Abstract
Objective: To investigate the value of amplitude-integrated electroencephalography (aEEG) combined with neuron-specific enolase (NSE) in the diagnosis and prognostic assessment of neonatal brain injury. Methods: Clinical data from 94 neonates with brain injury and 90 neonates without, admitted to Baoji Maternity and Child Healthcare Hospital between September 2022 and February 2024, were retrospectively analyzed. The relationship between aEEG score, NSE level, and Neurobehavioral Neurological Assay (NBNA) score was analyzed by Pearson’s correlation analysis. Independent factors affecting the prognosis of the neonates were identified by unifactorial and multifactorial analyses, and the predictive value was assessed using ROC curves. Results: The aEEG score was significantly lower while the NSE level was considerably higher in newborns with brain injury compared to those without (both P<0.001). aEEG score was positively correlated with the NBNA score (r=0.718, P<0.001), NSE level was negatively correlated with NBNA score (r=-0.785, P<0.001), and aEEG score was negatively correlated with NSE level (r=-0.749, P<0.001). The aEEG score and NSE level demonstrated good predictive value for neonatal brain injury, with AUC values of 0.903 and 0.897, respectively. The AUC of combined assessment was 0.917. Multifactorial analysis showed that intrauterine distress (OR: 3.385, 95% CI: 1.033-11.903, P=0.048) and higher NSE level (OR: 1.516, 95% CI: 1.117-2.136, P=0.011) were independent risk factors for poor prognosis of neonates with brain injury, while higher aEEG scores (OR: 0.587, 95% CI: 0.370-0.884, P=0.015) was an independent protective factor. Intrauterine distress, aEEG score, and NSE predicted poor prognostic outcomes with AUCs of 0.639, 0.809, and 0.827, and the combined diagnosis had an AUC of 0.871. Conclusion: aEEG combined with NSE level can effectively predict neonatal brain injury and prognosis, providing a valuable reference for early diagnosis and intervention.
Keywords: Amplitude-integrated electroencephalography, neuron-specific enolase, neonatal brain injury, prognosis
Introduction
The perinatal period is an extremely vulnerable stage for newborns, as they are exposed to numerous high-risk factors such as preterm labor, infection, hemorrhage, trauma, hypoxia, and metabolic disorders [1,2]. These factors can lead to neonatal brain damage, resulting in severe complications like mental retardation, muscle spasms, seizures, and deficits in vision, hearing, and language development [3]. In severe cases, affected children may develop cerebral palsy, significantly reducing their quality of life and self-care abilities. This condition not only places a substantial emotional and financial burden on the family but can also disrupt family harmony and strain societal resources [4,5]. Therefore, early identification, diagnosis and timely intervention for neonatal brain injury are crucial in improving the prognosis of affected children. Early detection and treatment are essential in preventing long-term complications; however, neonatal brain injuries are sometimes asymptomatic or present with atypical clinical features, making them easy to overlook or misdiagnosis [6,7].
Magnetic resonance imaging (MRI) is the primary imaging tool for the evaluation of neonatal brain injury, but it has limitations, including low positivity rates for early diagnosis and unsuitability for rapid bedside examination [8]. In recent years, amplitude-integrated electroencephalography (aEEG) has emerged as a valuable bedside brain function monitoring technique [9]. aEEG simplifies conventional EEG signals through filtering, integration, and time compression, allowing for a single-channel representation of overall brain activity [10]. aEEG can detect pathological changes such as edema caused by cerebral insufficiency or brain injury, manifested in abnormal waveforms like disrupted voltages, absent sleep-wake cycles, and amplitude irregularities [11]. Despite its usefulness, aEEG has limitations regarding sensitivity and specificity, leading some researchers to recommend combining it with biochemical markers for more accurate diagnosis [12]. In neonatal brain injury, abnormal aEEG patterns can provide insight into the extent and progression of brain damage, aiding in assessing the severity of the injury and predicting long-term neurodevelopmental outcomes [13].
Neuron-specific enolase (NSE), an enzyme found in neurons of brain tissue, is widely recognized as a biomarker of brain injury. When brain injury occurs, NSE levels significantly increase and are released into the bloodstream, making it a useful tool for assessing the extent of brain damage [14]. Measuring NSE levels helps in the prognosis of various conditions such as acute stroke, cerebral venous thrombosis, hypoxic brain injury after cardiopulmonary resuscitation, and traumatic brain injury [15,16]. Studies have shown that elevated NSE levels are often associated with more severe neurodevelopmental disorders [17], and its correlation with long-term neurodevelopmental outcomes in children has been noted, aiding in the stratification of infants who may experience adverse neurodevelopmental outcomes [18]. Although NSE is widely used in the assessment of brain injury in adults, relatively few studies have been conducted on the combined use of NSE and aEEG for diagnosing neonatal brain injury. aEEG provides real-time electrophysiological information of the brain activity, while NSE offers biochemical information following brain injury. Together, they can offer a more comprehensive assessment, enhancing the accuracy of predicting the neurodevelopmental prognosis in infants with hypoxic-ischemic encephalopathy (HIE).
In this study, we analyzed data from newborns between September 2022 and February 2024 to examine the application of aEEG and NSE in diagnosing neonatal brain injury and predicting prognosis. The goal was to provide valuable reference information for enhancing clinical diagnosis and prognostic assessment in neonatal brain injury cases.
