis in sepsis patients [11]. Therefore, identifying drugs that can inhibit NET formation in sepsis is critical for its treatment.
LPS is a major pathogenic molecule associated with sepsis. It activates neutrophils to initiate NADPH oxidase-dependent NETosis and induces NETs formation through TLR4-dependent and JNK-mediated molecular sensing mechanisms [21]. Additionally, both the dose and strain of LPS can influence NETs production levels [22,23]. This study confirmed that LPS induced neutrophil-like NETs release in vitro, with the amounts of NETs produced closely related to the LPS concentration. The expression of the key NETs protein Cit-H3 peaked in dHL-60 cells in response to 200 ng/ml LPS. Therefore, 200 ng/ml was selected as the optimal concentration of LPS for subsequent experiments.
GL is a selective inhibitor of HMGB1 discovered in recent years [24]. GL exerts significant glucocorticoid-like effects, including anti-inflammatory properties, tumor growth inhibition, and immune enhancement. HMGB1 primarily interacts with receptors such as TLR-like receptors and receptors for advanced glycation end products (RAGE), with TLR9 being one of the most important intracellular receptors. Recent research shows that TLR9 is closely related to the formation and function of NETs. In a liver ischemia/reperfusion model, NET formation was driven by danger-associated molecular patterns (DAMPs) (such as HMGB1) released from stressed hepatocytes and mediated through TLR4 and TLR9 signaling pathways, which exacerbated liver injury progression [18]. Sabbione et al. [25] cultured airway cells with HMGB1 antibodies or DNase in combination with NETs and found that HMGB1 antibodies could inhibit the production of CXCL8/IL-8 and IL-6, while DNase had no significant effect. This suggests that inhibiting HMGB1 may reduce NETs-induced secretion of inflammatory factors from epithelial cells, thus attenuating the proinflammatory effects of NETs, whereas DNase was ineffective in preventing the inflammatory response. Activated platelets secrete HMGB1, which can activate neutrophils through the RAGE products, TLR2, and TLR4, leading to NET formation. Studies have also shown that in sepsis, HMGB1, miR-15b-5p, and miR-378a-3p induce NET formation through the Akt/mTOR autophagy pathway [26]. Recent research has focused on targeting HMGB1 and NETs to prevent and treat sepsis. PAD4 inhibitors such as C1-amidine can interfere with histone citrullination, inhibit NETs generation, and reduce organ damage [27]. HMGB1 inhibitors, such as anti-HMGB1 antibodies [28], can prevent NET release by blocking upstream signaling. Given the strong connection between HMGB1 and NETs, and the significant regulatory effect of GL on HMGB1, it is important to explore whether GL also regulates NETs, which play a critical role in sepsis pathogenesis, or whether GL further affects NET release by regulating HMGB1. This scientific question has prompted our research group to investigate further. Yildiz et al. [29] used the HMGB1 inhibitor GL in a ventilator-related lung injury model and found no significant decrease in the expression of DNA, CitH3, or other NET markers in bronchoalveolar lavage fluid.
In this study, the classical sepsis pathogen molecule, LPS, was used to stimulate differentiated neutrophils derived from HL-60 cells to construct a neutrophil-like sepsis model. HL-60 cells were successfully differentiated into dHL-60 cells after 5 days of induction, as confirmed by flow cytometry. Following the successful construction of the neutrophil-like sepsis model, we assessed the expression of HMGB1 and key components of NETs, such as Cit-H3 and free DNA, using Western blot, cellular immunofluorescence, and fluorescence microplate assays. The results indicated that in the neutrophil-like sepsis model, the expression levels of Cit-H3 and HMGB1 were significantly increased, suggesting that LPS promoted HMGB1 expression and NETs release, thereby validating the stability of our neutrophil-like sepsis model. We then conducted GL intervention on the neutrophil-like sepsis model to determine if GL could modulate HMGB1 expression and NET release. The results showed that after GL intervention, HMGB1 protein levels were significantly lower compared to the LPS group, and notably, NET release was also significantly reduced. This confirmed that GL not only inhibited HMGB1 expression but also regulated NETs release. To further explore the mechanism by which GL regulates NETs, we measured TLR9 expression by Western blot and administered exogenous HMGB1. The study results indicate that in a neutrophil-like sepsis model, TLR9 expression was significantly increased. Exogenous HMGB1 further promoted this increase, while GL inhibited this process. This suggests that TLR9 may be involved in the relationship between HMGB1 and NETs. Our findings preliminarily suggest that GL may inhibit NET release in sepsis via the HMGB1/TLR9 pathway.
There are a few shortcomings in this study. First, the dose of GL used was based on previous reports, and an in-depth investigation of the dose-response relationship was not conducted. The clinical applicability of GL needs further evaluation through toxicological studies and pharmacokinetic analyses. Second, cells were harvested 12 hours after GL treatment, but the time-dependent effects of GL were not studied, leaving the optimal treatment duration undetermined. Third, GL was administered immediately after model construction, which may not fully reflect clinical scenarios, thus limiting its applicability. Finally, while our results suggest that GL may inhibit NET release through the HMGB1/TLR9 pathway, the underlying mechanism was not further investigated. Therefore, the molecular mechanism by which GL regulates NETs warrants further study.
