Abstract
Objective: To identify risk factors for bone metastasis in patients with newly diagnosed malignant tumor and to develop a prediction model. Methods: Clinical data from 232 patients with newly diagnosed malignant tumors were analyzed to screen for risk factors associated with bone metastasis. A nomogram prediction model was constructed using R software. The model’s performance was evaluated using Receiver Operating Characteristic (ROC) analysis, Bootstrap sampling, and Decision Curve Analysis (DCA). Results: The incidence of bone metastasis in the 232 cases with newly diagnosed malignant tumors was 21.98% (51/232). Multivariate logistic regression analysis revealed that tumor staging III-IV, lymph node metastasis, high Eastern Cancer Collaboration Group Physical Status (ECOG-PS) score, high alkaline phosphatase (ALP) expression, and high SII index were risk factors for bone metastasis at initial diagnosis (all P<0.05). The area under the curve (AUC) of the nomogram model was 0.893. Bootstrap sampling validation showed a small error of 0.017 between predicted and actual probabilities. DCA supported the utility of the model in clinical practice. Conclusion: Bone metastasis in newly diagnosed malignant tumors is associated with advanced tumor staging, lymph node metastasis, high ECOG-PS score, elevated ALP expression, and a high SII index. A nomogram model based on these factors can effectively predict the risk of bone metastasis in these patients.
Keywords: Initial diagnosis, malignant tumors, bone metastasis, risk factors, prediction model
Introduction
Bone is a common site for metastasis in various malignant tumors [1]. Patients with bone metastasis often develop bone-related complications such as bone pain, pathological fractures, myelosuppression, and hypercalcemia [2]; affecting their quality of life and the prognosis of primary disease and increasing their medical burden. Currently, the clinical diagnosis of malignant tumor patients with bone metastasis mainly depends on imaging modalities, including X-ray, computed tomography (CT), magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), positron emission computed tomography (PET-CT), and radionuclide bone scans [3,4]. However, early-stage bone metastasis often presents subtle imaging alterations, and by the time imaging manifestations and clinical symptoms are significant, the disease has usually advanced. In addition, imaging examination can be costly and carry risks of radiation exposure, making frequent screening impractical. As a result, imaging technologies have limitations in the detection of bone metastases in newly diagnosed malignant tumors. The uncertainty in risk stratification for bone metastases can complicate decision-making in clinical practice, leading to potential missed diagnoses [5].
Identifying the influencing factors for bone metastasis in newly diagnosed malignant tumor patients and constructing a predictive model could help address this issue. Clinicians could use such models to stratify patients according to their risk of bone metastasis, reducing the likelihood of missed diagnoses. Moreover, based on the estimated risk, preventive strategies could be implemented to minimize the incidence of bone metastasis in the later stages of disease. The purpose of this study is to analyze the risk factors for bone metastasis in patients with newly diagnosed malignant tumors and to construct a predictive model, providing clinicians with a useful tool to assess risk and implement preventive measures.
Material and methods
Research subjects
In this retrospective study, the clinical data of 232 patients with newly diagnosed malignant tumors were collected and analyzed. Patients were divided into a bone metastasis group and non-bone metastasis group, based on imaging examination or bone biopsy confirmation of metastasis after admission. This study was approved by the Ethic Committee of Suzhou Ninth People’s Hospital.
Inclusion criteria: (1) Diagnosed as a malignant tumor based on histopathology and imaging, meeting the diagnostic criteria of the International Union Against Cancer (UICC), as outlined in the “UICC Clinical Oncology Handbook” [6]; (2) Age ≥18 years old, first visit, without any treatment (such as surgery, radiotherapy, chemotherapy, or other anti-tumor drugs) upon admission. Exclusion criteria: (1) Patients with dual or multiple cancers; (2) Patients with diseases affecting bone metabolism, such as thyroid and parathyroid diseases, rickets, osteoporosis, traumatic fractures, and rheumatoid arthritis; (3) Patients with infectious diseases within 3 months prior to the initial diagnosis; (4) Patients taking bisphosphate, hormones, or calcium supplements within 1 month before the initial diagnosis.