Methodology and information
Patients’ selection
This retrospective study involved 94 neonates with brain injury (observation group) and 90 neonates without (control group), who were admitted to Baoji Maternity and Child Healthcare Hospital from September 2022 to February 2024. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Baoji Maternity and Child Healthcare Hospital.
The neonates were diagnosed with brain injury based on cranial ultrasound, CT, or MRI findings conducted 1 to 5 d after birth [19]. The neonates included in this group had gestational ages of 34-42 weeks, birth weights of ≥1500 g, and complete clinical profiles. Neonates with central nervous system (CNS) infections, congenital brain defects, or traumatic brain injury were excluded from the study. The research methodology is outlined in Figure 1.
Figure 1.
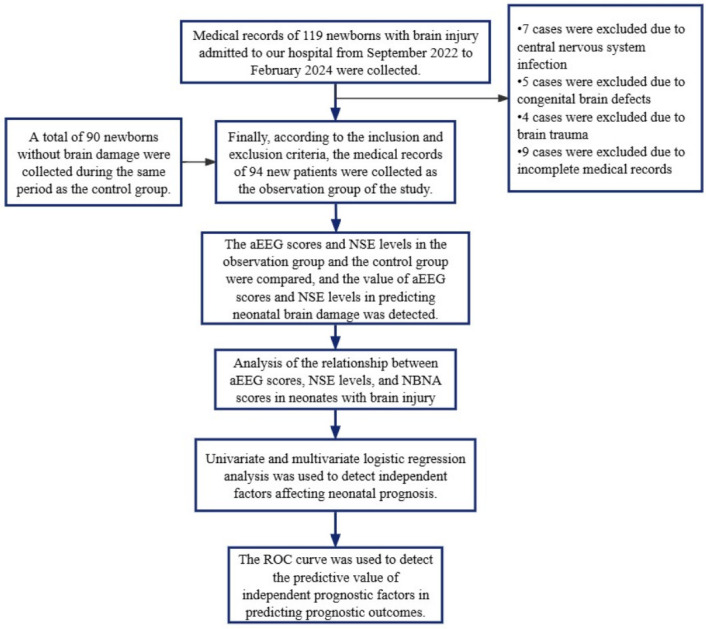
Research flow chart. aEEG: amplitude-integrated electroencephalography.
Data extraction
General information was extracted from electronic medical records of newborns, including age, birth weight, sex, gestational age, preterm birth, singleton, mode of delivery, intrauterine distress, 1-min Apgar score, 5-min Apgar score, and Neurobehavioral Neurological Assay (NBNA) score. In addition, aEEG brain monitoring, serum NSE levels, and Gesell developmental diagnostic scale scores at 3 months were collected for both groups of newborns.
Newborns were monitored using a neonatal brain function monitor for 6 hours within the first 12 hours of birth. Prior to testing, the scalp area of the neonate was cleaned with an alcoholic cotton ball. An appropriate elastic mesh skullcap, sized according to the head circumference, was selected. Conductive paste was applied into the grooves of the disc-shaped electrodes on the skullcap, and the connecting wires were secured, ensuring no contact with skin lesions or hematomas. Additionally, a sensor belt for recording breathing patterns was placed around the neonate’s lumbar abdomen, avoiding the umbilical cord area, and connected to the corresponding electrodes. Once the devices were connected, the parameters of the EEG instrument were appropriately adjusted for the observation and recording of the EEG images. Evaluation was performed according to the aEEG scoring system, with key observation indexes including continuity, sleep-wake cycle, lower border amplitude, and bandwidth. The aEEG score ranged from 3 to 12 points [20].
Within 12 hours after birth, 2 mL of venous blood was drawn under strict aseptic conditions. The serum NSE level was measured immediately using a Swiss Roche automatic analyzer, employing the electrochemiluminescence method, strictly following the instructions provided with the kit.
The neurological function of the newborns was assessed using the NBNA on the day of blood collection. The NBNA, assessed by a pediatrician, evaluates brain function through 20 entries, with a total possible score of 40 points. A score of 36 or higher was indicative of normal neurobehavioral function [21].
The neonates in the observation group were further categorized into a good prognosis group and a poor prognosis group based on the developmental quotient (DQ) results from the Gesell Developmental Diagnostic Scale at 3 months of birth. DQ>75 is defined as a good prognosis, while DQ≤75 is considered a poor prognosis [22].
Observation indicators
Primary observation indicators
(1) aEEG scores and NSE levels were compared between the observation and control groups, and performance of aEEG scores and NSE levels in predicting neonatal brain injury was assessed. (2) Independent factors affecting neonatal prognosis were identified by univariate and multivariate analyses.
Secondary observation indicators
(1) The difference in baseline information was compared between the observation and control groups. (2) The relationship between aEEG scores, NSE levels, and NBNA scores in brain-injured neonates was analyzed. (3) Predictive value of independent prognostic factors for neonate’s prognosis was analyzed using ROC curves.
Statistical methods
SPSS 20.0 software and R version 3.6.1 were utilized for data analysis. Count data were expressed as number (%) and analyzed using the chi-square test. Measurement data conforming to normal distribution were expressed as mean ± standard deviation, and comparisons between groups were made using the independent samples t-test. Pearson’s correlation test was used to analyze the correlations among aEEG scores, NSE levels, and NBNA scores in neonates with brain injury. Independent prognostic factors were analyzed using a multivariate logistic regression model. The receiver operating characteristic (ROC) curve was employed to evaluate the predictive value of aEEG scores, NSE levels, and their combination for neonatal brain injury. The area under the curve (AUC), along with sensitivity and specificity, were calculated to determine the efficacy of these predictive models. Statistical significance was defined as P<0.05.
