Abstract
Objective: To explore the influencing factors of spontaneous preterm birth (sPTB) in patients with gestational diabetes mellitus (GDM) and construct a nomogram model. Methods: A retrospective analysis was conducted on the clinical data of 289 GDM patients who gave birth at Yangzhou University Affiliated Hospital from January 2021 to December 2022. The patients were divided into the sPTB (n = 52) and non-sPTB (n = 237) groups based on whether sPTB occurred. Logistic multivariate analysis was used to explore the influencing factors of sPTB in GDM patients and construct a nomogram model. The predictive performance of the nomogram model was evaluated using ROC curves and calibration curves in internal validation. Additionally, 62 GDM patients who visited Yangzhou University Affiliated Hospital from January 2023 to June 2023 were retrospectively selected for external validation of the prediction model. Results: Logistic analysis showed that maternal age ≥30 years, pre-pregnancy BMI ≥26.3 kg/m2, history of spontaneous abortion, premature rupture of membranes, and oral glucose tolerance test (OGTT) fasting blood glucose ≥5.1 mmol/L were independent risk factors for sPTB in GDM patients (all P<0.05). In internal validation, the AUC value of the model’s ROC curve was 0.901, and in external validation, the AUC value was 0.939. The calibration curve showed that the predicted probability was consistent with the actual probability. In addition, the sensitivity, specificity, and accuracy of the model in external validation were 84.21%, 81.40%, and 82.26%, respectively. Conclusion: Maternal age ≥30 years, pre-pregnancy BMI ≥26.3 kg/m2, history of spontaneous abortion, premature rupture of membranes, and OGTT fasting blood glucose ≥5.1 mmol/L are independent risk factors for sPTB in GDM patients. The nomogram model based on these risk factors has high discrimination and accuracy in predicting sPTB in GDM patients.
Keywords: Gestational diabetes mellitus, spontaneous preterm birth, logistic regression analysis, nomogram model
Introduction
Gestational diabetes mellitus (GDM) is a condition characterized by abnormal glucose metabolism first detected during pregnancy [1]. This condition is associated with an increased risk of metabolic disorders in both pregnant women and their babies, as well as adverse outcomes such as spontaneous preterm labor and fetal developmental abnormalities. These issues significantly impact the health of both mother and child [2]. Premature birth is the leading cause of neonatal mortality, with approximately 70% attributed to spontaneous preterm birth (sPTB) [3]. Research indicates that women with GDM have a significantly higher incidence of sPTB, up to 20%, thereby substantially elevating the risk of adverse pregnancy outcomes [4]. Furthermore, premature birth not only adversely affects perinatal outcomes but also imposes increased burdens on families and society. Therefore, preventing and treating sPTB in GDM patients is crucial to improving pregnancy outcomes and reducing the incidence of sPTB.
sPTB is a complex pregnancy outcome influenced by multiple factors. Genetic factors play a significant role, with specific genetic variations associated with an increased risk of PTB [5]. Maternal health status is another important factor, with complications such as hypertension and kidney disease being associated with an increased risk of PTB in GDM patients [6]. Additionally, pregnancy-related infections and inflammation are key factors promoting PTB, potentially increasing risk by inducing inflammatory responses and altering the cervical environment [7]. Environmental factors, including socio-economic status, residential environment, and occupational exposure, also significantly impact the risk of sPTB. For example, women exposed to high stress, adverse living conditions, or harmful chemicals over a long period have a higher risk of PTB [8]. Lifestyle factors, such as diet, weight management, and exercise habits, also play an essential role in preventing PTB. An unbalanced diet and excessive weight gain can increase the risk of PTB [9]. Women with a history of PTB are at a higher risk of experiencing PTB again [10]. Cervical abnormalities, such as cervical shortening or insufficiency, are also significant factors leading to spontaneous PTB [11]. Finally, psychosocial factors such as psychological stress, anxiety, and depression in pregnant women are associated with an increased risk of PTB, potentially by affecting hormone levels and immune system function [12].
In summary, sPTB is a complex pathological process involving multiple factors and pathways, necessitating comprehensive consideration for effective prevention and intervention. Currently, the prediction of sPTB in GDM patients mainly relies on clinical indicators and biochemical tests, which have limited sensitivity and specificity. With the development of big data and artificial intelligence, medical prediction models have become more accurate and efficient. The nomogram, as a visual prediction model, has been widely used in the risk assessment of various diseases. Nomograms can comprehensively analyze multiple variables, thereby promoting personalized healthcare and assisting in clinical decision-making [13]. Based on this, our study analyzes the risk factors affecting sPTB in GDM patients and constructs a nomogram prediction model to make reasonable predictions. Additionally, clinical validation is conducted to provide theoretical guidance for improving pregnancy outcomes in GDM patients.