Research methods
Data collection
A structured data sheet was used to collect patient information, including age, sex, body mass index (BMI), comorbidities (hypertension, diabetes, hyperlipidemia etc.), malignant tumor type, tumor stage, lymph node metastasis, primary tumor diameter, physical status, serum biomarkers [lactate dehydrogenase (LDH), alkaline phosphatase (ALP), blood calcium (Ca), carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), systemic immune inflammation index (SII)]. The SII value was calculated as platelet count * neutrophil count/lymphocyte count [7]. The physical fitness status was evaluated using the Eastern Cancer Collaboration Group Physical Status (ECOG-PS) scale [8]. This scale classifies patients’ physical condition into six levels, ranging from 0 to 5, with higher scores indicating poorer physical condition; 0 points: fully active, with no restriction on activities, similar to pre-disease condition; 1 point: mild symptoms, able to engage in light physical activities; 2 points: able to care for themselves and tolerate tumor-related symptoms but limited to being active for at least half of the day; 3 points: severe symptoms, able to care for themselves partially, and spend more than half of the day in bed or in a wheelchair; 4 points: completely bedridden and unable to take care of themselves; 5 points: deceased.
Diagnosis of bone metastasis
First, other benign bone lesions were excluded prior to the diagnosis of bone metastasis. The diagnosis criteria of bone metastasis [9]: 1) A pathological biopsy indicates malignant tumor bone metastasis; 2) If the biopsy does not confirm bone metastasis, imaging evidence from at least two different modalities (such as CT, MRI, ECT, or PET-CT) is required to support the diagnosis of bone metastasis.
Research process
First, the general data, blood test data at the time of diagnosis, and pathological examination data of all cases were organized. The clinical characteristics of patients with bone metastasis were compared to those without bone metastasis. Statistically significant indicators were included in multivariate analysis to determine independent risk factors for bone metastasis in newly diagnosed malignant tumor patients. Then, based on the results of multivariate analysis, a predictive model for bone metastasis risk was constructed. The model was developed in strict adherence to the TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis) guidelines, and visualized as a nomogram. Internal validation of the model was conducted, focusing on evaluating its discrimination, calibration, and clinical applicability.
Statistical analysis
SPSS 27.0 software was used for data analysis. Measurement data were expressed by (mean ± SD), and compared by t-test between the two groups. Categorical variables were presented as counts and percentages [n (%)], and the χ2 test was used for comparison between groups. Multivariate logistic regression analysis was used to identify the risk factors for bone metastasis in patients with newly diagnosed malignant tumors. A P value less than 0.05 was considered with statistically significant difference. A nomogram prediction model for bone metastasis risk was constructed using R3.4.3 software. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the discrimination performance of the model. The Hosmer Lemeshow (H-L) goodness-of-fit test and Bootstrap resampling were applied to verify the model’s fit and calibration, respectively. Decision Curve Analysis (DCA) was used to evaluate the model’s practical value in clinical practice.
Results
Bone metastasis status and univariate analysis
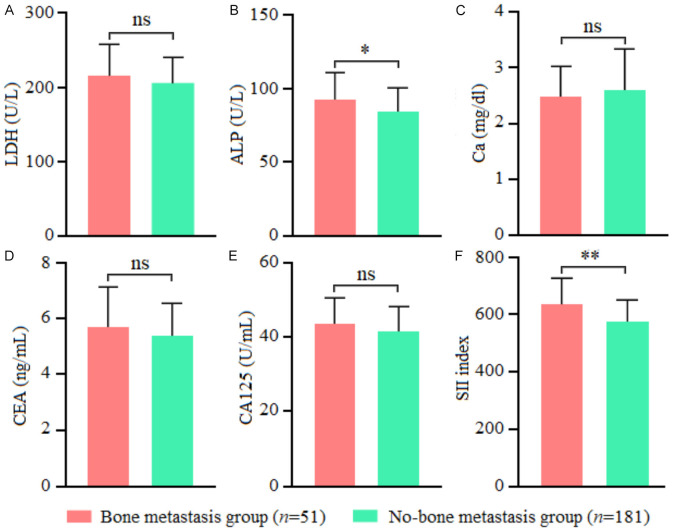
Of the 232 cases with newly diagnosed malignant tumors, 51 were confirmed with bone metastasis (bone metastasis group), with an incidence of 21.98% (51/232). Among them, there were 12 cases of multiple bone metastases and 39 cases of single bone metastases. The remaining 181 cases were confirmed without bone metastasis (non-bone metastasis group). Univariate analysis showed significant differences between the two groups in tumor staging, lymph node metastasis, ECOG-PS score, ALP, and SII index (all P<0.05) (Table 1; Figure 1).
Table 1.