Results
Comparison of baseline information between the two groups
Statistical analysis of the baseline data for the newborns in the two groups showed that there was no statistical difference between the two groups in terms of day age, birth weight, gender, gestational age, singleton, mode of delivery, and mothers’ of deliveries numbers (all P>0.05), but there was a statistical differences between the two groups in terms of intrauterine distress, 1-min Apgar score, 5-min Apgar score, and NANB score (all P<0.05), as shown in Table 1.
Table 1.
Comparison of baseline information between the two groups
| Observation group (n=94) | Control group (n=90) | t/χ2 | P | |
|---|---|---|---|---|
| Age (days) | 5.93±1.08 | 6.13±0.90 | 1.419 | 0.158 |
| Birth weight (kg) | 3.31±0.19 | 3.34±0.20 | 1.262 | 0.209 |
| Gender | 0.736 | 0.391 | ||
| Male | 55 (58.51) | 47 (52.22) | ||
| Female | 39 (41.49) | 43 (47.78) | ||
| Gestational age | 2.922 | 0.087 | ||
| Preterm birth | 15 (15.96) | 7 (7.78) | ||
| Full-term | 79 (84.04) | 83 (92.22) | ||
| Singleton | 1.374 | 0.241 | ||
| Yes | 73 (77.66) | 76 (84.44) | ||
| No | 21 (22.34) | 14 (15.56) | ||
| Mode of delivery | 3.267 | 0.071 | ||
| Natural birth | 56 (59.57) | 65 (72.22) | ||
| Cesarean section | 38 (40.43) | 25 (27.78) | ||
| Parity | 0.729 | 0.393 | ||
| Primiparous | 38 (40.43) | 42 (46.67) | ||
| Multiparous | 56 (59.57) | 48 (53.33) | ||
| Intrauterine distress | 11.206 | <0.001 | ||
| Yes | 28 (29.79) | 9 (10.00) | ||
| No | 66 (70.21) | 81 (90.00) | ||
| 1-min Apgar score | 25.208 | <0.001 | ||
| >7 points | 57 (60.64) | 83 (92.22) | ||
| ≤7 points | 37 (39.36) | 7 (7.78) | ||
| 5-min Apgar score | 20.589 | <0.001 | ||
| >7 points | 71 (74.74) | 88 (97.78) | ||
| ≤7 points | 24 (25.26) | 2 (2.22) | ||
| NANB score | 31.96±1.70 | 35.96±0.91 | 19.772 | <0.001 |
NBNA: Neurobehavioral Neurological Assay.
Comparison of aEEG scores and NSE levels between the two groups
The aEEG scores of the observation group were significantly lower than those of the control group. Conversely, the NSE levels in the observation group were significantly higher than those in the control group (all P<0.001), as illustrated in Figure 2.
Figure 2.
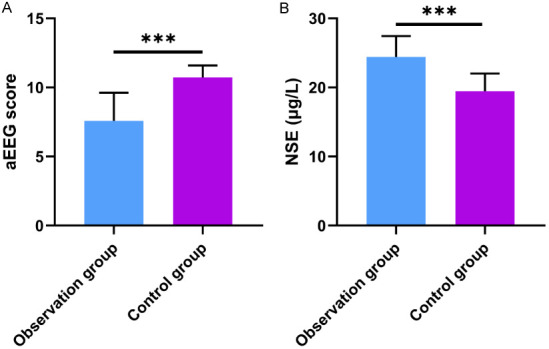
aEEG scores and NSE levels in both groups. aEEG: amplitude-integrated electroencephalography; NSE: neuron-specific enolase. P***<0.001.
Predictive value of aEEG scores and NSE levels in brain injury
The predictive value of aEEG score and NSE level in neonatal brain injury was assessed by plotting ROC curves. The AUC of aEEG score in predicting brain injury was 0.903, indicating a strong predictive accuracy. Similarly, the AUC of NSE level was 0.897, also demonstrating strong predictive accuracy. Notably, their combined detection increased the AUC to 0.019, further enhancing diagnostic precision (Table 2 and Figure 3).
Table 2.
Performance of aEEG scores and NSE levels in predicting neonatal brain injury analyzed by ROC curve
| AUC | 95% CI | Specificity (%) | Sensitivity (%) | Cut off | |
|---|---|---|---|---|---|
| aEEG score | 0.903 | 0.857-0.950 | 81.91% | 93.33% | 9.500 |
| NSE | 0.897 | 0.851-0.943 | 76.60% | 92.22% | 22.310 |
| Joint diagnosis | 0.917 | 0.872-0.961 | 79.79% | 97.78% | 0.646 |
aEEG: amplitude-integrated electroencephalography; NSE: neuron-specific enolase; ROC: receiver operating characteristic; AUC: area under the curve.
Figure 3.
