Abstract
Objective: To evaluate the effect of Tirofiban combined with endovascular treatment on hemorrhagic transformation (HT) and neurological function in patients with ischemic stroke. Methods: A retrospective analysis was performed on 94 patients with ischemic stroke treated at Xi’an International Medical Center Hospital from January 2020 to January 2023. Among them, 45 patients underwent endovascular treatment only and served as the control group. Another 49 patients received Tirofiban in addition to endovascular treatment and they formed the study group. The pre-treatment and 14-day post-treatment NIHSS scores were compared between the two groups. The 24-hour HT rate and the incidence of post-treatment adverse events were also compared between the two groups. Multivariate Cox regression analysis was used to identify factors influencing patient prognosis. Results: The NIHSS scores in the study group were significantly lower than those in the control group 14 days after treatment (P<0.05). There was no statistically significant difference in the 24-hour HT rate between the two groups (P>0.05). In addition, there were no significant differences in the incidence of adverse events between the two groups (P>0.05). Multivariate Cox regression analysis showed that treatment regimen, age, and time from onset to admission were independent factors affecting 90-day prognosis (P<0.01). A prognostic model based on β-coefficients was constructed: Cox risk = 2.729 * treatment regimen + 2.881 * age + 2.795 * time from onset to admission. The Cox risk was significantly lower in the good prognosis group compared to the poor prognosis group (P<0.0001), with an area under the curve of 0.925 in predicting patient prognosis. Conclusions: Tirofiban combined with endovascular treatment can improve quality of life, neurological function, and short-term prognosis in patients with ischemic stroke without increasing the incidence of adverse effects and HT.
Keywords: Tirofiban, endovascular treatment, ischemic stroke, hemorrhagic transformation, neurological function
Introduction
Stroke, also known as cerebrovascular injury, is a common cardiovascular and cerebrovascular disease [1]. It is primarily caused by the obstruction or sudden rupture of the cerebral blood vessels, resulting in impaired blood flow to the brain and subsequent damage to the brain tissue [2]. According to statistics, approximately 15 million people worldwide suffer from strokes each year, making it the second leading cause of death [3]. Ischemic stroke, characterized by a sudden and sustained reduction in local cerebral blood flow, resulting in brain cell death, is the most common type of stroke [4]. Within a few hours, patients may experience irreversible brain tissue damage, resulting in neurological or physical functional impairment due to brain tissue softening and local necrosis [5]. The complications of ischemic stroke are numerous and severe, as damaged neuronal tissues can lead to reduced limb function [6]. Currently, the pathogenesis of ischemic stroke is not fully understood, and treatment options are limited. In clinical practice, the diagnosis of ischemic stroke is mainly based on clinical symptoms and imaging studies, due to the lack of early diagnostic methods.
The primary treatment strategy for ischemic stroke is to rapidly recanalize the occluded blood vessels, restore cerebral blood flow, and salvage the ischemic penumbra [7]. Rapid restoration of cerebral blood flow is critical to treatment. Although endovascular treatment of acute stroke is increasingly promoted, current guidelines still recommend intravenous thrombolysis with alteplase within 4.5 hours of stroke onset to salvage ischemic tissue [8]. However, due to the limitations of primary healthcare in China and lack of patient awareness, some patients miss the optimal time window for alteplase thrombolysis when seeking medical care.
Tirofiban, a novel antiplatelet and GP IIb/IIIa receptor antagonist, inhibits platelet aggregation by blocking the binding of fibrinogen and other adhesion proteins to GP IIb/IIIa receptors on the platelet membrane, thereby preventing thrombosis [9,10]. Tirofiban has demonstrated safety and efficacy in the treatment of acute coronary syndromes, but its use in acute ischemic stroke requires further research and evaluation [11]. After mechanical thrombectomy, Tirofiban may help prevent platelet overactivation and reocclusion, offering a new treatment option for acute ischemic stroke. However, current data on the safety and efficacy of Tirofiban in patients with acute ischemic stroke are limited and further clinical trials are needed for validation [12].
Hemorrhagic transformation (HT) is a common and serious complication during treatment of ischemic stroke, mainly due to blood-brain barrier disruption, reperfusion injury and endothelial damage, resulting in leakage of blood components into brain tissue and hemorrhage [13]. Therefore, the risk of HT must be considered in the treatment of acute ischemic stroke, and patients must be closely monitored to improve treatment outcomes and reduce adverse prognosis.
Despite the increasing number of treatment options for acute ischemic stroke, many patients still fail to achieve ideal treatment outcomes due to the time of onset, complexity of the disease, and limitations of current treatment modalities [14]. In particular, patients who miss the time window for alteplase thrombolysis lack effective treatment options. This study introduces Tirofiban, a novel antiplatelet drug, to evaluate its efficacy and safety in patients with acute ischemic stroke, particularly its role in bridging thrombolysis and mechanical thrombectomy. We systematically analyzed the potential benefits of Tirofiban in reducing platelet overactivation, preventing vascular reocclusion, and improving clinical outcomes. This study fills existing research gaps by providing new insights and evidence for future treatment of acute ischemic stroke.
