Abstract
Objective: To investigate the biological role of miR-132 in a murine model of chronic obstructive pulmonary disease (COPD) via activation of the SIRT1/FoxO1 axis. Methods: COPD was induced in C57BL/6J male mice by exposing them to cigarette smoke (CS) for 8 weeks. A miR-132 knockout mouse model was used to assess the role of miR-132 in CS-induced COPD. Lung tissue apoptosis was evaluated using TUNEL assays and histopathology, along with lung functional tests which were performed to assess CS-induced lung injury. Results: Elevated miR-132 expression was observed in lung tissues and bronchoalveolar lavage fluid in COPD mice. miR-132 depletion improved lung function, restored lung tissue morphology, and reduced apoptosis. Target prediction software identified miR-132 as a potential repressor of SIRT1. In COPD mice, SIRT1 and FoxO1 expression were reduced, but miR-132 knockout restored their levels. Conclusion: Inhibition of miR-132 may serve as a therapeutic strategy for CS-induced COPD.
Keywords: Chronic obstructive pulmonary disease, SIRT1/FoxO-1 axis, microRNA-132
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease characterized by chronic airflow obstruction that is irreversible [1-3]. MicroRNA (miRNA), a class of non-coding RNA, can silence target gene mRNA post-transcriptionally [4,5]. Recent studies have increasingly highlighted the role of miRNAs in COPD development and progression [6]. Elevated levels of miR-132 have been observed in the blood of COPD patients, and a negative correlation has been reported between serum miR-132 levels and FEV1/FVC% [7,8]. However, the precise role of miR-132 in COPD remains unclear in murine models.
In this study, we hypothesized that miR-132 removal could attenuate lung injury caused by cigarette smoke (CS) exposure. To test this, we generated miR-132 knockout (KO) mice and investigated the role of miR-132 in a CS-induced murine model of COPD. Our findings could improve the understanding of COPD pathogenesis and underscore the therapeutic potential of targeting miR-132 in COPD treatment.
Materials and methods
CS-exposed mice
Eight-week-old male C57BL/6J mice (20-25 g) were randomly assigned to a CS-exposure group or a control group (n=8 per group). The CS-exposed group was used to assess miR-132 expression in a COPD model. miR-132KO and wild-type (WT) mice were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). Both miR-132KO and WT mice (n=8 per group) were exposed to CS for 2 hours, twice a day, 6 days a week, for 8 weeks to induce COPD, as described previously [9]. At the end of the experiment, mice were euthanized via intraperitoneal injection of an overdose of pentobarbital. The study was approved by the Hunan Provincial People’s Hospital Animal Experiments Committee.
Lung function testing
Lung function in miR-132KO and WT mice was measured using the Buxco Fine Pointe Series Whole Body Plethysmography system. Each test took approximately 30 minutes per mouse. The following parameters were recorded: peak inspiratory flow (PIF), peak expiratory flow (PEF), end-inspiratory phase (EIP), minute ventilation (MV), inspiratory duration (Ti), and expiratory duration (Te).
Histopathology analysis
Lung tissues from each group were rinsed with 0.9% saline, fixed in 10% neutral buffered formalin, and embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin and eosin (H&E) for histological evaluation. Lung tissue injuries were assessed using a microscope and graded based on the modified 0-5 Jablonsky scale, as previously described, with 10 fields examined per lung sample [10,11].
Immunohistochemistry
Sections were incubated overnight at 4°C with a primary antibody against SIRT1 (sc-74465; 1:100; Santa Cruz Biotechnology, CA, USA) and a rat monoclonal antibody against FoxO-1 (sc-515244; 1:100; Santa Cruz Biotechnology, CA, USA). After washing, sections were treated with a secondary antibody (FSX-100; 1:100; Olympus, Tokyo, Japan) for 30 minutes at room temperature. The percentage of cells positively stained for SIRT1 and FoxO-1 was calculated by multiplying the staining intensity by the number of positively stained cells using ImageJ software [12].
Bronchoalveolar lavage fluid (BALF) collection
Each mouse underwent three lavages with 0.8 mL saline, collecting approximately 1.8-2.0 mL in total. Saline was instilled into the lungs via a cannula inserted into the trachea, and after lavage, the supernatant was collected and stored at -80°C for subsequent miR-132 analysis.