Materials and methods
General information
This study was approved by the Ethics Committee of The Affiliated Hospital of Yangzhou University. A retrospective analysis was conducted on the clinical data of 289 patients with GDM who gave birth at The Affiliated Hospital of Yangzhou University between January 2021 and December 2022. The patients were categorized into two groups based on the occurrence of sPTB: a sPTB group (n = 52) and a non-sPTB group (n = 237).
The sample size calculation method was as follows: According to reports, the incidence of PTB in GDM patients is 25%. Assuming the same incidence in this study, it is expected to include 6 variables in a multivariate regression model, and the sample size was calculated using the empirical method (EPV). When EPV = 10, the required number of GDM patients is 10 × 6/0.25 = 240. Considering a dropout rate of about 20% and the specific situation of mothers with GDM admitted to our hospital, a final sample size of 289 cases was included.
Inclusion criteria: (1) Diagnosis of GDM in accordance with the guidelines for the diagnosis and treatment of hyperglycemia during pregnancy [14]; (2) Meeting the diagnostic criteria of sPTB in the “Guidelines for the Diagnosis and Treatment of PTB” [15]: 28 to 37 weeks of gestation, regular uterine contractions, accompanied by cervical canal shortening ≥80% and cervical dilation.
Exclusion criteria: (1) Incomplete clinical data; (2) Presence of infectious diseases; (3) Complications with heart, liver, or kidney dysfunction; (4) Iatrogenic PTB.
Furthermore, 62 GDM patients who met the criteria at the Affiliated Hospital of Yangzhou University from January 2023 to June 2023 were selected for clinical validation of the prediction model (Figure 1).
Figure 1.
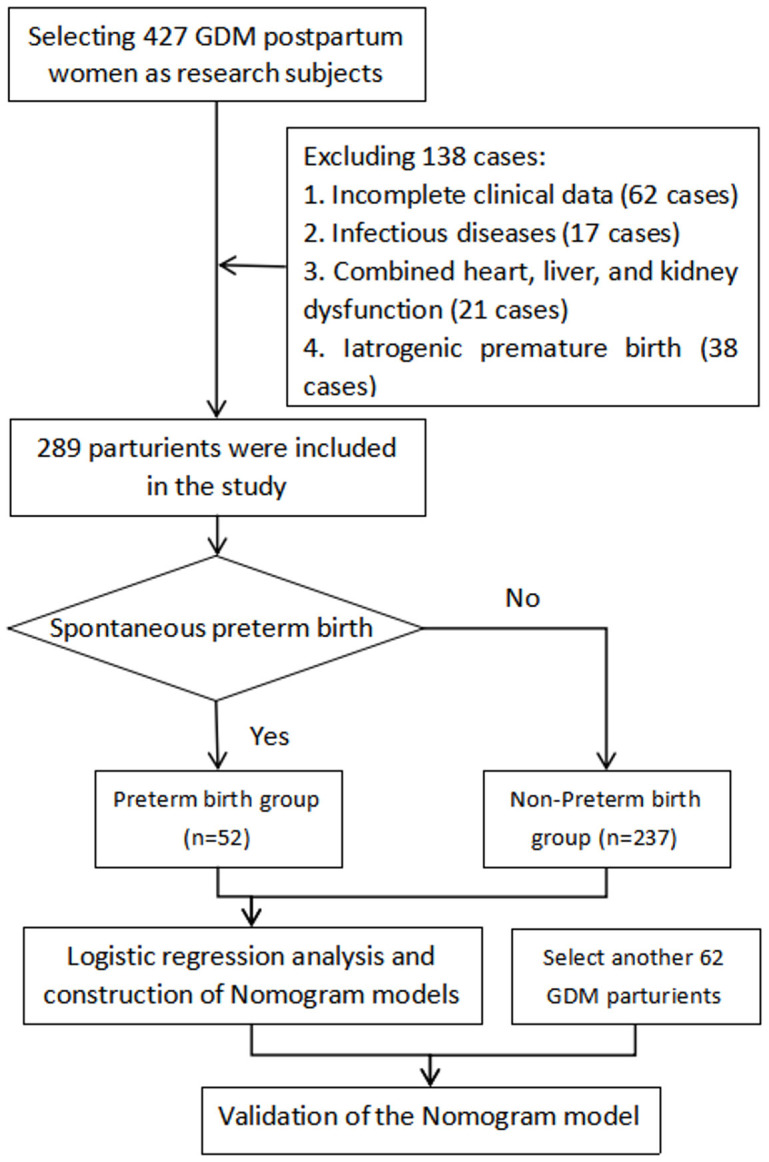
Flow chart of sample inclusion and exclusion.