Methods and materials
Ethical statement
This study was approved by the Medical Ethics Committee of Xi’an International Medical Center Hospital.
Sample collection
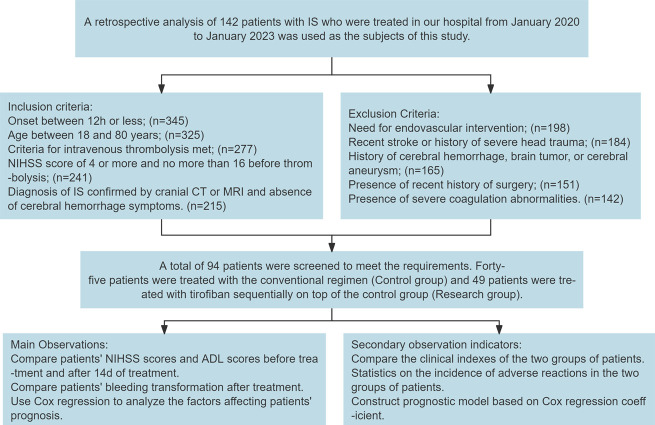
A retrospective analysis was conducted on 142 patients with ischemic stroke treated at Xi’an International Medical Center Hospital from January 2020 to January 2023.
Inclusion and exclusion criteria
The following criteria were used to determine which patients were eligible for inclusion in the study. 1) The onset of symptoms was within a 12-hour period upon admission. 2) The participants were between the ages of 18 and 80 years. 3) In order to be eligible for intravenous thrombolysis, patients met the criteria set forth in the 2018 Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke [15]. 4) The patients presented with neurological deficits caused by ischemic stroke. 5) A National Institutes of Health Stroke Scale (NIHSS) score between four and 16 was observed prior to thrombolysis [16]. 6) A diagnosis of confirmed ischemic stroke was made based on the results of a head CT or MRI scan, which did not show any signs of cerebral hemorrhage.
Patients were excluded from the study if they required endovascular treatment, had a history of cerebrovascular accident or severe head trauma within the past three months, had history of cerebral hemorrhage, brain tumor, or cerebral aneurysm, did not have detailed account of recent surgical history, or had severe coagulation disorders.
Sample screening
A total of 94 patients met the inclusion and exclusion criteria. Of these, 45 patients received the conventional treatment regimen and served as the control group. The other 49 patients were administered Tirofiban in addition to the conventional regimen, thus forming the study group.
Treatment protocol
All participants received the standard treatment regimen, which included the following components: lipid regulation, plaque stabilization, collateral circulation improvement, free radical scavenging, neuroprotection, and rehabilitation. If a head CT scan 24 hours after thrombolysis revealed no evidence of intracranial, dermal, mucosal, or organ bleeding, and the routine blood and coagulation function indicators were within the normal range, antiplatelet therapy with enteric-coated aspirin (H53021845, Yunnan Baiyao Group Co., Ltd.) was continued.
The control group received recombinant tissue plasminogen activator (rt-PA, Actilyse), produced by Boehringer Ingelheim in Germany. The dosage was 0.9 mg/kg, with a maximum of 90 mg. The initial dose, comprising 10% of the total, was administered via intravenous bolus, with the remaining 90% administered via continuous intravenous infusion over a period of 60 minutes.
The study group received the following treatment in addition to the measures in the control group. Patients at high risk of reocclusion (e.g., stent placement, balloon dilation, major vascular stenosis, vascular dissection) received Tirofiban (Chinese medicine approval H20041165, China Grand Pharmaceutical and Healthcare Holdings Limited). The initial dosage was a bolus of 10 μg/kg administered over a period of three minutes, followed by a maintenance dose of [0.12 × (weight in kg)] mL/h. In the case of patients with severe renal insufficiency, both the bolus and maintenance doses were reduced by half, with administration continuing for a period of 24 hours. In the absence of any evidence of hemorrhage at the 20-hour reexamination, a regimen of aspirin 300 mg and clopidogrel (H20056410, Sanofi Pharmaceutical Co., Ltd.) 300 mg was initiated, with bridging with tirofiban for a period of 4 hours. If a head CT/MRI scan 24 hours after thrombolysis confirmed the absence of hemorrhage, oral antiplatelet aggregation drugs were prescribed.
Data collection
Patient data were obtained from a combination of electronic medical records, outpatient follow-up records, and telephone follow-up records. The clinical data set included the subject’s age, gender, body mass index (BMI), time from onset to admission, history of hypertension, hyperlipidemia, diabetes, smoking status, and alcohol consumption. The assessment scales included the activity of daily living (ADL) scale, NIHSS, and the modified Rankin Scale (mRS) scores before and 14 days after treatment. Additionally, the incidence of adverse reactions, including vascular reocclusion, skin bleeding, respiratory tract bleeding, and mucosal bleeding, was documented. HT was classified according to the following criteria: hemorrhagic infarction, parenchymal hemorrhage, or symptomatic hemorrhage.