Conclusion
LPS induces overexpression of HMGB1 and excessive release of NETs in dHL-60 cells. GL can inhibit LPS-induced HMGB1 expression in dHL-60 cells and further suppress the release of NETs. GL may reduce NETs release in sepsis through the HMGB1/TLR9 pathway.
Acknowledgements
This work was supported by Guizhou Provincial Science and Technology Projects ([2021]041); Guizhou Provincial Science and Technology Projects ([2024]126); and National Natural Science Foundation Cultivation Project of Guizhou Medical University (22NSFCP42).
Disclosure of conflict of interest
None.
References
- 1.Leiding JW. Neutrophil evolution and their diseases in humans. Front Immunol. 2017;8:1009. doi: 10.3389/fimmu.2017.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in sepsis. Front Immunol. 2019;10:2536. doi: 10.3389/fimmu.2019.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klopf J, Brostjan C, Eilenberg W, Neumayer C. Neutrophil extracellular traps and their implications in cardiovascular and inflammatory disease. Int J Mol Sci. 2021;22:559. doi: 10.3390/ijms22020559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J Clin Cell Immunol. 2013;4:139. doi: 10.4172/2155-9899.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virzì GM, Mattiotti M, de Cal M, Ronco C, Zanella M, De Rosa S. Endotoxin in sepsis: methods for lps detection and the use of omics techniques. Diagnostics (Basel) 2022;13:79. doi: 10.3390/diagnostics13010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashiba M, Huq A, Tomino A, Hirakawa A, Hattori T, Miyabe H, Tsuda M, Takeyama N. Neutrophil extracellular traps in patients with sepsis. J Surg Res. 2015;194:248–254. doi: 10.1016/j.jss.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Ito T. PAMPs and DAMPs as triggers for DIC. J Intensive Care. 2014;2:67. doi: 10.1186/s40560-014-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Yan B, Liu Y. Neutrophil extracellular traps-induced endothelial cell damage in the pathogenesis of dermatomyositis-associated interstitial lung disease. Zhonghua Nei Ke Za Zhi. 2017;56:650–654. doi: 10.3760/cma.j.issn.0578-1426.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Cerletti C, Tamburrelli C, Izzi B, Gianfagna F, de Gaetano G. Platelet-leukocyte interactions in thrombosis. Thromb Res. 2012;129:263–266. doi: 10.1016/j.thromres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida T, Abe K, Ikeda T, Matsushita T, Wake K, Sato T, Sato T, Inoue H. Inhibitory effect of glycyrrhizin on lipopolysaccharide and d-galactosamine-induced mouse liver injury. Eur J Pharmacol. 2007;576:136–142. doi: 10.1016/j.ejphar.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Kimura M, Watanabe H, Abo T. Selective activation of extrathymic T cells in the liver by glycyrrhizin. Biotherapy. 1992;5:167–176. doi: 10.1007/BF02171049. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, Tsung A. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–614. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau A, Wang S, Liu W, Haig A, Zhang ZX, Jevnikar AM. Glycyrrhizic acid ameliorates HMGB1-mediated cell death and inflammation after renal ischemia reperfusion injury. Am J Nephrol. 2014;40:84–95. doi: 10.1159/000364908. [DOI] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan MA, Farahvash A, Douda DN, Licht JC, Grasemann H, Sweezey N, Palaniyar N. JNK activation turns on LPS- and gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Sci Rep. 2017;7:3409. doi: 10.1038/s41598-017-03257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoppenbrouwers T, Autar ASA, Sultan AR, Abraham TE, van Cappellen WA, Houtsmuller AB, van Wamel WJB, van Beusekom HMM, van Neck JW, de Maat MPM. In vitro induction of NETosis: comprehensive live imaging comparison and systematic review. PLoS One. 2017;12:e0176472. doi: 10.1371/journal.pone.0176472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan MA, Philip LM, Cheung G, Vadakepeedika S, Grasemann H, Sweezey N, Palaniyar N. Regulating NETosis: increasing pH promotes NADPH oxidase-dependent NETosis. Front Med (Lausanne) 2018;5:19. doi: 10.3389/fmed.2018.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Sabbione F, Keitelman IA, Iula L, Ferrero M, Giordano MN, Baldi P, Rumbo M, Jancic C, Trevani AS. Neutrophil extracellular traps stimulate proinflammatory responses in human airway epithelial cells. J Innate Immun. 2017;9:387–402. doi: 10.1159/000460293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao Y, Li W, Wang W, Tong X, Xia R, Fan J, Du J, Zhang C, Shi X. Platelet-derived exosomes promote neutrophil extracellular trap formation during septic shock. Crit Care. 2020;24:380. doi: 10.1186/s13054-020-03082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutua V, Gershwin LJ. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin Rev Allergy Immunol. 2021;61:194–211. doi: 10.1007/s12016-020-08804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SW, Lee H, Lee HK, Kim ID, Lee JK. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun. 2019;7:94. doi: 10.1186/s40478-019-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildiz C, Palaniyar N, Otulakowski G, Khan MA, Post M, Kuebler WM, Tanswell K, Belcastro R, Masood A, Engelberts D, Kavanagh BP. Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology. 2015;122:864–875. doi: 10.1097/ALN.0000000000000605. [DOI] [PubMed] [Google Scholar]