Data collection
Data collected included gestational age, pre-pregnancy body mass index (BMI), education level, gestational age at delivery, gravidity, parity, age of menarche, history of cesarean section, lower reproductive tract infection, history of spontaneous abortion, family history of diabetes, premature rupture of membranes (PROM), hypertension, and oral glucose tolerance test (OGTT) fasting blood glucose levels.
Statistical analysis
Data were analyzed using SPSS 27.0 and R 4.2.1 software. The factors affecting spontaneous preterm birth in GDM patients were analyzed using univariate analysis. Categorical data were expressed as frequencies and analyzed using the Chi-square test. Continuous data were represented as means and standard deviations and analyzed using the independent sample t-test. A P-value of <0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve for a single continuous variable was obtained using MedCalc software, and the area under the curve (AUC) and the optimal cutoff value were calculated. Logistic regression analysis was used to identify independent risk factors for sPTB in GDM patients. R software was used to create a nomogram of predictive factors, illustrating the proportion of factors affecting sPTB in GDM patients. ROC and calibration curves were used to validate the predictive ability of the model.
Results
Comparison of clinical data
Among the 289 patients, there were 52 sPTB and 237 non-sPTB. In the sPTB group, the mean maternal age was 33.52±3.84 years, the mean pre-pregnancy BMI was 26.73±3.37 kg/m2, 24 cases (46.15%) had a history of cesarean section, 22 cases (42.31%) had a history of spontaneous abortion, and 28 cases (53.85%) experienced PROM. Additionally, 18 cases (34.62%) had OGTT fasting blood glucose ≥5.1 mmol/L.
In the non-sPTB group, the mean maternal age was 28.47±3.30 years, the mean pre-pregnancy BMI was 24.36±1.57 kg/m2, 74 cases (31.22%) had a history of cesarean section, 50 cases (21.10%) had a history of spontaneous abortion, 70 cases (29.54%) experienced PROM, and 41 cases (17.30%) had OGTT fasting blood glucose levels ≥5.1 mmol/L. The proportions of maternal age, pre-pregnancy BMI, history of cesarean section, history of spontaneous abortion, PROM, and OGTT fasting blood glucose levels ≥5.1 mmol/L were significantly higher in the preterm birth group than in the non-sPTB group (all P<0.05), as shown in Table 1.
Table 1.
Comparison of the clinical data between the groups
| Index | Non-spontaneous preterm birth group (n = 237) | Spontaneous preterm birth (n = 52) | χ2/t | P |
|---|---|---|---|---|
| Gestational age (years) | 28.47±3.30 | 33.52±3.84 | 9.701 | <0.001 |
| Pre-pregnancy BMI | 24.36±1.57 | 26.73±3.37 | 8.795 | <0.001 |
| Degree of education | 0.517 | 0.472 | ||
| College or below | 101 (42.62) | 25 (48.08) | ||
| College or above | 136 (57.38) | 27 (51.92) | ||
| Pregnant times (times) | 2.63±0.98 | 2.63±0.99 | 0.039 | 0.969 |
| Production times (times) | 1.51±0.76 | 1.62±0.87 | 0.840 | 0.401 |