Comparison of baseline data between the two groups
| Characteristics | Bone metastasis group (n = 51) | Non-bone metastasis group (n = 181) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 59.65±9.54 | 57.93±9.16 | 1.174 | 0.242 |
| Gender | 0.198 | 0.657 | ||
| Male | 23 (45.10) | 88 (48.62) | ||
| Female | 28 (54.90) | 93 (51.38) | ||
| BMI (kg/cm2) | 22.17±2.08 | 22.49±2.14 | 0.949 | 0.344 |
| Combined hypertension | 0.820 | 0.365 | ||
| Yes | 6 (11.76) | 14 (7.73) | ||
| No | 45 (88.24) | 167 (92.27) | ||
| Combined diabetes | 2.439 | 0.118 | ||
| Yes | 8 (15.69) | 15 (8.29) | ||
| No | 43 (84.31) | 166 (91.71) | ||
| Combined with hyperlipidemia | 0.590 | 0.442 | ||
| Yes | 5 (9.80) | 12 (6.63) | ||
| No | 46 (90.20) | 169 (93.37) | ||
| Malignant tumor type | 0.428 | 0.934 | ||
| Digestive system tumors | 16 (31.37) | 52 (28.73) | ||
| Respiratory system tumors | 14 (27.45) | 47 (25.97) | ||
| Genitourinary neoplasms | 11 (21.57) | 39 (21.55) | ||
| Breast system tumors | 10 (19.61) | 43 (23.75) | ||
| Tumor staging | 47.050 | <0.001 | ||
| I-II | 3 (5.88) | 109 (60.22) | ||
| III-IV | 48 (94.12) | 72 (39.78) | ||
| Lymph node metastasis | 40.072 | <0.001 | ||
| Yes | 42 (82.35) | 59 (32.60) | ||
| No | 9 (17.65) | 122 (67.40) | ||
| Primary tumor diameter (cm) | 2.71±0.84 | 2.54±0.63 | 1.667 | 0.097 |
| ECOG-PS score (points) | 2.18±0.75 | 1.46±0.46 | 8.465 | <0.001 |
Note: BMI: body mass index; ECOG-PS: Eastern Cancer Collaboration Group Performance Status.
Figure 1.
Comparison of blood indicators between the two groups. A: LDH, lactate dehydrogenase; B: ALP, alkaline phosphatase; C: Ca, calcium; D: CEA, carcinoembryonic antigen; E: CA125, carbohydrate antigen 125; F: SII, systemic immune inflammatory index. Note: ns, P>0.05; *, P<0.05; **, P<0.01.
Multivariate logistic regression analysis
Bone metastasis in patients with newly diagnosed malignant tumor (0 = no; 1 = yes) was taken as the dependent variable, and the variables with P<0.05 from Table 1 were assigned as an independent variables, see Table 2. Multivariate logistic analysis showed that tumor stages III-IV, lymph node metastasis, high ECOG-PS score, elevated ALP levels, and high SII index were all risk factors for bone metastasis in newly diagnosed malignant tumor patients (all P<0.05), see Table 3.
Table 2.
Variable assignment table
| Variables | Assignment |
|---|---|
| Tumor staging | 0 = I-II, 1 = III-IV |
| Lymph node metastasis | 0 = No, 1 = Yes |
| ECOG-PS score | Continuous variable |
| ALP | Continuous variable |
| SII index | Continuous variable |
Note: ECOG-PS: Eastern Cancer Collaboration Group Performance Status; ALP: Alkaline phosphatase; SII: systemic immune inflammation index.
Table 3.
Multivariate logistic analysis
| Variable | Β | SE | Wald χ2 | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Tumor stage III-IV | 1.198 | 0.388 | 9.533 | 0.002 | 3.313 (1.549-7.085) |
| Lymph node metastasis | 1.054 | 0.384 | 7.534 | 0.006 | 2.869 (1.351-6.092) |
| ECOG-PS score is high | 0.738 | 0.256 | 8.311 | 0.004 | 2.092 (1.266-3.457) |
| High expression of ALP | 0.876 | 0.312 | 7.883 | 0.005 | 2.401 (1.302-4.428) |
| SII index high | 1.125 | 0.289 | 15.153 | <0.001 | 3.080 (1.749-5.425) |
Note: ECOG-PS: Eastern Cancer Collaboration Group Performance Status; ALP: Alkaline phosphatase; SII: systemic immune inflammation index.
Construction of a nomogram model for bone metastasis risk in patients with newly diagnosed malignant tumors
A prediction model was constructed based on the regression coefficients and constant terms derived from the multiple regression analysis results: Z = -9.543 + 1.198 × tumor stage (0 = I-II, 1 = III-IV) + 1.054 × lymph node metastasis (0 = no, 1 = yes) + 0.738 × ECOG-PS score (actual value) + 0.876 × ALP (actual value) + 1.125 × SII index (actual value). This logistic regression model was visualized using R software to create a nomogram. Interpretation: The total score was obtained by summing the individual scores for each factor in the nomogram. The corresponding prediction probability of bone metastasis risk is then determined by locating the total score on the vertical axis, which provides the predicted probability of bone metastasis in patients with newly diagnosed malignant tumors. See Figure 2.