TUNEL assay for lung tissues
An apoptosis detection kit was used to assess cell death in lung tissues using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay (11684817910; Roche, California, USA) [13].
Quantitative real-time polymerase chain reaction (qRT-PCR)
Quantitative real-time PCR (qRT-PCR) was used to determine gene expression levels, employing SYBR Premix Ex Taq (Takara) in the IQ5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The following primers were used: miR-132: forward 5’-GCCGCTAACAGTCTACAGCCAT-3’; reverse 5’-GTGCAGGGTCCGAGGT-3’. U6: forward 5’-CTCGCTTCGGCAGCACA-3’; reverse 5’-AACGCTTCACGAATTTGCGT-3’.
The comparative Ct method was used to calculate relative expression levels, with U6 serving as the internal control for miR-132 expression.
Western blotting analysis
Proteins of interest, including Bax (ab216494; 1:1000; Abcam, Boston, USA), Bcl-2 (BLL100187E; 1:1000; Baililai, Shanghai, China), and Caspase-3 (ab13847; 1:500; Abcam, Boston, USA), were analyzed. After washing and incubation with secondary antibodies (LBW00151; Yiji, Shanghai, China), membranes were treated with an ECL kit (E422; Vazyme, Nanjing, China), and the resulting bands were analyzed using Image Lab™ Software.
Statistical analysis
Statistical analyses were performed using Prism 9 software (GraphPad, USA). Data are presented as mean ± SD (Standard Deviation). A t-test was used to compare differences between the two groups, while one-way analysis of variance (ANOVA) and two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was applied for comparisons among three or more groups. A p-value of < 0.05 was considered statistically significant.
Results
Elevated miR-132 levels in the lung tissues and BALF of mice exposed to CS
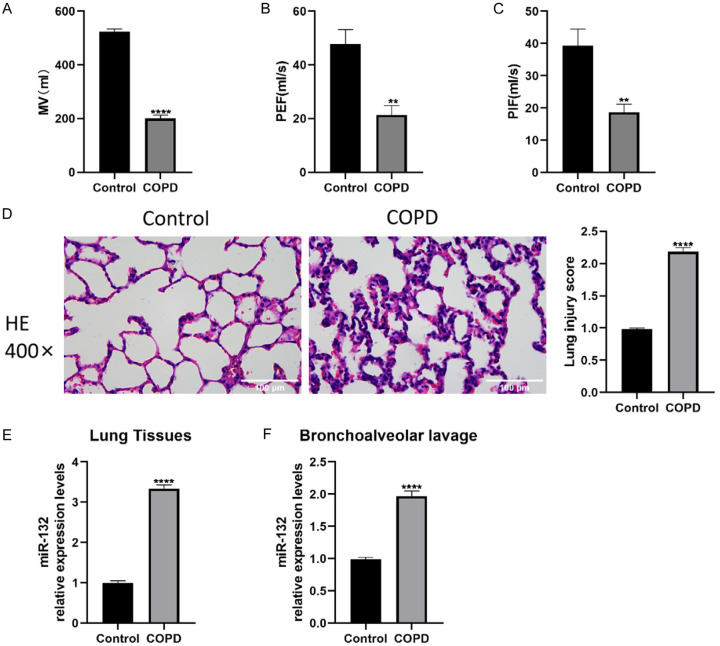
A mouse model of CS-induced COPD was constructed, and H&E staining along with lung function assays were conducted to assess lung damage. Figure 1A-D shows that the COPD group exhibited significant airflow obstruction and lung function decline, while the control group maintained normal lung function and structure. Additionally, miR-132 expression was measured in lung tissues and BALF. As expected, miR-132 levels were elevated in both BALF and lung tissues of COPD mice (Figure 1E, 1F), indicating the involvement of miR-132 in COPD progression.
Figure 1.
The increased expression of miR-132 in lung tissues and Bronchoalveolar lavage fluid (BALF) of smoking cigarettes (CS)-exposed mice. C57BL/6J mice were administrated with CS exposure 2 hours per day for 8 weeks, n=8 per group. A-C. Lung function text including MV, PEF, PIF. D. Haematoxylin and eosin-stained sections showing the lung injury in vivo (400×, scale bars =100 µm). E, F. The mRNA expression levels of miR-132 in lung tissues and BALF of mice exposed to CS. Results are expressed as mean ± SD, **P < 0.01, ****P < 0.0001, vs respective controls. Abbreviations: PIF: peak inspiratory flow; PEF: peak expiratory flow; MV: minute ventilation; CS: smoking cigarettes; BALF: Bronchoalveolar lavage fluid; SD: Standard Deviation; p: p value.