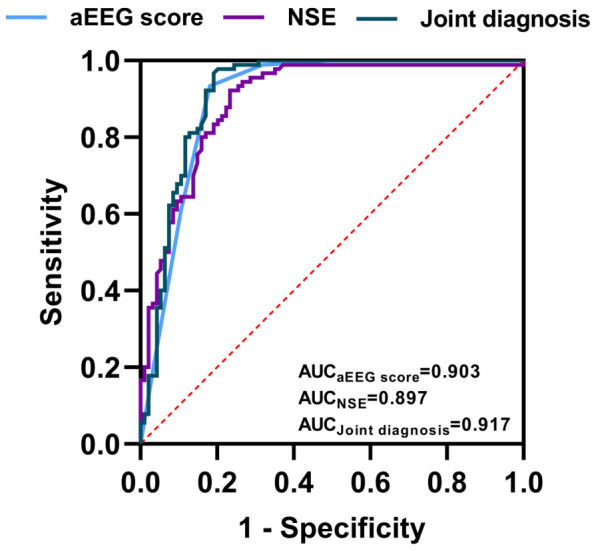
ROC curves of aEEG scores and NSE levels in predicting neonatal brain injury. aEEG: amplitude-integrated electroencephalography; NSE: neuron-specific enolase; AUC: area under the curve.
Correlation of aEEG scores and NSE levels with NBNA scores in brain-injured neonates
The degree of brain injury in neonates was assessed using the NBNA score. Pearson correlation analysis was conducted to examine the relationships between aEEG score, NSE level, and NBNA score in brain-injured neonates. The results indicated that the aEEG score was positively correlated with the NBNA score (r=0.718, P<0.001). Conversely, the NSE level was negatively correlated with the NBNA score (r=-0.785, P<0.001). Additionally, the aEEG score was negatively correlated with the NSE level (r=-0.749, P<0.001), suggesting that as aEEG scores increase, NSE levels tend to decrease. These relationships are detailed in Figure 4.
Figure 4.
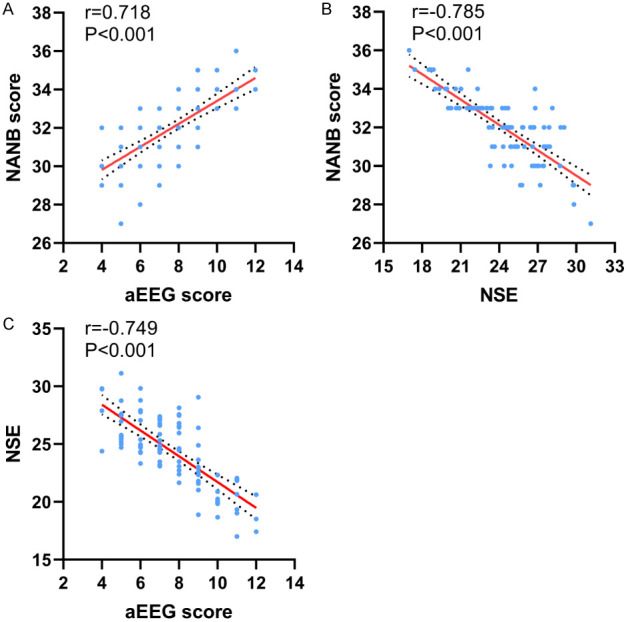
Correlation of aEEG scores and NSE levels with NBNA scores. aEEG: amplitude-integrated electroencephalography; NSE: neuron-specific enolase; NBNA: Neurobehavioral Neurological Assay.
Univariate analysis of factors affecting the prognosis of neonates with brain injury
Statistical analysis of the prognosis of neonates with brain injury at 3 months old revealed a total of 31 cases with unfavorable prognosis and 63 cases with favorable prognosis. Univariate analysis revealed significant differences in mode of delivery, intrauterine distress, aEEG scores, and NSE levels between the two subgroups, suggesting these factors may influence the prognosis of neonates with brain injuries. Detailed statistics are presented in Table 3.
Table 3.
Univariate analysis for identifying factors associated with prognosis in neonates with brain injury
| Favorable outcome group (n=64) | Unfavorable outcome group (n=31) | t/χ2 | P | |
|---|---|---|---|---|
| Age (days) | 5.98±1.05 | 5.81±1.14 | 0.729 | 0.469 |
| Birth weight (kg) | 3.32±0.20 | 3.29±0.15 | 0.845 | 0.401 |
| Gender | 0.176 | 0.675 | ||
| Male | 38 (59.38) | 17 (54.84) | ||
| Female | 26 (40.62) | 14 (45.16) | ||
| Gestational age | 1.596 | 0.206 | ||
| Preterm birth | 8 (12.5) | 7 (22.58) | ||
| Full-term | 56 (87.5) | 24 (77.42) | ||
| Singleton | 0.181 | 0.670 | ||
| Yes | 50 (78.12) | 23 (74.19) | ||
| No | 14 (21.88) | 8 (25.81) | ||
| Mode of delivery | 5.503 | 0.019 | ||
| Natural birth | 43 (67.19) | 13 (41.94) | ||
| Cesarean section | 21 (32.81) | 18 (58.06) | ||
| Parity | 0.032 | 0.858 | ||
| Primiparous | 26 (40.62) | 12 (38.71) | ||
| Multiparous | 38 (59.38) | 19 (61.29) | ||
| Intrauterine distress | 7.919 | 0.005 | ||
| Yes | 13 (20.31) | 15 (48.39) | ||
| No | 51 (79.69) | 16 (51.61) | ||
| 1-min Apgar score | 0.511 | 0.475 | ||
| >7 points | 40 (62.5) | 17 (54.84) | ||
| ≤7 points | 24 (37.5) | 14 (45.16) | ||
| Birth 5min Apgar score | 1.192 | 0.275 | ||
| >7 points | 50 (78.12) | 21 (67.74) | ||
| ≤7 points | 14 (21.88) | 10 (32.26) | ||
| aEEG score | 8.30±1.91 | 6.13±1.43 | 6.175 | <0.001 |
| NSE (μg/L) | 23.30±2.88 | 26.68±1.88 | 6.830 | <0.001 |
aEEG: amplitude-integrated electroencephalography; NSE: neuron-specific enolase.