Figure 2.
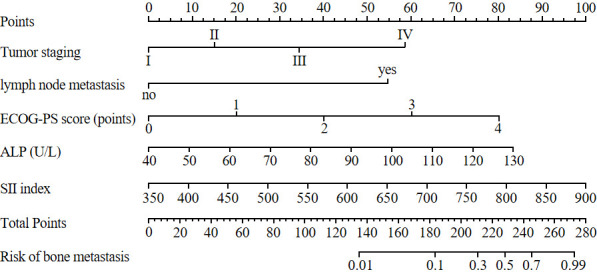
Nomogram prediction model for bone metastasis risk in patients with malignant tumor. Note: ECOG-PS: Physical strength rating scale of the eastern United States cancer cooperation group; ALP: Alkaline phosphatase; SII: systemic immune inflammation index.
Performance analysis of nomogram prediction model for bone metastasis risk in newly diagnosed malignant tumor patients
According to the ROC curve analysis results, the AUC for the nomogram model in predicting bone metastasis risk in newly diagnosed malignant tumor patients was 0.893, with a sensitivity of 0.941 and a specificity of 0.792 (Figure 3). The H-L goodness-of-fit test showed no significant difference between the predicted probability and the actual occurrence (χ2 = 2.665, P = 0.954), indicating no fitting phenomenon. The Bootstrap method was used for validation with 1,000 internal sampling, the error between the predicted probability of the model and the actual value was 0.017, indicating good consistency between predicted and actual probabilities of bone metastasis in newly diagnosed malignant tumor patients (Figure 4). Decision curve analysis further indicated that the net benefit of the prediction model was higher than the other two extreme curves, suggesting that the model is practical and valuable in clinical practice (Figure 5).
Figure 3.
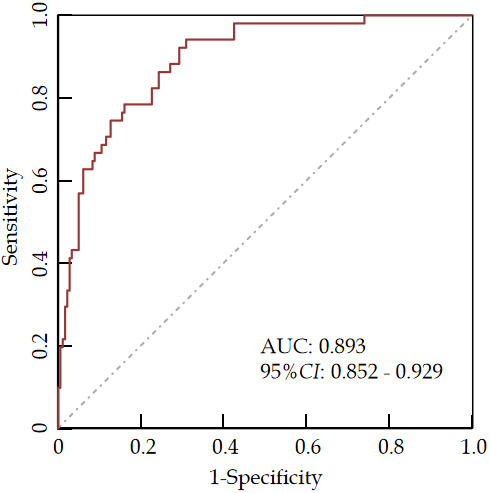
ROC curve analysis of the predictive model for bone metastasis in malignant tumor patients.
Figure 4.
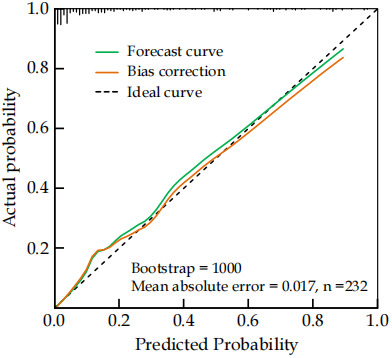
Calibration curve analysis of prediction model for bone metastasis in malignant tumor patients.
Figure 5.
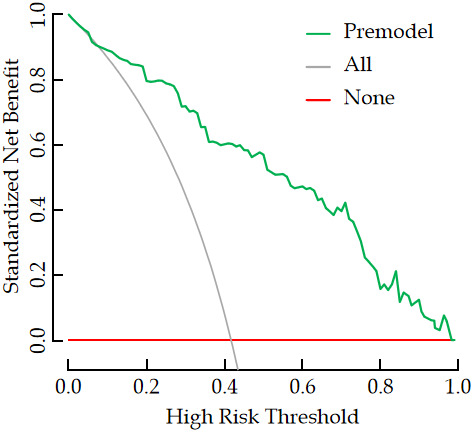
Decision curve analysis of prediction model for bone metastasis in patients with malignant tumors.
Discussion
Malignant tumors are known for their invasive nature, and metastasis is common. Related studies have shown that, about 80% of malignant tumors involve bones [10]. The incidence of bone metastasis in this study was 21.98%, lower than previously reported figures. This may be related to the selection of newly diagnosed malignant tumor patients, the sample size, and the specific inclusion and exclusion criteria in this study. Once bone metastasis occurs, patients may experience severe and persistent pain, along with various skeletal complications. This is because, when cancer cells metastasize to the bone, they can colonize and grow, causing damage to the bone and promoting excessive osteoclast activity, leading to local osteolytic destruction [11]. Therefore, early accurate assessment and timely intervention are crucial to managing these patients effectively.