| Age at menarche (years) | 13.71±1.09 | 13.62±1.01 | 0.591 | 0.555 |
| History of cesarean section | 4.242 | 0.039 | ||
| Yes | 74 (31.22) | 24 (46.15) | ||
| No | 163 (68.78) | 28 (53.85) | ||
| Lower genital tract infections | 1.378 | 0.240 | ||
| Yes | 93 (39.24) | 25 (48.08) | ||
| No | 144 (60.76) | 27 (51.92) | ||
| History of spontaneous abortion | 10.256 | 0.001 | ||
| Yes | 50 (21.10) | 22 (42.31) | ||
| No | 187 (78.90) | 30 (57.69) | ||
| Family history of diabetes | 0.537 | 0.464 | ||
| Yes | 40 (16.88) | 11 (21.15) | ||
| No | 197 (83.12) | 41 (78.85) | ||
| Premature rupture of membranes | 11.245 | <0.001 | ||
| Yes | 70 (29.54) | 28 (53.85) | ||
| No | 167 (70.46) | 24 (46.15) | ||
| Combined hypertension | 2.717 | 0.099 | ||
| Yes | 33 (13.92) | 12 (23.08) | ||
| No | 204 (86.08) | 40 (76.92) | ||
| OGTT fasting blood glucose (mmol/L) | 7.870 | 0.005 | ||
| ≥5.1 | 41 (17.30) | 18 (34.62) | ||
| <5.1 | 196 (82.70) | 34 (65.38) | ||
| OGTT 1 h blood glucose (mmol/L) | 0.896 | 0.344 | ||
| ≥10 | 115 (48.52) | 29 (55.77) | ||
| <10 | 122 (51.48) | 23 (44.23) | ||
| OGTT 2 h blood glucose (mmol/L) | 0.560 | 0.454 | ||
| ≥8.5 | 96 (40.51) | 24 (46.15) | ||
| <8.5 | 141 (59.49) | 28 (53.85) |
Note: BMI: body mass index; OGTT: Glucose tolerance test.
ROC curve analysis of correlated variables
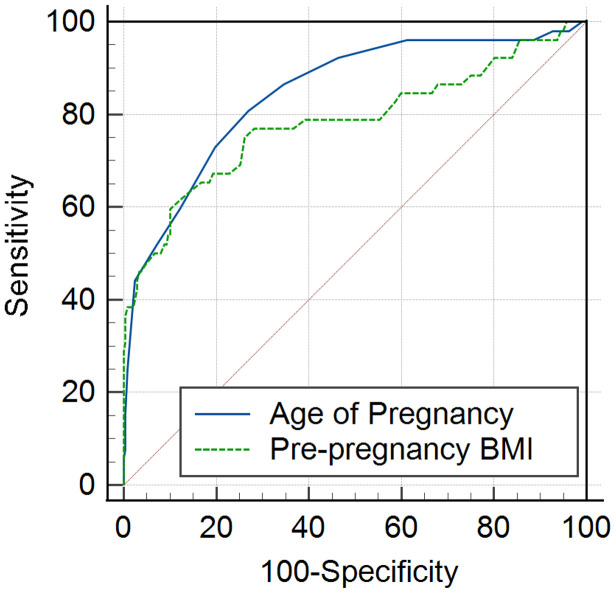
The ROC curve analysis was conducted on three statistically significant continuous variables: maternal age, pre-pregnancy BMI, and gestational week at delivery. ROC curves were obtained using MedCalc software, and the AUC and optimal cutoff values were calculated. The AUC for maternal age, pre-pregnancy BMI, and gestational week at delivery were 0.847 and 0.783, with optimal cutoff values of 30 and 26.3, respectively, as shown in Table 2 and Figure 2.
Table 2.
ROC curve analysis of the related variables
| Variable | AUC | SE | 95% CI | P | Youden index | Optimal critical value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|
| Gestational age (years) | 0.847 | 0.032 | 0.800-0.886 | 0.000 | 0.528 | 30.0 | 80.80 | 73.00 |
| Pre-pregnancy BMI (kg/m2) | 0.783 | 0.043 | 0.731-0.829 | 0.000 | 0.491 | 26.3 | 59.60 | 89.50 |
Note: BMI: body mass index.
Figure 2.
ROC curves for relevant variables. Note: BMI: body mass index.
Multivariate logistic analysis
Multivariate logistic regression analysis was employed to investigate the factors influencing sPTB in patients with GDM. Variables included in the analysis were maternal age, pre-pregnancy BMI, gestational week at delivery, history of cesarean section, history of spontaneous abortion, PROM, and OGTT fasting blood glucose. The variable assignments are shown in Table 3. Logistic regression analysis showed that maternal age (≥30 years), pre-pregnancy BMI (≥26.3 kg/m2), history of cesarean section, history of spontaneous abortion, PROM, and OGTT fasting blood glucose (≥5.1 mmol/L) were independent risk factors for spontaneous preterm birth in GDM patients (all P<0.05), as demonstrated in Table 4.
Table 3.