NIHSS score
The NIHSS is a clinical scale utilized to assess neurological deficits in patients experiencing acute stroke. The NIHSS score ranges from 0 to 42, with higher scores indicating a greater severity of deficits and 0 indicating no deficits [17].
mRS score
The mRS assesses daily living skills and functional impairment in stroke patients and ranges from 0 to 6. Higher scores indicate severer disability, with 0 indicating no symptoms, 1-2 indicating mild disability but independent living, 3-5 indicating moderate to severe disability, and 6 indicating death. mRS ≤2 indicates good long-term prognosis and mRS ≥3 indicates poor prognosis [18].
Follow-up
A 14-day follow-up was conducted at the 14-day mark post-treatment, at which point patients were still hospitalized. During this period, all necessary assessments and observations were conducted within the hospital setting. This approach permitted close monitoring of the patients’ conditions and timely intervention if necessary.
90-day follow-up: At 90 days post-treatment, patients returned for an outpatient visit. During this follow-up, patients underwent a series of evaluations to assess their long-term recovery and the efficacy of the treatment. Outpatient visits were scheduled to ensure comprehensive assessments while minimizing the burden on patients who had returned to their regular activities.
Evaluation of HT
Hemorrhagic infarction: These are small petechial hemorrhages at the margin of the infarct or confluent petechiae within the infarct area, which do not have a space-occupying effect. The classification is the presence of small petechiae or multiple confluent petechiae.
Parenchymal hemorrhage is defined as a hemorrhage with a significant space-occupying effect. Classification: A hemorrhage comprising 30% or less of the infarct size is classified as having a mild space-occupying effect. In contrast, a hemorrhage exceeding 30% of the infarct size is regarded as having a significant space-occupying effect, or as occurring in a region distant from the infarct area.
Observational indicators
Primary indicators: The NIHSS and the ADL scores before and 14 days after treatment, the incidence of HT following treatment, and the 90-day mRS scores were compared between the two groups. An mRS score of 2 or less indicated a favorable long-term prognosis and an mRS score of 3 or greater indicated an unfavorable prognosis. Patients were classified into two groups based on their prognosis: good and poor. The factors that influence prognosis were then analyzed using Cox regression.
Secondary indicators: Comparison of clinical indicators between the two groups was conducted.
The incidence of adverse reactions was compared between the two groups. A prognostic model was constructed based on the Cox regression coefficients (Figure 1).
Figure 1.
Flow chart of patient inclusion and study design.
Statistical analysis
The data were subjected to statistical analysis using the SPSS 26.0 software. Categorical data were expressed as percentages and compared using the chi-square test. Continuous data were expressed as mean ± standard deviation (Mean ± SD) and compared using either an independent sample t-test or a paired t-test. Cox regression was employed to ascertain the factors influencing the short-term prognosis. The receiver operating characteristic (ROC) curve was employed to assess the value of prognostic factors in predicting short-term prognosis. The rms package in R version 3.6.1 was utilized to construct a nomogram. The concordance index and calibration curve were employed to evaluate the accuracy of the regression-based validation model. The Hosmer-Lemeshow test was used to evaluate model calibration. A P-value of less than 0.05 was considered statistically significant.
Results
Clinical data
Comparison of clinical data between the two groups showed no statistically significant differences in age (P=0.491), gender (P=0.561), BMI (P=0.742), time from onset to admission (P=0.176), history of hypertension (P=0.340), history of hyperlipidemia (P=0.393), history of diabetes (P=0.410), smoking history (P=0.561), and alcohol consumption history (P=0.736) (Table 1).
Table 1.
Comparison of clinical data
| Factors | Control Group (n=45) | Study Group (n=49) | x2 Value | P Value |
|---|---|---|---|---|
| Age | ||||
| ≥65 years | 25 | 30 | 0.311 | 0.577 |
| <65 years | 20 | 19 | ||
| Gender | ||||
| Male | 20 | 25 | 0.406 | 0.524 |
| Female | 25 | 24 | ||
| BMI | ||||
| ≥25 kg/m2 | 11 | 14 | 0.205 | 0.651 |
| <25 kg/m2 | 34 | 35 | ||
| Time from Onset to Admission | ||||
| <5 hours | 34 | 30 | 2.217 | 0.136 |
| ≥5 hours | 11 | 19 | ||
| History of Hypertension | ||||
| Yes | 34 | 32 | 1.178 | 0.278 |
| No | 11 | 17 | ||
| History of Hyperlipidemia | ||||
| Yes | 26 | 24 | 0.729 | 0.393 |
| No | 19 | 25 | ||
| History of Diabetes | ||||
| Yes | 8 | 12 | 0.631 | 0.427 |
| No | 37 | 37 | ||
| Smoking History | ||||
| Yes | 20 | 25 | 0.406 | 0.524 |
| No | 25 | 24 | ||
| Alcohol Consumption History | ||||
| Yes | 4 | 5 | 0.047 | 0.829 |
| No | 41 | 44 |
Note: BMI: Body Mass Index.