Tumor staging reflects the extent of tumor infiltration. As staging progresses, the range of tumor invasion increases, and cancer cells become more actively proliferative, which is closely related to the poor prognosis of tumor patients [12]. Numerous studies have confirmed a significant correlation between clinical staging of tumors and the occurrence of bone metastasis in cancers such as non-small cell lung cancer [13], colorectal cancer [14], prostate cancer [15], primary liver cancer [16], and breast cancer [17]. This study also found that advanced tumor stage was a risk factor for bone metastasis in patients with newly diagnosed malignant tumors, aligning with previous reports. It is possible that as the tumor metastasizes to distant sites, it simultaneously invades surrounding tissues, capillaries, and lymphatic vessels, resulting in an escalation of tumor staging. The more advanced the stage, the greater the number of lymph nodes affected, and the higher the likelihood of bone metastasis. Studies have shown that, when lymph nodes are involved, tumor cells can evade immune-mediated cytotoxicity and induce the production of regulatory T cells, leading to immune tolerance and promoting tumor metastasis [18]. This suggests that lymph node involvement increases the risk of bone metastasis. This study found that lymph node metastasis is a risk factor for bone metastasis in newly diagnosed malignant tumor patients. This may be due to the involvement of lymph nodes, which indicates a shorter doubling time of cancer cells, a higher proliferation rate, and a broader dissemination range, making bone metastasis more likely. Additionally, tumor cells involved in lymph nodes can spread through the venous system in a hematogenous manner, involving bones, or occasionally directly invading adjacent bones through soft tissue, further increasing the risk of bone metastasis. Therefore, for patients with advanced-stage malignant tumors and lymph node involvement at initial diagnosis, close clinical attention should be paid to the risk of bone metastasis.
Decreased physical fitness is the most common symptom in patients with malignant tumors. The higher the ECOG-PS score, the worse their physical status [19]. Research has shown that bone related events such as bone pain and spinal cord compression caused by bone metastasis in patients with malignant tumors - especially when metastasis occurs in load-bearing bones - directly affects the patient’s physical condition, leading to reduced mobility and limited daily activities [20]. This suggests that poor physical fitness in patients with malignant tumor is associated with bone metastasis. In this study, a high ECOG-PS score was identified as a risk factor for bone metastasis in patients with newly diagnosed malignant tumors, similar to the findings in previous research [21]. This may be due to the high malignancy of the tumor and the excessive consumption of the patient’s physical fitness caused by the rapid development of tumor. During the aggressive phase of malignant tumor development, the rapid proliferation of cancer cells can drive metastasis, including bone metastasis. Therefore, for malignant tumor patients with high initial ECOG-PS scores, clinical attention should be paid to the possibility of existing bone metastases to avoid missed diagnosis and missed treatment.
ALP is a phosphohydrolase enzyme primarily derived from bone and liver [22], and it reflects the activity of osteoblasts. Wymenga et al. [23] showed that serum ALP exceeding 90 U/L could be a marker for positive bone metastasis in about 60% of prostate cancer patients. Bayrak et al. [24] found that, the serum ALP levels in lung cancer patients with bone metastasis were significantly higher than those without bone metastasis. Jiang et al. [25] showed that, elevated serum ALP level was significantly associated with bone metastasis in breast cancer patients. These suggest a correlation between elevated serum ALP levels and bone metastases in various malignant tumors. This study identified elevated serum ALP expression as a risk factor for bone metastasis of newly diagnosed malignant tumors, consistent with previously reported findings [26]. It is possible that cancer cells metastasize to bone tissue via blood circulation and lymphatic circulation, leading to the activation of osteoclasts and subsequent osteolytic destruction, which contributes to the formation of bone metastases. When bone cells are stimulated by growth factors from tumor cells, it may cause increased ALP secretion, resulting in elevated serum ALP levels. However, studies have shown that using serum ALP index alone to evaluate bone metastasis lacks specificity [27], as bone fractures, abnormal bone metabolism and other conditions affecting the bone can also significantly increase serum ALP levels. Inflammation is an important component of the tumor microenvironment and plays a crucial role in promoting tumor development and progression [28,29]. Several cellular markers associated with inflammation and immunity are routinely measured in peripheral blood tests, such as platelets, neutrophils, and lymphocytes. Among them, platelets have been proven to promote tumor progression and metastasis through various interactions with tumor cells, including helping them evade immune surveillance [30]. Neutrophils can promote tumor proliferation, invasion, and metastasis by releasing cytokines and chemokines, such as interleukin-6 and tumor necrosis factor-alpha [31]. Lymphocytes are essential for eliminating tumor cells, as they induce cytotoxic cell death [32], effectively inhibiting tumor growth and migration. Recent research has established that the SII index, calculated from platelets, neutrophils and lymphocyte counts, can predict disease progression in various malignancies [33-35]. He et al. [36] demonstrated that a high SII index is an independent risk factor for bone metastasis in lung cancer patients, which aligns with the results of this study. SII index reflects the balance between inflammation and immunity by representing three coexisting immune and inflammatory pathways in the body [37]. It is easily obtainable in clinical practice and serves as a composite index, making it a more comprehensive and accurate tool for assessing the risk of bone metastasis in newly diagnosed malignant tumor patients.