Deletion of miR-132 improved pulmonary function of COPD mice
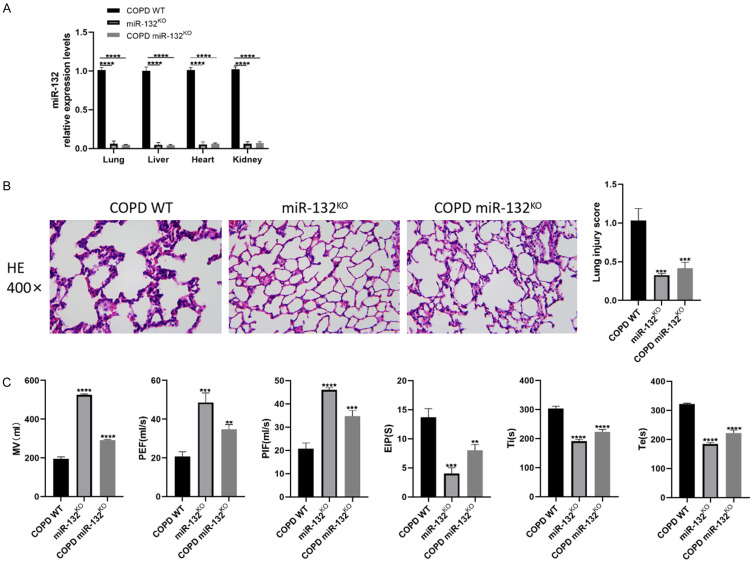
To investigate the effect of miR-132 on lung tissues affected by COPD, miR-132KO mice were generated and exposed to CS, alongside WT mice. qRT-PCR confirmed the effective KO of miR-132. Figure 2A shows significantly reduced miR-132 levels in the lungs, liver, heart, and kidneys of miR-132KO mice. Lung function tests revealed that PEF, PIF, and MV were significantly increased in miR-132KO mice compared to COPD WT controls (Figure 2C). Furthermore, miR-132KO COPD mice exhibited reduced pathological damage and inflammatory cell infiltration (Figure 2B). These findings suggest that miR-132 deletion enhances respiratory function in COPD mice.
Figure 2.
The deletion of miR-132 improved pulmonary function of Chronic obstructive pulmonary disease (COPD) mice. miR-132KO mice and WT mice were administrated with CS exposure 2 hours per day for 8 weeks, n=8 per group. A. qRT-PCR analysis of miR-132 expression in the lung tissues, liver, heart, and kidney of miR-132KO mice and WT mice. B. H&E staining sections showing the lung injury in vivo (400×, scale bars =100 µm). C. Pulmonary function parameters including MV, PIF, PEF, EIP, Ti and Te were determined. Results are expressed as mean ± SD, **P < 0.01, ***P < 0.001, ****P < 0.0001, vs COPD WT mice. Abbreviations: PIF: peak inspiratory flow; PEF: peak expiratory flow; EIP: end-inspiratory phase; MV: minute ventilation; Ti: inspiratory duration; Te: expiratory duration; SD: Standard Deviation; CS: smoking cigarettes; COPD: Chronic obstructive pulmonary disease; WT: wild type; p: p value.