Multivariate analysis of factors affecting the prognosis of neonates with brain injury
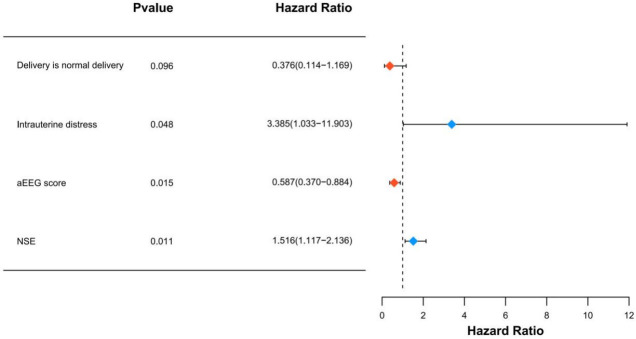
Significant variables in the univariate analysis were used as independent variables, while neonatal prognostic outcomes were used as dependent variables (see Table 4 for variable assignments). Multifactorial logistic regression analysis was conducted to identify the factors affecting the prognosis of neonates with brain injury, with the results displayed in Figure 5. The analysis revealed that the mode of delivery was not an independent factor influencing the prognosis of neonates with brain injury (OR: 0.376, 95% CI: 0.114-1.169, P=0.096). In contrast, intrauterine distress (OR: 3.385, 95% CI: 1.033-11.903, P=0.048) and higher levels of NSE (OR: 1.516, 95% CI: 1.117-2.136, P=0.011) were identified as independent risk factors for poor prognosis in brain-injured neonates. Conversely, a higher aEEG score (OR: 0.587, 95% CI: 0.370-0.884, P=0.015) was found to be an independent protective factor.
Table 4.
The assignment table
| Variable | Assignment |
|---|---|
| Mode of delivery | Natural birth = 1, cesarean section = 0 |
| Intrauterine distress | Yes = 1, no = 0 |
| aEEG score | Continuous variables are analyzed using raw data |
| NSE | Continuous variables are analyzed using raw data |
| Prognostic outcome | Poor prognosis = 1, good prognosis = 0 |
NSE: neuron-specific enolase.
Figure 5.
Forest plot of independent prognostic factors analyzed by multifactor logistic regression. aEEG: amplitude-integrated electroencephalography; NSE: neuron-specific enolase.
Predictive value of independent prognostic factors for prognostic outcomes
The predictive value of the independent prognostic factors identified through multifactorial analysis was assessed using ROC curves. The AUC for intrauterine distress, aEEG score, and NSE in predicting poor prognostic outcomes were 0.639, 0.809, and 0.827, respectively. Notably, the AUC for the combined diagnosis using all three factors increased to 0.871, indicating a significant improvement in predictive accuracy. The details are illustrated in Table 5 and Figure 6.
Table 5.
Predictive performance of independent factors for the outcome of neonates with brain injury analyzed using ROC
| Marker | AUC | 95% CI | Specificity (%) | Sensitivity (%) | Cut off |
|---|---|---|---|---|---|
| Intrauterine distress | 0.639 | 0.515-0.762 | 48.39 | 79.37 | 0.500 |
| aEEG score | 0.809 | 0.722-0.895 | 83.87 | 65.08 | 7.500 |
| NSE | 0.827 | 0.745-0.909 | 87.10 | 73.02 | 24.880 |
| Combined diagnosis | 0.871 | 0.801-0.942 | 83.87 | 80.95 | 0.368 |
ROC: receiver operating characteristic; AUC: area under the curve; aEEG: amplitude-integrated electroencephalography; NSE: neuron-specific enolase.
Figure 6.
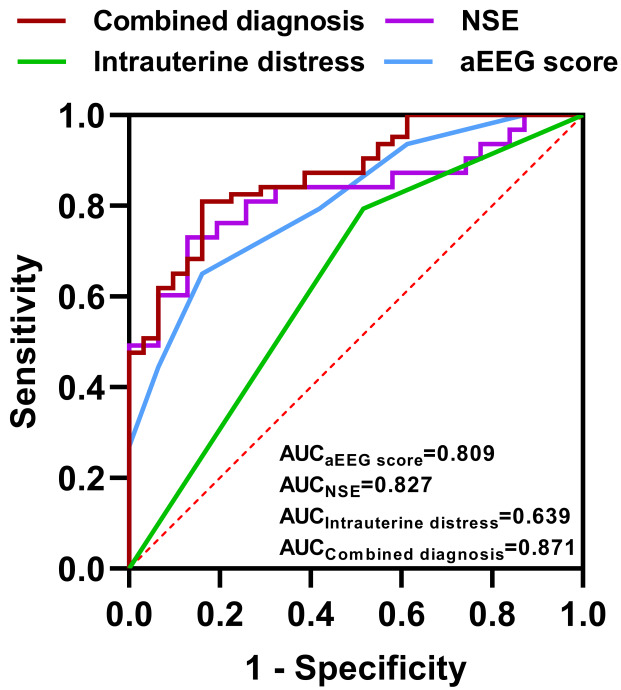
ROC curves for independent prognostic factors in predicting outcomes in neonates with brain injury. AUC: area under the curve; aEEG: amplitude-integrated electroencephalography; NSE: neuron-specific enolase.