There are many factors influencing bone metastasis in patients with newly diagnosed malignant tumors, each with complex mechanisms. It is essential to combine these factors into a predictive model to better evaluate the risk of bone metastasis. In this study, multivariate logistic regression analysis identified five risk factors, including tumor stage, lymph node metastasis, physical condition, ALP, and SII index. Based on these factors, a predictive model was constructed. Subsequent analysis yielded an AUC value of 0.893, and the H-L goodness-of-fit test showed no overfitting, indicating that the model effectively evaluates and distinguishes bone metastases in newly diagnosed malignant tumors. Decision curve analysis found that the net benefit curve of this model was superior to the extreme curve in evaluating bone metastasis, indicating that this model has timeliness in clinical practice. Therefore, clinicians can stratify the patients into different bone metastasis risk groups according to the prediction model, reducing the missed diagnosis rate. Additionally, based on the assessed probability of bone metastasis, preventive measures can be implemented to minimize the risk of bone metastasis in these patients during the later stages of their disease.
Conclusion
In summary, bone metastasis in newly diagnosed malignant tumor patients is associated with advanced tumor stage, lymph node metastasis, poor physical condition, elevated ALP levels, and high SII index. The predictive model constructed based on these factors can provide guidance for clinical assessment of bone metastasis risk in newly diagnosed malignant tumor patients and the development of preventive measures. This study does have a few limitations. First, it is a single center retrospective study, with a single sample source, which may limit the model’s generalizability. Second, the model has not been externally validated, and its reliability still needs to be verified through future multi-center studies with large sample sizes.
Disclosure of conflict of interest
None.
References
- 1.Zhao H, Han KL, Wang ZY, Chen Y, Li HT, Zeng JL, Shen Z, Yao Y. Value of C-telopeptide-cross-linked Type I collagen, osteocalcin, bone-specific alkaline phosphatase and procollagen Type I N-terminal propeptide in the diagnosis and prognosis of bone metastasis in patients with malignant tumors. Med Sci Monit. 2011;17:CR626–633. doi: 10.12659/MSM.882047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okabe H, Aoki K, Yogosawa S, Saito M, Marumo K, Yoshida K. Downregulation of CD24 suppresses bone metastasis of lung cancer. Cancer Sci. 2018;109:112–120. doi: 10.1111/cas.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng C, Tang C, Liang J. Progress of biomarkers in diagnosis of bone metastases of lung cancer. Zhongguo Fei Ai Za Zhi. 2018;21:615–619. doi: 10.3779/j.issn.1009-3419.2018.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuji I, Yamane T, Seto A, Yasumizu Y, Shirotake S, Oyama M. Skeletal standardized uptake values obtained by quantitative SPECT/CT as an osteoblastic biomarker for the discrimination of active bone metastasis in prostate cancer. Eur J Hybrid Imaging. 2017;1:2. doi: 10.1186/s41824-017-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaac A, Dalili D, Dalili D, Weber MA. State-of-the-art imaging for diagnosis of metastatic bone disease. Radiologe. 2020;60(Suppl 1):1–16. doi: 10.1007/s00117-020-00666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertero L, Massa F, Metovic J, Zanetti R, Castellano I, Ricardi U, Papotti M, Cassoni P. Eighth edition of the UICC classification of malignant tumours: an overview of the changes in the pathological TNM classification criteria-what has changed and why? Virchows Arch. 2018;472:519–531. doi: 10.1007/s00428-017-2276-y. [DOI] [PubMed] [Google Scholar]