miR-132 deletion mitigated CS-induced lung apoptosis
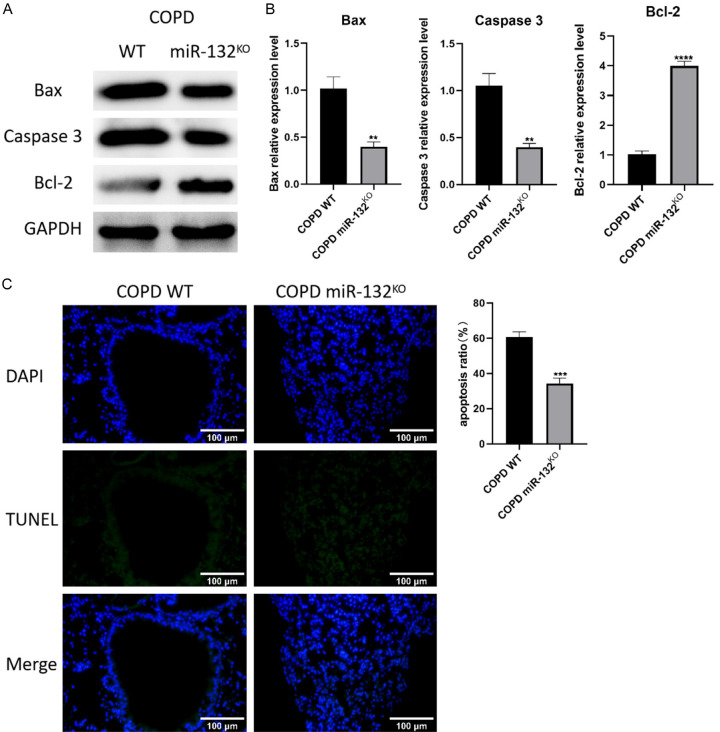
In COPD, miRNAs can influence lung function by regulating apoptosis and inflammation in lung tissues. We examined the effect of miR-132 deletion on apoptosis using TUNEL assays and western blotting. As anticipated, TUNEL staining showed a significant reduction in apoptotic cells in the lung tissues of miR-132KO COPD mice (Figure 3C). Additionally, miR-132 deletion altered the expression of apoptosis-related proteins, further attenuating the apoptotic response (Figure 3A, 3B).
Figure 3.
The deletion of miR-132 alleviated CS-induced lung apoptotic response. miR-132KO mice and WT mice were administered CS exposure 2 hours per day for 8 weeks, n=8 per group. (A) The western blot images and (B) protein quantification of Bax, Caspase-3, Bcl-2 in the lung tissues of WT and miR-132KO mice. (C) Apoptosis ratio of Lung tissues was determined by TUNEL staining (green). n=3 (400×, scale bars =100 µm). Results are expressed as mean ± SD, **P < 0.01, ***P < 0.001, ****P < 0.0001, vs COPD WT mice. Abbreviations: SD: Standard Deviation; COPD: Chronic obstructive pulmonary disease; WT: wild type; CS: smoking cigarettes; p: p value.
miR-132 deletion protected against CS-related COPD via activation of the SIRT1/FoxO-1 axis
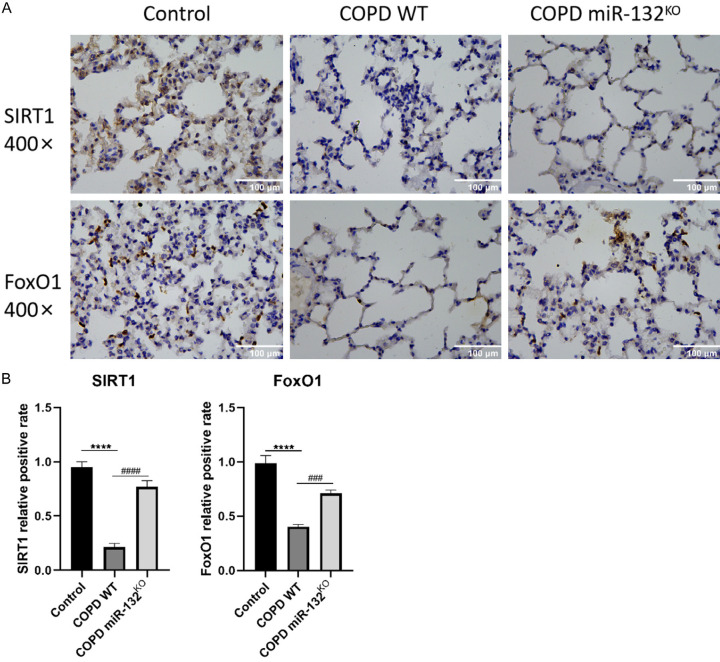
Although our data suggest that miR-132 deletion delays COPD progression by reducing apoptosis and lung tissue damage in COPD mice, the precise molecular mechanism remains unclear. We hypothesized that the SIRT1/FoxO-1 signaling pathway is involved in miR-132-mediated COPD progression. To test this, we performed immunohistochemistry to detect SIRT1 and FoxO-1 in lung tissues of miR-132KO and WT mice. We assessed the effects of CS exposure on SIRT1 and FoxO-1 expression in COPD and control groups. The COPD group exhibited a significant decrease in SIRT1 and FoxO-1 levels compared to controls (Figure 4A, 4B). However, miR-132 deletion restored SIRT1 and FoxO-1 expression in COPD mice (Figure 4A, 4B). These findings indicate that miR-132 deletion protects against cell death by activating the SIRT1/FoxO-1 signaling pathway in the lung tissues of COPD mice.