Discussion
In this study, we investigated the value of amplitude-integrated electroencephalography (aEEG) combined with neuron-specific enolase (NSE) in the diagnosis and prognostic assessment of neonatal brain injury by retrospectively analyzing the clinical data of 94 neonates with brain injury and 90 neonates without.
aEEG, as a bedside brain function monitoring technique, can reflect pathological changes such as cerebral edema due to cerebral insufficiency or brain injury by processing conventional EEG signals through filtering, integration, and time compression [23]. In this study, aEEG scores were significantly lower in brain-injured neonates compared to the controls, and positively correlated with NBNA scores, suggesting that aEEG is an effective tool for identifying brain injury. Similarly, Mires et al. [24] reported that aEEG assessment during childbirth can improve the detection of fetal hypoxia, enabling early intervention, and thus reducing the risk of hypoxic brain damage. NSE is an enzyme present in neurons of brain tissues, and its levels increase significantly when brain injury occurs, releasing into the bloodstream [25]. Therefore, NSE is widely recognized as a biomarker for brain injury. In this study, NSE levels in brain-injured neonates were significantly higher than those in the control group, and NSE levels were negatively correlated with NBNA scores, further validating the value of NSE in the diagnosis of brain injury. Additionally, in this study, we found that the AUCs of aEEG scores and NSE levels in predicting neonatal brain injury were 0.903 and 0.897, respectively, indicating good predictive value. Moreover, their combined detection increased the AUC to 0.917, further enhancing diagnostic precision.
Various diagnostic tools, such as CT, intrapartum ultrasound, and the Apgar score, are commonly used in assessing neonatal brain injury. However, neonates with brain injury are often in severe and unstable clinical conditions, limiting the applicability of many neuroimaging techniques [26-28]. Locatelli et al. [29] also noted that the Apgar score is not a specific diagnostic tool for neonatal brain damage, and even prenatal fetal heart rate monitoring may not improve the diagnostic decision. Magnetic resonance imaging (MRI) plays an important role in the diagnosis of neonatal brain injury, providing an objective basis for the assessment of brain development, brain injury severity, and prognosis [30]. Griesmaier et al. [31] compared aEEG scores with MRI findings in 523 preterm infants and found that aEEG could effectively predict the severity of brain injury detected by MRI at full-term age. This underscores the utility of aEEG as a valuable tool for neonatal brain injury, complementing traditional diagnostic methods like MRI by providing timely functional insights at the bedside.
The aEEG score and NSE level also showed significant value in prognostic assessment. Multifactorial analysis revealed that intrauterine distress and higher NSE levels were independent risk factors for poor prognosis in brain-injured neonates, while higher aEEG scores were independent protective factors. A study by Efstathiou et al. [32] found that in preterm infants, elevated NSE levels were found to have strong predictive value for the children’s prognosis at two years of age, aligning with our findings. Similarly, Metallinou et al. [33] investigated the prognostic value of NSE levels in preterm infants during the first three days following birth and discovered that serum NSE levels in newborns with intraventricular hemorrhage were significantly higher than those in normal newborns and preterm infants with periventricular leukomalacia. Therefore, measuring NSE levels during the first three days after birth can effectively predict the risk of brain injury in preterm infants. Tan et al. [34] reported that higher theta power during cognitive tasks in aEEG was associated with better cognitive performance in children and adolescents. In our study, the assessment of the accuracy of independent prognostic factors revealed that intrauterine distress had an AUC of 0.639, aEEG score had an AUC of 0.809, and NSE level had an AUC of 0.827. Their combination increased the AUC to 0.871, indicating a notable improvement in the accuracy of predicting poor prognostic outcomes.
The findings of this study provide critical implications for clinical practice. First, by performing aEEG monitoring and measuring NSE levels in the early postnatal period, brain-injured newborns can be recognized early, allowing for timely intervention to improve the prognosis of the children. Second, the combined application of aEEG and NSE levels improves diagnostic accuracy and reduces the likelihood of misdiagnosis and underdiagnosis. Additionally, the independent influencing factors identified through multifactorial analysis can help clinicians better assess the prognosis of newborns and provide a basis for developing individualized treatment plans. This approach not only enhances early detection and intervention but also supports tailored therapeutic strategies, ultimately aiming to improve long-term outcomes for neonates with brain injury.
Although this study achieved some valuable results, several limitations must be acknowledged. First, as a retrospective analysis, it may be prone to selection bias. Moreover, with the advancement of medical technology, more new biomarkers and monitoring techniques could further refine the diagnosis and prognostic assessment of neonatal brain injury. For example, proteomics-based biomarkers and advanced imaging techniques may offer novel perspectives for the diagnosis and treatment of neonatal brain injury. These advancements could further enhance the accuracy and effectiveness of early diagnosis and individualized treatment plans, ultimately improving outcomes for affected neonates. While aEEG and NSE have shown predictive value in this study, they may not fully replace other diagnostic methods such as MRI. Furthermore, the sample size and inclusion period limit the ability to analyze long-term trends and potential seasonal variations in neonatal brain injury.