Quality of life and neurological function assessment
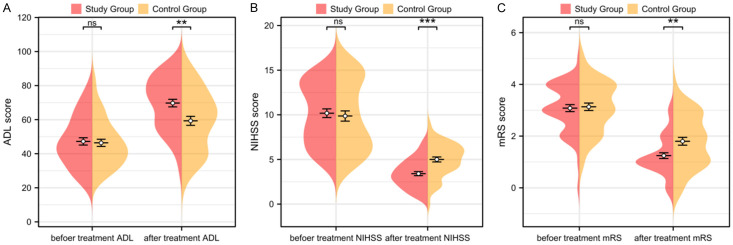
Comparison of ADL and NIHSS scores before and 14 days after treatment between the two groups showed no statistically significant differences in ADL and NIHSS scores before treatment (P>0.05, Figure 2A, 2B). However, the study group had significantly higher ADL scores and lower NIHSS scores than the control group 14 days after treatment (P<0.05, Figure 2A, 2B).
Figure 2.
Changes in ADL, NIHSS, and mRS scores. A. Comparison of ADL scores before and 14 days after treatment in both groups. B. Comparison of NIHSS scores before and 14 days after treatment in both groups. C. Comparison of mRS scores before and 90 days after treatment in both groups. Note: NIHSS: National Institute of Health Stroke Scale, ADL: Activity of Daily Living, mRS: Modified Rankin Scale; nsP>0.05, **P<0.01, ***P<0.001.
Prognosis assessment
The mRS scores before treatment and 90 days after treatment were compared between the two groups. Before treatment, there were no significant differences in the mRS scores between the two groups (P>0.05, Figure 2C). However, the study group had significantly lower mRS scores than the control group 90 days after treatment (P<0.05, Figure 2C).
HT incidence
The incidence of HT within 24 hours of treatment was not significantly different between the control and study groups (P>0.05, Table 2).
Table 2.
HT incidence
| Group | Hemorrhagic Infarction | Parenchymal Hemorrhage | Total Incidence |
|---|---|---|---|
| Control Group (n=45) | 17 (37.78%) | 10 (22.22%) | 27 (60.00%) |
| Study Group (n=49) | 16 (32.65%) | 6 (12.25%) | 22 (44.90%) |
| Χ2 Value | 0.27 | 1.653 | 2.144 |
| P Value | 0.603 | 0.199 | 0.143 |
Note: HT: Hemorrhagic Transformation.
Adverse reactions
The incidence of adverse reactions was compared between the two groups, but no statistically significant differences were found between the two groups (P>0.05, Table 3).
Table 3.
Incidence of adverse reactions
| Group | Skin Bleeding | Respiratory Tract Bleeding | Mucosal Bleeding |
|---|---|---|---|
| Control Group (n=45) | 2 | 2 | 2 |
| Study Group (n=49) | 6 | 5 | 4 |
| Χ2 Value | 1.833 | 1.129 | 0.543 |
| P Value | 0.176 | 0.288 | 0.461 |
Prognostic factor analysis
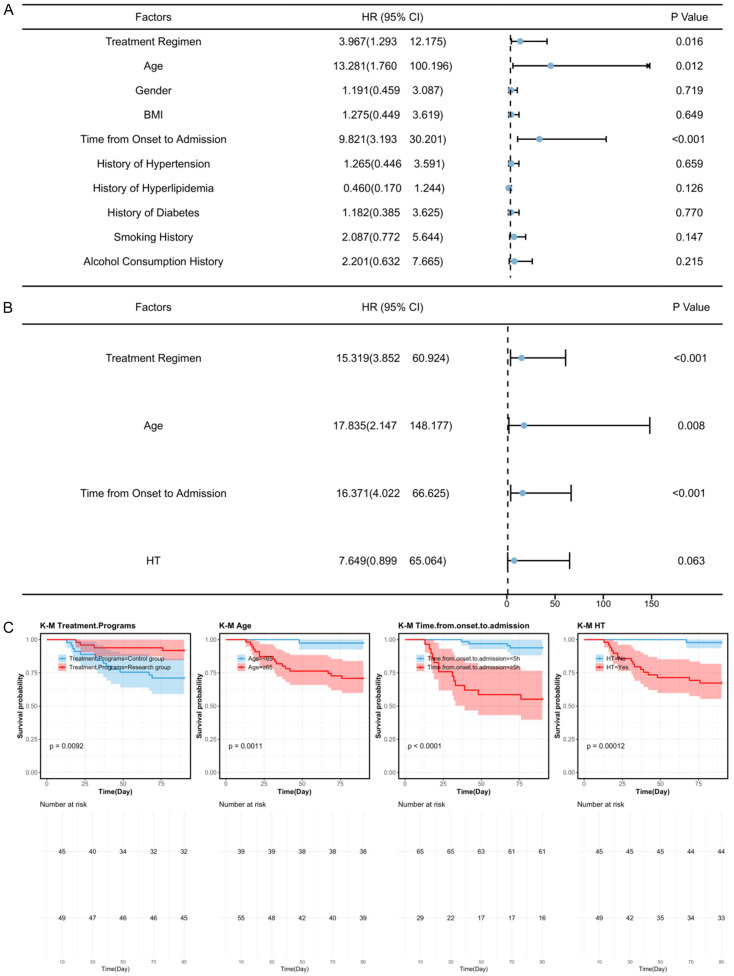
A total of 17 patients had poor prognosis at 90 days, with 13 in the control group and 4 in the study group. Patients were redivided into good prognosis (n=77) and poor prognosis (n=17) groups based on their mRS scores. Univariate analysis showed that treatment regimen, age, time from onset to admission, and NIHSS score were factors influencing patient prognosis (P<0.05, Figure 3). Multivariate Cox regression analysis further identified treatment regimen, age, and time from onset to admission as independent prognostic factors (P<0.01, Figure 3).