Figure 4.
The deletion of micRNA-132 protects against CS-related COPD via activation of SIRT1/FoxO-1 axis. SIRT1 and FoxO-1 expression in lung tissues of mice in each group, n=3 per group. (A) Immunohistochemistry staining and (B) quantification of SIRT1 and FoxO-1 in lung tissues of mice in each group (400×, scale bars =100 µm). Results are expressed as mean ± SD, ###P < 0.001, ####P < 0.0001, vs Control mice; ****P < 0.0001, vs COPD WT mice. Abbreviations: SD: Standard Deviation; CS: smoking cigarettes; COPD: Chronic obstructive pulmonary disease; WT: wild type; p: p value.
Discussion
Previous studies have linked abnormal miRNA expression to COPD [14-17]. In this study, we investigated the biological role and regulatory mechanism of miR-132 in a CS-induced COPD mouse model. Our data suggest that miR-132 deletion alleviates CS-induced lung apoptosis and injury by activating the SIRT1/FoxO-1 signaling pathway.
miR-132 is a multifunctional miRNA involved in regulating various pathological processes, including immune response and inflammation [18]. It also regulates apoptosis in lung endothelial cells [7]. In this study, we assessed miR-132 levels in lung tissues and BALF of CS-exposed mice. CS exposure significantly increased miR-132 expression in both lung tissues and BALF, indicating that CS induces miR-132 production and release. Notably, previous studies have shown that miR-132 can be transported via exosomes [19]. Emerging evidence suggests that exosomes protect miRNAs in solution, ensuring their stability in body fluids [20-24]. Moreover, miRNAs can use exosomes for long-distance communication, modulating biological functions. It would be intriguing to explore whether exosome-mediated miR-132 transport plays a role in COPD progression by regulating intercellular communication in lung tissues.
Mice exposed to CS for 2 months exhibited COPD-like symptoms, including lung function decline and structural damage [25-27]. Deletion of miR-132 significantly improved these manifestations, reducing the progression of pulmonary function decline and structural alterations caused by CS exposure.
Higher levels of apoptotic cells have been observed in the lung tissues of COPD patients, particularly in alveolar, bronchiolar, and endothelial cells [28]. The TUNEL assay and western blot analysis confirmed that miR-132 deletion reduced CS-induced apoptosis in lung tissues. These findings are consistent with previous reports demonstrating the pro-apoptotic effects of miR-132 in lung endothelial cells [8].
SIRT1, a NAD+-dependent histone deacetylase, is known to regulate aging, stress resilience, and inflammation [18,29]. miR-132 has been reported to specifically target SIRT1, negatively impacting its expression in neuronal and endothelial cells [30]. Previous studies have shown reduced SIRT1 levels in COPD patients [31,32], and activation of SIRT1 has been found to protect against CS-induced COPD in mice [33-35]. Thus, SIRT1 plays a critical role in COPD prevention. In this study, we found that the reduced expression of SIRT1 caused by CS exposure was partially restored by miR-132 deletion. SIRT1 activation leads to FoxO-1 expression, which improves apoptotic responses in myocardial tissues [36,37]. Therefore, we propose that miR-132 deletion increases SIRT1 expression, which subsequently promotes FoxO-1 expression, alleviating apoptosis in lung tissues. Our findings showed that miR-132KO partially restored the decreased expression of FoxO-1 caused by CS exposure, similar to its effects on SIRT1. Collectively, the pro-apoptotic function of miR-132 is linked to its inhibition of the SIRT1/FoxO-1 signaling pathway (Figure 5).
Figure 5.