Variable assignments
| Variable | Assignment condition |
|---|---|
| SPTB | 1 = occurred, 0 = did not occur |
| Age of Pregnancy | 1 = ≥30 years old, 0 = <30 years old |
| Pre-pregnancy BMI | 1 = ≥26.3 kg/m2, 0 = <26.3 kg/m2 |
| History of cesarean section | 1 = yes, 0 = no |
| History of spontaneous abortion | 1 = yes, 0 = no |
| Premature rupture of membranes | 1 = yes, 0 = no |
| OGTT fasting blood glucose | 1 = ≥5.1 mmol/L, 0 = <5.1 mmol/L |
Note: BMI: body mass index; OGTT: Glucose tolerance test.
Table 4.
Multivariate logistic-analysis of factors affecting spontaneous preterm birth in patients with GDM
| Variables of interest | B | SE | Wald | OR | P | 95% CI |
|---|---|---|---|---|---|---|
| Age of Pregnancy | 2.840 | 0.543 | 27.387 | 17.118 | <0.001 | 5.909-49.590 |
| Pre-pregnancy BMI | 2.902 | 0.505 | 32.989 | 18.214 | <0.001 | 6.766-49.035 |
| History of cesarean section | 0.575 | 0.440 | 1.708 | 1.778 | 0.191 | 0.750-4.214 |
| History of spontaneous abortion | 1.739 | 0.523 | 11.050 | 5.694 | <0.001 | 2.042-15.881 |
| Premature rupture of membranes | 1.589 | 0.464 | 11.716 | 4.900 | <0.001 | 1.972-12.173 |
| OGTT fasting blood glucose | 1.498 | 0.500 | 8.983 | 4.473 | 0.003 | 1.679-11.913 |
| constant | -5.978 | 0.761 | 61.676 | - | - | - |
Note: BMI: body mass index; OGTT: Glucose tolerance test; GDM: gestational diabetes mellitus.
Construction of nomogram prediction model
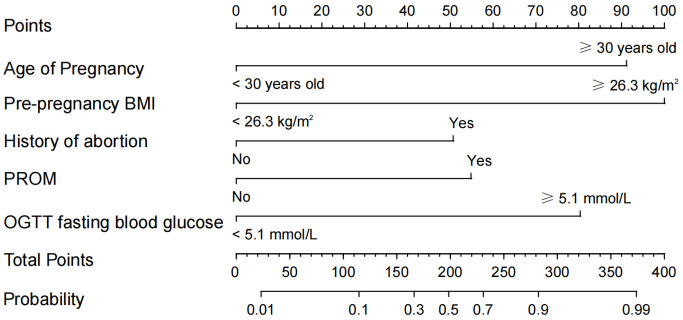
A nomogram was constructed using maternal age, pre-pregnancy BMI, gestational age at delivery, history of cesarean section, history of spontaneous abortion, PROM, and fasting plasma glucose during OGTT as predictors. The logistic regression equation was: Logit (P) = 2.840 × gestational age + 32.902 × pre-pregnancy BMI + 1.739 × history of spontaneous abortion + 1.589 × PROM + 1.498 × OGTT fasting blood-glucose - 5.978, as demonstrated in Figure 3, by mapping each clinical feature of the patient to the upper scale, the corresponding score can be obtained, and the total score can be calculated. The total score can then be mapped to the lower scale to determine the probability of sPTB.
Figure 3.
Nomogram prediction model for SPTB in women with GDM. Note: BMI: body mass index; PROM: premature rupture of membranes; GDM: gestational diabetes mellitus; OGTT: Glucose tolerance test.