Figure 3.
Prognostic factor analysis. A. Cox univariate analysis of factors affecting patient prognosis. B. Cox multivariate analysis of factors affecting patient prognosis. C. Survival curve for prognostic factors. Note: NIHSS: National Institute of Health Stroke Scale, ADL: Activity of Daily Living, mRS: Modified Rankin Scale, HT: Hemorrhagic Transformation.
Prognostic model construction
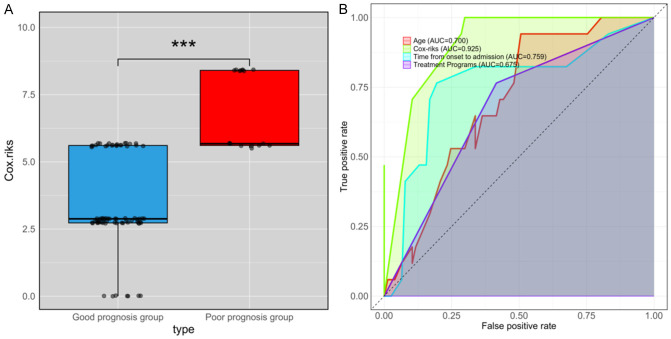
Cox regression identified treatment regimen, age, and time from onset to admission as prognostic factors. To further predict patient prognosis, a model was constructed based on β coefficients: Cox-risk = 2.729 * treatment regimen + 2.881 * age + 2.795 * time from onset to admission. The Cox-risk was significantly lower in the good prognosis group compared to the poor prognosis group (P<0.001, Figure 4A). ROC curve analysis showed an AUC of 0.925 for Cox-risk in predicting patient prognosis (Figure 4B). The Delong test indicated that Cox-risk had a significantly higher AUC in predicting 90-day prognosis compared to other individual factors (Tables 4, 5).
Figure 4.
Value of Cox-risk in predicting 90-day prognosis. A. Cox-risk levels in both groups. B. ROC curve assessing Cox-risk in predicting patient prognosis. Note: ROC: Receiver Operating Characteristic; ***P<0.001.
Table 4.
ROC parameters
| Marker | AUC | 95% CI | Specificity | Sensitivity | Youden Index | Cut off | Accuracy | Precision | F1 Score |
|---|---|---|---|---|---|---|---|---|---|
| Treatment Programs | 0.675 | 0.557-0.792 | 58.44% | 76.47% | 34.91% | 0.5 | 61.70% | 76.47% | 41.94% |
| Age | 0.7 | 0.580-0.820 | 49.35% | 94.12% | 43.47% | 64.5 | 57.45% | 94.12% | 44.44% |
| Time from Onset to Admission | 0.759 | 0.616-0.902 | 80.52% | 76.47% | 56.99% | 5.5 | 79.79% | 76.47% | 57.78% |
| Cox-risk | 0.925 | 0.870-0.980 | 70.13% | 100.00% | 70.13% | 4.202 | 75.53% | 100.00% | 59.65% |
Table 5.
Delong test results
| Marker1 | Marker2 | Z Value | P Value | AUC Difference | 95% CI |
|---|---|---|---|---|---|
| Treatment Programs | Cox-risk | -3.639 | <0.001 | -0.25 | -0.27 |
| Age | Cox-risk | -4.1 | <0.001 | -0.225 | -0.216 |
| Time from Onset to Admission | Cox-risk | -2.77 | 0.006 | -0.166 | -0.235 |
Discussion
Platelet activation plays a crucial role in the pathogenesis of ischemic stroke. The fundamental mechanism of stroke is atherosclerosis [19]. In the acute phase of ischemic stroke, a large number of platelets are activated in the blood, resulting in changes in their ultrastructure, which enhances their adhesiveness and aggregation, further leading to thrombus formation [20]. Currently, the most effective method is the ultra-early use of intravenous thrombolytic drugs to rapidly recanalize occluded cerebral blood vessels. However, the recanalization rate of blood vessels after thrombolytic drug use is very limited, and even when recanalization occurs, the reocclusion rate is relatively high [21,22]. The medical community still lacks effective treatment methods for stroke patients who cannot restore blood flow within the thrombolysis time window. Therefore, this disease places higher demands on clinical treatment. Since many treatment methods are limited by the time window, finding an efficient and safe treatment method is of great importance.