- 7.Ciocan A, Ciocan RA, Al Hajjar N, Gherman CD, Bolboaca SD. Abilities of pre-treatment inflammation ratios as classification or prediction models for patients with colorectal cancer. Diagnostics (Basel) 2021;11:566. doi: 10.3390/diagnostics11030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neeman E, Gresham G, Ovasapians N, Hendifar A, Tuli R, Figlin R, Shinde A. Comparing physician and nurse eastern cooperative oncology group performance status (ECOG-PS) ratings as predictors of clinical outcomes in patients with cancer. Oncologist. 2019;24:e1460–e1466. doi: 10.1634/theoncologist.2018-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, Pan Q, Huang F, Hu H, Shao Z. Research progress of bone metastases: from disease recognition to clinical practice. Front Oncol. 2023;12:1105745. doi: 10.3389/fonc.2022.1105745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu NN, Shen DL, Chen XQ, He YL. Clinical analysis of 355 patients with bone metastasis of malignant tumors. Zhonghua Zhong Liu Za Zhi. 2010;32:203–207. [PubMed] [Google Scholar]
- 11.Porta-Sales J, Garzon-Rodriguez C, Llorens-Torrome S, Brunelli C, Pigni A, Caraceni A. Evidence on the analgesic role of bisphosphonates and denosumab in the treatment of pain due to bone metastases: a systematic review within the European Association for Palliative Care guidelines project. Palliat Med. 2017;31:5–25. doi: 10.1177/0269216316639793. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Yu W, Zhang C, Li H, Li X, Song F, Li S, Jiang G, Li H, Mao M, Wang X. Reclassified the phenotypes of cancer types and construct a nomogram for predicting bone metastasis risk: a pan-cancer analysis. Cancer Med. 2024;13:e7014. doi: 10.1002/cam4.7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song Q, Shang J, Zhang C, Zhang L, Wu X. Impact of the homogeneous and heterogeneous risk factors on the incidence and survival outcome of bone metastasis in NSCLC patients. J Cancer Res Clin Oncol. 2019;145:737–746. doi: 10.1007/s00432-018-02826-7. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, Zhang C, Ma W, Tian F, Xu G, Han X, Sun P, Baklaushev VP, Bryukhovetskiy AS, Wang G, Ma Y, Wang X. Patterns of bone metastases in newly diagnosed colorectal cancer: a real-world analysis in the SEER database. Int J Colorectal Dis. 2019;34:533–543. doi: 10.1007/s00384-018-3213-5. [DOI] [PubMed] [Google Scholar]
- 15.Afriansyah A, Hamid ARA, Mochtar CA, Umbas R. Survival analysis and development of a prognostic nomogram for bone-metastatic prostate cancer patients: a single-center experience in Indonesia. Int J Urol. 2019;26:83–89. doi: 10.1111/iju.13813. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Guo Z, Yu H, Sun Y, Yang X, Zhang W. Clinicopathological features and survival of bone metastasis from primary hepatic carcinoma. Zhonghua Yi Xue Za Zhi. 2015;95:3324–3327. [PubMed] [Google Scholar]
- 17.Zhong X, Lin Y, Zhang W, Bi Q. Predicting diagnosis and survival of bone metastasis in breast cancer using machine learning. Sci Rep. 2023;13:18301. doi: 10.1038/s41598-023-45438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiappetta M, Lococo F, Leuzzi G, Sperduti I, Bria E, Petracca Ciavarella L, Mucilli F, Filosso PL, Ratto G, Spaggiari L, Facciolo F, Margaritora S. Survival analysis in single N2 station lung adenocarcinoma: the prognostic role of involved lymph nodes and adjuvant therapy. Cancers (Basel) 2021;13:1326. doi: 10.3390/cancers13061326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulas A, Bilici A, Durnali A, Tokluoglu S, Akinci S, Silay K, Oksuzoglu B, Alkis N. Risk factors for skeletal-related events (SREs) and factors affecting SRE-free survival for nonsmall cell lung cancer patients with bone metastases. Tumour Biol. 2016;37:1131–1140. doi: 10.1007/s13277-015-3907-z. [DOI] [PubMed] [Google Scholar]
- 20.Khor RC, Bressel M, Tedesco J, Tai KH, Ball DL, Duchesne GM, Farrugia H, Yip WK, Foroudi F. Tolerability and outcomes of curative radiotherapy in patients aged 85 or more years. Med J Aust. 2015;202:153–155. doi: 10.5694/mja14.00441. [DOI] [PubMed] [Google Scholar]
- 21.Pruksakorn D, Phanphaisarn A, Settakorn J, Arpornchayanon U, Tantraworasin A, Chaiyawat P, Klangjorhor J, Teeyakasem P. Prognostic score for life expectancy evaluation of lung cancer patients after bone metastasis. J Bone Oncol. 2017;10:1–5. doi: 10.1016/j.jbo.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu X, Ye K, Wang L, Lin Y, Du D. A review on emerging principles and strategies for colorimetric and fluorescent detection of alkaline phosphatase activity. Anal Chim Acta. 2019;1086:29–45. doi: 10.1016/j.aca.2019.07.068. [DOI] [PubMed] [Google Scholar]