In conclusion, this study demonstrated that aEEG combined with NSE level can effectively predict neonatal brain injury and prognosis, providing a valuable reference for early diagnosis and intervention.
Disclosure of conflict of interest
None.
References
- 1.Duncan AF, Matthews MA. Neurodevelopmental outcomes in early childhood. Clin Perinatol. 2018;45:377–392. doi: 10.1016/j.clp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Bolk J, Kallen K, Farooqi A, Hafstrom M, Fellman V, Aden U, Serenius F. Perinatal risk factors for developmental coordination disorder in children born extremely preterm. Acta Paediatr. 2023;112:675–685. doi: 10.1111/apa.16651. [DOI] [PubMed] [Google Scholar]
- 3.Leavy A, Jimenez Mateos EM. Perinatal brain injury and inflammation: lessons from experimental murine models. Cells. 2020;9:2640. doi: 10.3390/cells9122640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang XL, Wang X, Shao L, Jiang GT, Min JW, Mei XY, He XH, Liu WH, Huang WX, Peng BW. TRPV1 mediates astrocyte activation and interleukin-1beta release induced by hypoxic ischemia (HI) J Neuroinflammation. 2019;16:114. doi: 10.1186/s12974-019-1487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alken J, Hakansson S, Ekeus C, Gustafson P, Norman M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in sweden. JAMA Netw Open. 2019;2:e190858. doi: 10.1001/jamanetworkopen.2019.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinan D, Daneman A, Guimaraes CV, Chauvin NA, Victoria T, Epelman M. Easily overlooked sonographic findings in the evaluation of neonatal encephalopathy: lessons learned from magnetic resonance imaging. Semin Ultrasound CT MR. 2014;35:627–651. doi: 10.1053/j.sult.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Araki T, Yokota H, Morita A. Pediatric traumatic brain injury: characteristic features, diagnosis, and management. Neurol Med Chir (Tokyo) 2017;57:82–93. doi: 10.2176/nmc.ra.2016-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programs provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015;2015:CD005495. doi: 10.1002/14651858.CD005495.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah DK, Mathur A. Amplitude-integrated EEG and the newborn infant. Curr Pediatr Rev. 2014;10:11–15. doi: 10.2174/157339631001140408115859. [DOI] [PubMed] [Google Scholar]
- 10.Pollack R, Rana D, Purvis J, Pollard L, Pourcyrous M. Effect of prenatal marijuana exposure on sleep wake cycles and amplitude-integrated electroencephalogram (aEEG) J Perinatol. 2021;41:1355–1363. doi: 10.1038/s41372-020-00911-9. [DOI] [PubMed] [Google Scholar]
- 11.Park KH, Kim YM, Lee YJ, Bae MH, Lee NR, Han YM, Kim HY, Kim SY, Byun SY. Predictive value of an early amplitude-integrated electroencephalogram for short-term neurologic outcomes in preterm infants. Turk J Pediatr. 2020;62:367–378. doi: 10.24953/turkjped.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Pisani F, Pavlidis E. The role of electroencephalogram in neonatal seizure detection. Expert Rev Neurother. 2018;18:95–100. doi: 10.1080/14737175.2018.1413352. [DOI] [PubMed] [Google Scholar]
- 13.Griesmaier E, Schreiner C, Winkler I, Posod A, Sappler M, Kiechl-Kohlendorfer U, Neubauer V. Association of aEEG and brain injury severity on MRI at term-equivalent age in preterm infants. Acta Paediatr. 2024;113:229–238. doi: 10.1111/apa.17017. [DOI] [PubMed] [Google Scholar]
- 14.Babkina AS, Lyubomudrov MA, Golubev MA, Pisarev MV, Golubev AM. Neuron-specific enolase-what are we measuring? Int J Mol Sci. 2024;25:5040. doi: 10.3390/ijms25095040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurakina AS, Semenova TN, Guzanova EV, Nesterova VN, Schelchkova NA, Mukhina IV, Grigoryeva VN. Prognostic value of investigating neuron-specific enolase in patients with ischemic stroke. Sovrem Tekhnologii Med. 2021;13:68–72. doi: 10.17691/stm2021.13.2.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Meng R, Zhang X, Guo L, Li S, Wu Y, Duan J, Ding Y, Ji X. Serum neuron specific enolase may be a marker to predict the severity and outcome of cerebral venous thrombosis. J Neurol. 2018;265:46–51. doi: 10.1007/s00415-017-8659-9. [DOI] [PubMed] [Google Scholar]
- 17.Bagnato S, Andriolo M, Boccagni C, Lucca LF, De Tanti A, Pistarini C, Barone T, Galardi G. Reduced neuron-specific enolase levels in chronic severe traumatic brain injury. J Neurotrauma. 2020;37:423–427. doi: 10.1089/neu.2019.6449. [DOI] [PubMed] [Google Scholar]
- 18.Mercier E, Tardif PA, Cameron PA, Emond M, Moore L, Mitra B, Ouellet MC, Frenette J, de Guise E, Le Sage N. Prognostic value of neuron-specific enolase (NSE) for prediction of post-concussion symptoms following a mild traumatic brain injury: a systematic review. Brain Inj. 2018;32:29–40. doi: 10.1080/02699052.2017.1385097. [DOI] [PubMed] [Google Scholar]