Tirofiban is a non-peptide IIb/IIIa receptor antagonist commonly used to inhibit platelet aggregation and thereby prevent thrombus formation. Tirofiban is widely used in the treatment of cardiovascular diseases such as acute coronary syndromes [23,24]. In this study, we found that both groups of patients showed improvements in quality of life and neurological function after treatment, with the study group showing better improvements in ADL and NIHSS scores compared to the control group. Additionally, the combined use of Tirofiban did not increase the incidence of adverse events. A meta-analysis by Gong et al. [25] also found that Tirofiban did not increase the incidence of adverse events such as symptomatic intracranial hemorrhage. Furthermore, a study by Du et al. [26] found that patients treated with Tirofiban showed significant improvements in quality of life and neurological function compared to the conventional treatment group. These results are consistent with our findings.
HT is a common and serious complication in ischemic stroke patients during or after treatment, significantly increasing morbidity and mortality. HT occurs mainly due to blood-brain barrier disruption, reperfusion injury, and endothelial damage, resulting in leakage of blood components into brain tissue and hemorrhage [27]. In this study, we found that the use of Tirofiban did not increase the incidence of HT, indicating its safety in patients with acute ischemic stroke. We speculate that this is mainly due to the rapid onset and short half-life of Tirofiban, which allows it to effectively inhibit platelet aggregation while reducing the risk of bleeding. A study by Yi et al. [28] found that the combined use of Tirofiban during mechanical thrombectomy did not increase the incidence of HT compared with mechanical thrombectomy alone. This is consistent with our findings, suggesting that Tirofiban not only has a high safety profile, but also significantly improves patient prognosis when combined with mechanical thrombectomy in the treatment of acute ischemic stroke.
The mRS is a commonly used tool to assess the independence of daily living function in stroke patients and to provide prognostic guidance [29,30]. In this study, we found that 90 days after treatment, the mRS scores of the study group were significantly lower than those of the control group, indicating that combined treatment positively affected the short-term prognosis of ischemic stroke patients. However, to further determine the factors influencing short-term prognosis, we performed Cox regression analysis and established a prognostic model. Our study found that treatment regimen, age, and time from onset to admission were independent factors affecting short-term prognosis. With increasing age, various physiological functions, including vascular elasticity and organ recovery ability, decline, affecting stroke treatment outcomes and prognosis [31,32]. Additionally, elderly patients may face more challenges during the stroke recovery period, such as muscle atrophy, pressure sores, pneumonia, and deep vein thrombosis, which may also affect prognosis [33].
Stroke is an acute event in which brain cells die rapidly under hypoxic conditions. “Time is brain” is a critical principle in stroke treatment. Research shows that for every hour that treatment is delayed, a stroke patient can lose 1.9 million brain cells [34]. Therefore, the longer the time from onset to treatment, the more severe the brain damage and the worse the prognosis. In this study, we found for the first time that the combined use of Tirofiban with endovascular treatment is a significant factor influencing short-term prognosis. In the final part of our study, we constructed a prognostic model based on Cox regression coefficients, which showed that the Cox risk (AUC: 0.925) was significantly higher in the poor prognosis group compared to the good prognosis group. The Cox risk showed high clinical value in predicting poor prognosis. Previously, Wang et al. [35] developed a prognostic model using logistic regression nomogram and machine learning to predict 3-month outcomes in patients with acute ischemic stroke, with an AUC of 0.865. Additionally, Lv et al. [36] used the N2H3 nomogram model to predict 3-month outcomes in acute ischemic stroke patients receiving intravenous alteplase thrombolysis, with an AUC of 0.9. In comparison, our prognostic model had a higher AUC value, suggesting that the Cox risk model has higher accuracy and clinical value in predicting 3-month outcomes in patients with acute ischemic stroke.
Although this study confirms that Tirofiban combined with endovascular treatment can improve ischemic stroke, it has certain limitations. First, this is a retrospective study with a limited sample size, so the results may be biased. Second, as a single-center study, the generalizability of the model needs further validation with more samples and data. Finally, long-term prognostic data were not obtained in this study, so the effect of the two treatment regimens on long-term prognosis remains unclear. Therefore, we hope to conduct more clinical trials in future research to strengthen our conclusions.
In conclusion, Tirofiban combined with endovascular treatment can improve the quality of life, neurological function, and short-term prognosis in ischemic stroke patients without increasing the incidence of adverse effects and HT.
Disclosure of conflict of interest
None.