- 23.Wymenga LF, Boomsma JH, Groenier K, Piers DA, Mensink HJ. Routine bone scans in patients with prostate cancer related to serum prostate-specific antigen and alkaline phosphatase. BJU Int. 2001;88:226–230. doi: 10.1046/j.1464-410x.2001.02275.x. [DOI] [PubMed] [Google Scholar]
- 24.Bayrak SB, Ceylan E, Serter M, Karadag F, Demir E, Cildag O. The clinical importance of bone metabolic markers in detecting bone metastasis of lung cancer. Int J Clin Oncol. 2012;17:112–118. doi: 10.1007/s10147-011-0266-7. [DOI] [PubMed] [Google Scholar]
- 25.Jiang C, Hu F, Xia X, Guo X. Prognostic value of alkaline phosphatase and bone-specific alkaline phosphatase in breast cancer: a systematic review and meta-analysis. Int J Biol Markers. 2023;38:25–36. doi: 10.1177/03936155231154662. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Yu C, Huang B, Zhou FL, Huang HD, Li Q. Correlation between bone metastasis and thrombocytosis in pulmonary adenocarcinoma patients. Oncol Lett. 2015;9:762–768. doi: 10.3892/ol.2014.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokkel MP, Linthorst MF, Borm JJ, Taminiau AH, Pauwels EK. A reassessment of bone scintigraphy and commonly tested pretreatment biochemical parameters in newly diagnosed osteosarcoma. J Cancer Res Clin Oncol. 2002;128:393–399. doi: 10.1007/s00432-002-0350-5. [DOI] [PubMed] [Google Scholar]
- 28.Zheng F, Meng Q, Zhang L, Chen J, Zhao L, Zhou Z, Liu Y. Prognostic roles of hematological indicators for the efficacy and prognosis of immune checkpoint inhibitors in patients with advanced tumors: a retrospective cohort study. World J Surg Oncol. 2023;21:198. doi: 10.1186/s12957-023-03077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J, Xin Y, Wang Y, Yang C, Cheng Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33:e22964. doi: 10.1002/jcla.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng C, Zhang N, Wang Y, Jiang S, Lu M, Huang Y, Ma J, Hu C, Hou T. High systemic immune-inflammation index predicts poor prognosis in advanced lung adenocarcinoma patients treated with EGFR-TKIs. Medicine (Baltimore) 2019;98:e16875. doi: 10.1097/MD.0000000000016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimini M, Casadei-Gardini A, Ravaioli A, Rovesti G, Conti F, Borghi A, Dall’Aglio AC, Bedogni G, Domenicali M, Giacomoni P, Tiribelli C, Bucchi L, Falcini F, Foschi FG Bagnacavallo Study Group. Could inflammatory indices and metabolic syndrome predict the risk of cancer development? Analysis from the Bagnacavallo Population Study. J Clin Med. 2020;9:1177. doi: 10.3390/jcm9041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Wang G, Zhang H, Song X, Cao J, Zhang X, Xue R, Wang W, Jia S, Li Z. Prognostic role of the systemic immune-inflammation index in brain metastases from lung adenocarcinoma with different EGFR mutations. Genes Immun. 2019;20:455–461. doi: 10.1038/s41435-018-0050-z. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Yue W, Li W, Luo Y, Li Z, Shao Y, He Z. Systemic immune-inflammation index and ultrasonographic classification of breast imaging-reporting and data system predict outcomes of triple-negative breast cancer. Cancer Manag Res. 2019;11:813–819. doi: 10.2147/CMAR.S185890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrom P, Zolnierek J, Bodnar L, Stec R, Szczylik C. External validation of the systemic immune-inflammation index as a prognostic factor in metastatic renal cell carcinoma and its implementation within the international metastatic renal cell carcinoma database consortium model. Int J Clin Oncol. 2019;24:526–532. doi: 10.1007/s10147-018-01390-x. [DOI] [PubMed] [Google Scholar]
- 36.He J, Liang G, Yu H, Lin C, Shen W. Evaluating the predictive significance of systemic immune-inflammatory index and tumor markers in lung cancer patients with bone metastases. Front Oncol. 2024;13:1338809. doi: 10.3389/fonc.2023.1338809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W, Luo J, Wen J, Jiang M. The relationship between systemic immune inflammatory index and prognosis of patients with non-small cell lung cancer: a meta-analysis and systematic review. Front Surg. 2022;9:898304. doi: 10.3389/fsurg.2022.898304. [DOI] [PMC free article] [PubMed] [Google Scholar]