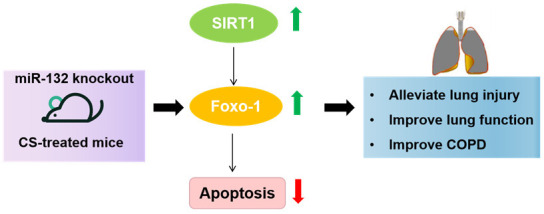
The deletion of micRNA-132 protects against CS-related COPD via activation of SIRT1/FoxO-1 axis. Abbreviations: SD: Standard Deviation; CS: smoking cigarettes; COPD: Chronic obstructive pulmonary disease.
In conclusion, miR-132 inhibited airway remodeling caused by CS in mice by reversing apoptosis and improving lung function, and this effect was partially mediated by the activation of the SIRT1/FoxO-1 axis. These results suggest that miR-132 may be a potential therapeutic target for the prevention and treatment of COPD.
Acknowledgements
This work is supported by the Natural Science Foundation of Hunan Province (No. 2022JJ70012) and Excellent Youth Funding of Hunan Provincial Education Department (No. 21B0069).
Disclosure of conflict of interest
None.
References
- 1.Roffel MP, Boudewijn IM, van Nijnatten JLL, Faiz A, Vermeulen CJ, van Oosterhout AJ, Affleck K, Timens W, Bracke KR, Maes T, Heijink IH, Brandsma CA, van den Berge M. Identification of asthma-associated microRNAs in bronchial biopsies. Eur Respir J. 2022;59:2101294. doi: 10.1183/13993003.01294-2021. [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, Huang K, Yao W, Sun T, Shan G, Yang T, Lin Y, Wu S, Zhu J, Wang R, Shi Z, Zhao J, Ye X, Song Y, Wang Q, Zhou Y, Ding L, Yang T, Chen Y, Guo Y, Xiao F, Lu Y, Peng X, Zhang B, Xiao D, Chen CS, Wang Z, Zhang H, Bu X, Zhang X, An L, Zhang S, Cao Z, Zhan Q, Yang Y, Cao B, Dai H, Liang L, He J China Pulmonary Health Study Group. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Li P, Yuan Q, Wang X, Ma HH, Zhuan B. Inhibition of miR-4640-5p alleviates pulmonary hypertension in chronic obstructive pulmonary disease patients by regulating nitric oxide synthase 1. Respir Res. 2023;24:92. doi: 10.1186/s12931-023-02387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim RY, Sunkara KP, Bracke KR, Jarnicki AG, Donovan C, Hsu AC, Ieni A, Beckett EL, Galvão I, Wijnant S, Ricciardolo FL, Di Stefano A, Haw TJ, Liu G, Ferguson AL, Palendira U, Wark PA, Conickx G, Mestdagh P, Brusselle GG, Caramori G, Foster PS, Horvat JC, Hansbro PM. A microRNA-21-mediated SATB1/S100A9/NF-kappaB axis promotes chronic obstructive pulmonary disease pathogenesis. Sci Transl Med. 2021;13:eaav7223. doi: 10.1126/scitranslmed.aav7223. [DOI] [PubMed] [Google Scholar]
- 5.Reid LV, Spalluto CM, Watson A, Staples KJ, Wilkinson TMA. The role of extracellular vesicles as a shared disease mechanism contributing to multimorbidity in patients with COPD. Front Immunol. 2021;12:754004. doi: 10.3389/fimmu.2021.754004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massara L, Gosset P. MicroRNA control lipid-laden alveolar macrophages in smokers: a potential therapeutic target for chronic obstructive pulmonary disease? Am J Respir Cell Mol Biol. 2022;67:619–620. doi: 10.1165/rcmb.2022-0338ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diao X, Zhou J, Wang S, Ma X. Upregulation of miR-132 contributes to the pathophysiology of COPD via targeting SOCS5. Exp Mol Pathol. 2018;105:285–292. doi: 10.1016/j.yexmp.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Shen Q, Zheng J, Wang X, Hu W, Jiang Y, Jiang Y. LncRNA SNHG5 regulates cell apoptosis and inflammation by miR-132/PTEN axis in COPD. Biomed Pharmacother. 2020;126:110016. doi: 10.1016/j.biopha.2020.110016. [DOI] [PubMed] [Google Scholar]