References
- 1.Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165260. doi: 10.1016/j.bbadis.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boursin P, Paternotte S, Dercy B, Sabben C, Maier B. Semantics, epidemiology and semiology of stroke. Soins. 2018;63:24–27. doi: 10.1016/j.soin.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(Suppl 2):S6–S16. doi: 10.1212/WNL.0000000000012781. [DOI] [PubMed] [Google Scholar]
- 4.Zhu H, Hu S, Li Y, Sun Y, Xiong X, Hu X, Chen J, Qiu S. Interleukins and ischemic stroke. Front Immunol. 2022;13:828447. doi: 10.3389/fimmu.2022.828447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335:113518. doi: 10.1016/j.expneurol.2020.113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinstein AA. Update on treatment of acute ischemic stroke. Continuum (Minneap Minn) 2020;26:268–286. doi: 10.1212/CON.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 7.Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. 2020;48:1654–1663. doi: 10.1097/CCM.0000000000004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jolugbo P, Ariens RAS. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke. 2021;52:1131–1142. doi: 10.1161/STROKEAHA.120.032810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao W, Li S, Li C, Wu C, Wang J, Xing L, Wan Y, Qin J, Xu Y, Wang R, Wen C, Wang A, Liu L, Wang J, Song H, Feng W, Ma Q, Ji X TREND Investigators. Effects of tirofiban on neurological deterioration in patients with acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2024;81:594–602. doi: 10.1001/jamaneurol.2024.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smilowska K, Smilowski M, Partyka R, Kokocinska D, Jalowiecki P. Personalised approach to diagnosing and managing ischemic stroke with a plasma-soluble urokinase-type plasminogen activator receptor. J Pers Med. 2022;12:457. doi: 10.3390/jpm12030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Jiang C, Tao Y, Gao Y, Xu Y, Zhang R, Liu K, Gu H, Wang Y, Xu Y, Song B. Rationale and design of a phase 3b, prospective, randomized, open label, blinded-endpoint, multicenter trial of the efficacy and safety of urokinase thrombolysis comparing with antiplatelet agents for patients with minor stroke. Int J Stroke. 2022;17:474–477. doi: 10.1177/17474930211014344. [DOI] [PubMed] [Google Scholar]
- 12.Tang L, Tang X, Yang Q. The application of tirofiban in the endovascular treatment of acute ischemic stroke: a meta-analysis. Cerebrovasc Dis. 2021;50:121–131. doi: 10.1159/000512601. [DOI] [PubMed] [Google Scholar]
- 13.Zubair AS, Sheth KN. Hemorrhagic conversion of acute ischemic stroke. Neurotherapeutics. 2023;20:705–711. doi: 10.1007/s13311-023-01377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauzier DC, Kansagra AP. Thrombectomy in acute ischemic stroke. N Engl J Med. 2022;386:1351. doi: 10.1056/NEJMicm2116727. [DOI] [PubMed] [Google Scholar]
- 15.Wolf A, Langmann T. Anti-VEGF-A/ANG2 combotherapy limits pathological angiogenesis in the eye: a replication study. EMBO Mol Med. 2019;11:e10362. doi: 10.15252/emmm.201910362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix P, Melamed I, Collins M, Lieberman N, Sharma V, Goren O, Zand R, Schirmer CM, Griessenauer CJ. NIHSS 24 h after mechanical thrombectomy predicts 90-day functional outcome. Clin Neuroradiol. 2022;32:401–406. doi: 10.1007/s00062-021-01068-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Xiong Y, Yu Q, Shen S, Chen L, Lei X. The activity of daily living (ADL) subgroups and health impairment among Chinese elderly: a latent profile analysis. BMC Geriatr. 2021;21:30. doi: 10.1186/s12877-020-01986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Tsang RC, Zhou J, Zhou M, Zha F, Long J, Wang Y. Relationship of barthel index and its short form with the modified rankin scale in acute stroke patients. J Stroke Cerebrovasc Dis. 2020;29:105033. doi: 10.1016/j.jstrokecerebrovasdis.2020.105033. [DOI] [PubMed] [Google Scholar]
- 19.Cheng TF, Zhao J, Wu QL, Zeng HW, Sun YT, Zhang YH, Mi R, Qi XP, Zou JT, Liu AJ, Jin HZ, Zhang WD. Compound Dan Zhi tablet attenuates experimental ischemic stroke via inhibiting platelet activation and thrombus formation. Phytomedicine. 2020;79:153330. doi: 10.1016/j.phymed.2020.153330. [DOI] [PubMed] [Google Scholar]
- 20.Kollikowski AM, Pham M, Marz AG, Papp L, Nieswandt B, Stoll G, Schuhmann MK. Platelet activation and chemokine release are related to local neutrophil-dominant inflammation during hyperacute human stroke. Transl Stroke Res. 2022;13:364–369. doi: 10.1007/s12975-021-00938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendelson SJ, Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA. 2021;325:1088–1098. doi: 10.1001/jama.2020.26867. [DOI] [PubMed] [Google Scholar]