- 19.Xin T, Li Z, Zhang X. Use of MRI and CT image indexing to assess cerebral injuries in neonates with hypoxic-ischemic encephalopathy. Minerva Pediatr. 2019;71:438–442. doi: 10.23736/S0026-4946.16.04349-8. [DOI] [PubMed] [Google Scholar]
- 20.Jia W, Lei X, Dong W, Li Q. Benefits of starting hypothermia treatment within 6 h vs. 6-12 h in newborns with moderate neonatal hypoxic-ischemic encephalopathy. BMC Pediatr. 2018;18:50. doi: 10.1186/s12887-018-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Jing J, Huang S, He X, Gao P, Li H, Lin Z, Sangild PT, Zhu Y. Relationship of early anemia with neurodevelopment and brain injury in very low birth weight preterm infants-a prospective cohort study. Nutrients. 2022;14:4931. doi: 10.3390/nu14224931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G, Cheng S, Wan L, Li Y, Zhao Q, Liu J, Jiang X. Correlation analysis of NT-proBNP (N-terminal probrain natriuretic peptide), 25-Hydroxyvitamin D, HMGB1(High-mobility group box 1), ACTA (endogenous activin A), blood glucose level, and electrolyte level with developmental quotient scores in neonates with hypoxic-ischemic encephalopathy. BMC Pediatr. 2022;22:739. doi: 10.1186/s12887-022-03606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruns N, Felderhoff-Muser U, Dohna-Schwake C. aEEG as a useful tool for neuromonitoring in critically ill children - current evidence and knowledge gaps. Acta Paediatr. 2021;110:1132–1140. doi: 10.1111/apa.15676. [DOI] [PubMed] [Google Scholar]
- 24.Mires S, Kerr RS, Denbow M, Dahnoun N, Tancock S, Osredkar D, Chakkarapani E. Foetal amplitude-integrated electroencephalography: proof of principle of a novel foetal monitoring technique in adult volunteers. J Obstet Gynaecol. 2022;42:2672–2679. doi: 10.1080/01443615.2022.2081797. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Wu J, Wang W, Zhang Y, He D, Xiao B, Zhang H, Song A, Xing Y, Li B. Reprogramming of rat fibroblasts into induced neurons by small-molecule compounds in vitro and in vivo. ACS Chem Neurosci. 2022;13:2099–2109. doi: 10.1021/acschemneuro.2c00078. [DOI] [PubMed] [Google Scholar]
- 26.Tefr Faridova A, Herman H, Danacikova S, Svoboda J, Otahal J. Serum biomarkers of hypoxic-ischemic brain injury. Physiol Res. 2023;72:S461–S474. doi: 10.33549/physiolres.935214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strzalko B, Karowicz-Bilinska A, Wyka K, Krajewski P, Kesiak M, Kociszewska-Najman B. Serum S100B protein concentrations in SGA/FGR newborns. Ginekol Pol. 2021 doi: 10.5603/GP.a2021.0119. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Elbayiyev S, Cevirici T, Gungor AA, Kadıoglu Simsek G, Kanmaz Kutman HG, Canpolat FE. A novel scoring system (YASHMA) predicting brain injury in asphyxiated newborns. J Trop Pediatr. 2022;68:fmac082. doi: 10.1093/tropej/fmac082. [DOI] [PubMed] [Google Scholar]
- 29.Locatelli A, Lambicchi L, Incerti M, Bonati F, Ferdico M, Malguzzi S, Torcasio F, Calzi P, Varisco T, Paterlini G. Is perinatal asphyxia predictable? BMC Pregnancy Childbirth. 2020;20:186. doi: 10.1186/s12884-020-02876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parmentier CEJ, de Vries LS, Groenendaal F. Magnetic resonance imaging in (Near-) term infants with hypoxic-ischemic encephalopathy. Diagnostics (Basel) 2022;12:645. doi: 10.3390/diagnostics12030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griesmaier E, Schreiner C, Winkler I, Posod A, Sappler M, Kiechl-Kohlendorfer U, Neubauer V. Association of aEEG and brain injury severity on MRI at term-equivalent age in preterm infants. Acta Paediatr. 2024;113:229–238. doi: 10.1111/apa.17017. [DOI] [PubMed] [Google Scholar]
- 32.Efstathiou N, Soubasi V, Koliakos G, Kantziou K, Kyriazis G, Slavakis A, Dermentzoglou V, Michalettou I, Drosou-Agakidou V. Beyond brain injury biomarkers: chemoattractants and circulating progenitor cells as biomarkers of endogenous rehabilitation effort in preterm neonates with encephalopathy. Front Pediatr. 2023;11:1151787. doi: 10.3389/fped.2023.1151787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metallinou D, Karampas G, Pavlou ML, Louma MI, Mantzou A, Sarantaki A, Nanou C, Gourounti K, Tzeli M, Pantelaki N, Tzamakos E, Boutsikou T, Lykeridou A, Iacovidou N. Serum neuron-specific enolase as a biomarker of neonatal brain injury-new perspectives for the identification of preterm neonates at high risk for severe intraventricular hemorrhage. Biomolecules. 2024;14:434. doi: 10.3390/biom14040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan E, Troller-Renfree SV, Morales S, Buzzell GA, McSweeney M, Antunez M, Fox NA. Theta activity and cognitive functioning: Integrating evidence from resting-state and task-related developmental electroencephalography (EEG) research. Dev Cogn Neurosci. 2024;67:101404. doi: 10.1016/j.dcn.2024.101404. [DOI] [PMC free article] [PubMed] [Google Scholar]