- 9.Shu J, Li D, Ouyang H, Huang J, Long Z, Liang Z, Chen Y, Chen Y, Zheng Q, Kuang M, Tang H, Wang J, Lu W. Comparison and evaluation of two different methods to establish the cigarette smoke exposure mouse model of COPD. Sci Rep. 2017;7:15454. doi: 10.1038/s41598-017-15685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roffel MP, Maes T, Brandsma CA, van den Berge M, Vanaudenaerde BM, Joos GF, Brusselle GG, Heijink IH, Bracke KR. MiR-223 is increased in lungs of patients with COPD and modulates cigarette smoke-induced pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. 2021;321:L1091–L1104. doi: 10.1152/ajplung.00252.2021. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Zhang L, Wang Q. MicroRNA-221-3p alleviates cell apoptosis and inflammatory response by targeting cyclin dependent kinase inhibitor 1B in chronic obstructive pulmonary disease. Bioengineered. 2021;12:5705–5715. doi: 10.1080/21655979.2021.1967837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki T, Sugihara F, Fukushima K, Matsuki T, Nabeshima H, Machida T, Mitsui Y, Fujimura S, Sagawa R, Gaheun L, Kuniyoshi K, Tanaka H, Narazaki M, Kumanogoh A, Akira S, Satoh T. Loss of FCHSD1 leads to amelioration of chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2021;118:e2019167118. doi: 10.1073/pnas.2019167118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas-Quintero J, Laucho-Contreras ME, Wang X, Fucci QA, Burkett PR, Kim SJ, Zhang D, Tesfaigzi Y, Li Y, Bhashyam AR, Li Z, Khamas H, Celli B, Pilon AL, Polverino F, Owen CA. CC16 augmentation reduces exaggerated COPD-like disease in Cc16-deficient mice. JCI Insight. 2023;8:e130771. doi: 10.1172/jci.insight.130771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirra D, Esposito R, Spaziano G, La Torre C, Vocca C, Tallarico M, Cione E, Gallelli L, D’Agostino B. Lung microRNAs expression in lung cancer and COPD: a preliminary study. Biomedicines. 2023;11:736. doi: 10.3390/biomedicines11030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Zhao H, Raman I, Yan M, Chen Q, Li QZ. Peripheral blood mononuclear cell gene expression in chronic obstructive pulmonary disease: miRNA and mRNA regulation. J Inflamm Res. 2022;15:2167–2180. doi: 10.2147/JIR.S337894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D, Jing J, Li Z, Wang J, Jiang M, Meng T. Association between genetic polymorphisms of miRNAs (miR-8079 and miR-5007) and susceptibility of chronic obstructive pulmonary disease in Chinese people. Microb Pathog. 2021;160:105160. doi: 10.1016/j.micpath.2021.105160. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang Y, Hobbs BD, Hersh CP, Kechris K. Identifying miRNA-mRNA networks associated with COPD phenotypes. Front Genet. 2021;12:748356. doi: 10.3389/fgene.2021.748356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Huang D, Wang Q, Shen D, Wang Y, Chen B, Zhang J, Gai L. MiR-132 inhibits expression of SIRT1 and induces pro-inflammatory processes of vascular endothelial inflammation through blockade of the SREBP-1c metabolic pathway. Cardiovasc Drugs Ther. 2014;28:303–11. doi: 10.1007/s10557-014-6533-x. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Li Y, Jiang R, Nie C, Zeng Z, Zhao N, Huang C, Shao Q, Ding C, Qing C, Xia L, Zeng E, Qian K. miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Exp Lung Res. 2015;41:261–9. doi: 10.3109/01902148.2015.1004206. [DOI] [PubMed] [Google Scholar]
- 20.Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, Abdul Roda M, Xu X, Rezonzew G, Viera L, Dobosh BS, Margaroli C, Abdalla TH, King RW, McNicholas CM, Wells JM, Dransfield MT, Tirouvanziam R, Gaggar A, Blalock JE. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019;176:113–126. e15. doi: 10.1016/j.cell.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez N, James V, Onion D, Fairclough LC. Extracellular vesicles and chronic obstructive pulmonary disease (COPD): a systematic review. Respir Res. 2022;23:82. doi: 10.1186/s12931-022-01984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Yin Z, Fan J, Zhang S, Yang W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther. 2019;4:47. doi: 10.1038/s41392-019-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell DW, Genschmer KR, Blalock JE. Extracellular vesicles as central mediators of COPD pathophysiology. Annu Rev Physiol. 