- 22.Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51:3440–3451. doi: 10.1161/STROKEAHA.120.029749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Yang Y, Liu H. Efficacy outcomes and safety measures of intravenous tirofiban or eptifibatide for patients with acute ischemic stroke: a systematic review and meta-analysis of prospective studies. J Thromb Thrombolysis. 2022;53:898–910. doi: 10.1007/s11239-021-02584-3. [DOI] [PubMed] [Google Scholar]
- 24.DeFilippis AP, Chapman AR, Mills NL, de Lemos JA, Arbab-Zadeh A, Newby LK, Morrow DA. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140:1661–1678. doi: 10.1161/CIRCULATIONAHA.119.040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong J, Shang J, Yu H, Wan Q, Su D, Sun Z, Liu G. Tirofiban for acute ischemic stroke: systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:475–481. doi: 10.1007/s00228-019-02817-8. [DOI] [PubMed] [Google Scholar]
- 26.Du N, Wang LX, Liu YL, Yin XL, Zhao JS, Yang L. Effect of tirofiban in treating patients with progressive ischemic stroke. Eur Rev Med Pharmacol Sci. 2022;26:2098–2105. doi: 10.26355/eurrev_202203_28357. [DOI] [PubMed] [Google Scholar]
- 27.Verkerk BS, Lesch C, Cham S, Berger K. Cryoprecipitate for alteplase-related hemorrhagic conversion of acute ischemic stroke. J Pharm Pract. 2023;36:1253–1259. doi: 10.1177/08971900221102116. [DOI] [PubMed] [Google Scholar]
- 28.Yi HJ, Sung JH, Lee DH. Safety and efficacy of intra-arterial tirofiban injection during mechanical thrombectomy for large artery occlusion. Curr Neurovasc Res. 2019;16:416–424. doi: 10.2174/1567202616666191023154956. [DOI] [PubMed] [Google Scholar]
- 29.Saver JL, Chaisinanunkul N, Campbell BCV, Grotta JC, Hill MD, Khatri P, Landen J, Lansberg MG, Venkatasubramanian C, Albers GW XIth Stroke Treatment Academic Industry Roundtable. Standardized nomenclature for modified rankin scale global disability outcomes: consensus recommendations from stroke therapy academic industry roundtable XI. Stroke. 2021;52:3054–3062. doi: 10.1161/STROKEAHA.121.034480. [DOI] [PubMed] [Google Scholar]
- 30.ElHabr AK, Katz JM, Wang J, Bastani M, Martinez G, Gribko M, Hughes DR, Sanelli P. Predicting 90-day modified Rankin scale score with discharge information in acute ischaemic stroke patients following treatment. BMJ Neurol Open. 2021;3:e000177. doi: 10.1136/bmjno-2021-000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bc PK, Somannavar VG. A study on the role of serum calcium, serum albumin and serum uric acid as markers of initial neurological severity and short term outcome indicators in acute ischemic stroke. J Assoc Physicians India. 2022;70:11–12. [PubMed] [Google Scholar]
- 32.Eren F, Ozguncu C, Ozturk S. Short-term prognostic predictive evaluation in female patients with ischemic stroke: a retrospective cross-sectional study. Front Neurol. 2022;13:812647. doi: 10.3389/fneur.2022.812647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, Wang CJ, Gu HQ, Meng X, Jiang Y, Yang X, Zhang J, Xiong YY, Zhao XQ, Liu LP, Wang YL, Wang YJ, Li ZX. Sex differences in short-term and long-term outcomes among patients with acute ischemic stroke in China. Stroke. 2022;53:2268–2275. doi: 10.1161/STROKEAHA.121.037121. [DOI] [PubMed] [Google Scholar]
- 34.Puig J, Shankar J, Liebeskind D, Terceno M, Nael K, Demchuk AM, Menon B, Dowlatshahi D, Leiva-Salinas C, Wintermark M, Thomalla G, Silva Y, Serena J, Pedraza S, Essig M. From “time is brain” to “imaging is brain”: a paradigm shift in the management of acute ischemic stroke. J Neuroimaging. 2020;30:562–571. doi: 10.1111/jon.12693. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Yin J, Xu L, Lu J, Chen J, Chen Y, Wufuer A, Gong T. Development and validation of outcome prediction model for reperfusion therapy in acute ischemic stroke using nomogram and machine learning. Neurol Sci. 2024;45:3255–3266. doi: 10.1007/s10072-024-07329-7. [DOI] [PubMed] [Google Scholar]
- 36.Lv S, Song Y, Zhang FL, Yan XL, Chen J, Gao L, Guo ZN, Yang Y. Early prediction of the 3-month outcome for individual acute ischemic stroke patients who received intravenous thrombolysis using the N2H3 nomogram model. Ther Adv Neurol Disord. 2020;13:1756286420953054. doi: 10.1177/1756286420953054. [DOI] [PMC free article] [PubMed] [Google Scholar]