2022;84:631–654. doi: 10.1146/annurev-physiol-061121-035838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundar IK, Li D, Rahman I. Small RNA-sequence analysis of plasma-derived extracellular vesicle miRNAs in smokers and patients with chronic obstructive pulmonary disease as circulating biomarkers. J Extracell Vesicles. 2019;8:1684816. doi: 10.1080/20013078.2019.1684816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bi H, Wang G, Li Z, Zhou L, Zhang M, Ye J, Wang Z. Long noncoding RNA (lncRNA) maternally expressed gene 3 (MEG3) participates in chronic obstructive pulmonary disease through regulating human pulmonary microvascular endothelial cell apoptosis. Med Sci Monit. 2020;26:e920793. doi: 10.12659/MSM.920793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen P, Xu W, Luo Y, Zhang Y, He Y, Yang S, Yuan Z. MicroRNA 543 suppresses breast cancer cell proliferation, blocks cell cycle and induces cell apoptosis via direct targeting of ERK/MAPK. Onco Targets Ther. 2017;10:1423–1431. doi: 10.2147/OTT.S118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang T, Hu W, Zhang S, Ren C, Lin S, Zhou Z, Wu H, Yin J, Tan L. Fibroblast growth factor 10 attenuates chronic obstructive pulmonary disease by protecting against glycocalyx impairment and endothelial apoptosis. Respir Res. 2022;23:269. doi: 10.1186/s12931-022-02193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Q, Chen P, Liu XM. The role of cigarette smoke-induced pulmonary vascular endothelial cell apoptosis in COPD. Respir Res. 2021;22:39. doi: 10.1186/s12931-021-01630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Zhang Y, Kang Y, Liu S, Wang Y, Wang Y, Wang L. LncRNA MIAT inhibits MPP(+)-induced neuronal damage through regulating the miR-132/SIRT1 axis in PC12 cells. Neurochem Res. 2021;46:3365–3374. doi: 10.1007/s11064-021-03437-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhu HC, Song WW, Zhao ML, Zhang RM, Tian X. Effect of miR-132 on lung injury in sepsis rats via regulating Sirt1 expression. Eur Rev Med Pharmacol Sci. 2021;25:1042–1049. doi: 10.26355/eurrev_202101_24674. [DOI] [PubMed] [Google Scholar]
- 31.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–70. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanagisawa S, Papaioannou AI, Papaporfyriou A, Baker JR, Vuppusetty C, Loukides S, Barnes PJ, Ito K. Decreased serum sirtuin-1 in COPD. Chest. 2017;152:343–352. doi: 10.1016/j.chest.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng M, Tong R, Bian Y, Hou G. Astaxanthin attenuates cigarette smoking-induced oxidative stress and inflammation in a sirtuin 1-dependent manner. Biomed Pharmacother. 2023;159:114230. doi: 10.1016/j.biopha.2023.114230. [DOI] [PubMed] [Google Scholar]
- 34.He B, Zhang W, Qiao J, Peng Z, Chai X. Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can J Physiol Pharmacol. 2019;97:386–391. doi: 10.1139/cjpp-2018-0529. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Ma H, Wang L, Zhang H, Lu L, Xiao T, Cheng C, Wang P, Yang Y, Wu M, Wang S, Zhang J, Liu Q. Regulation of lung epithelial cell senescence in smoking-induced COPD/emphysema by microR-125a-5p via Sp1 mediation of SIRT1/HIF-1a. Int J Biol Sci. 2022;18:661–674. doi: 10.7150/ijbs.65861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taka C, Hayashi R, Shimokawa K, Tokui K, Okazawa S, Kambara K, Inomata M, Yamada T, Matsui S, Tobe K. SIRT1 and FOXO1 mRNA expression in PBMC correlates to physical activity in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:3237–3244. doi: 10.2147/COPD.S144969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K, Hu W. Oxypaeoniflorin improves myocardial ischemia/reperfusion injury by activating the Sirt1/Foxo1 signaling pathway. Acta Biochim Pol. 2020;67:239–245. doi: 10.18388/abp.2020_5206. [DOI] [PubMed] [Google Scholar]