Validation of nomogram prediction models
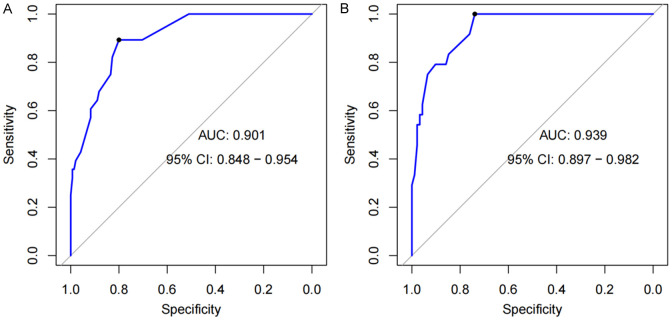
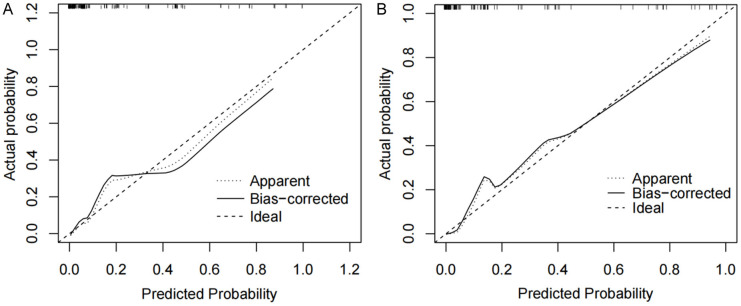
The nomogram was evaluated using both a training set and a validation set for discrimination and calibration. The results showed that the AUC value of the ROC curve in the training set was 0.901, and in the validation set, it was 0.939, indicating that the nomogram had good discrimination (Figure 4). The calibration curve was used to assess the model’s calibration accuracy. When the predicted probability closely aligns with the actual probability, the dashed line will closely follow the reference line. The results showed that the predicted probability closely aligned with the actual probability, indicating that the nomogram had good calibration (Figure 5).
Figure 4.
ROC Curves of Spontaneous preterm birth in GDM patients. (A) ROC of the training set and (B) ROC of the validation set. Note: GDM: gestational diabetes mellitus.
Figure 5.
Calibration evaluation curves of the nomogram prediction model for spontaneous preterm delivery in GDM patients. A. Calibration curve of the training set; B. Calibration curve of validation set. Note: GDM: gestational diabetes mellitus.
Clinical validation of a nomogram prediction model for sPTB in patients with GDM
A total of 62 GDM patients who met the criteria at The Affiliated Hospital of Yangzhou University from January 2023 to June 2023 were retrospectively selected for clinical validation of the prediction model. The results showed that the sensitivity was 84.21% (16/19), the specificity was 81.40% (35/43), and the accuracy was 82.26% ((16+35)/62). See Table 5 for details.
Table 5.
Clinical validation of the predictive model
| Spontaneous preterm birth | Models predicted spontaneous preterm birth | Total | |
|---|---|---|---|
|
| |||
| Yes | No | ||
| Yes | 16 | 3 | 19 |
| No | 8 | 35 | 43 |
| Total | 24 | 38 | 62 |
Discussion
PTB is one of the most complex and critical challenges in obstetrics, with sPTB being the most common type. In China, the incidence of PTB ranges from 5% to 15%, with premature infants often prone to respiratory distress syndrome, sepsis, and other conditions [16]. sPTB not only increases the medical burden during pregnancy but also raises maternal and infant morbidity and mortality rates [17]. Therefore, investigating and assessing the potential risk factors associated with spontaneous preterm labor in patients with GDM is crucial for developing effective management strategies.
This study analyzed the clinical data of 289 GDM patients. The results indicated that maternal age (≥30 years), pre-pregnancy BMI (≥26.3 kg/m2), history of cesarean section, history of spontaneous abortion, PROM, and OGTT fasting blood glucose (≥5.1 mmol/L) were independent risk factors for spontaneous preterm delivery in GDM patients. These findings enhance clinicians’ understanding of the risk of PTB in GDM patients and provide a basis for early intervention.
Advanced maternal age has multifaceted effects on the risk of spontaneous abortion in GDM patients [18]. Biologically, advanced age may be associated with changes such as decreased ovarian reserve and reduced egg quality, increasing the risk of spontaneous abortion. Zhu et al. [19] noted that as age increases, the quality and quantity of follicles decline, directly affecting fertility and pregnancy outcomes. Genetically and epigenetically, age-related accumulation of genetic damage and epigenetic changes may impair embryo growth, raising the risk of miscarriage. Kordowitzki et al. [20] found that with increasing maternal age, fertilized eggs exhibited lower quality and developmental ability, increasing the risk of adverse pregnancy outcomes due to aneuploidy, oxidative stress, and other factors.
From a metabolic and endocrine perspective, advanced maternal age may alter metabolic and endocrine systems, affecting placental function and fetal development, and thereby increasing the risk of miscarriage in GDM patients. GDM itself poses challenges of insulin resistance and glucose metabolism disorders. Aging-associated endocrine changes can exacerbate these issues, leading to hormonal imbalances that impair placental nutrition and oxygen supply, thus increasing the risk of miscarriage [21]. Clinically, special attention should be given to older GDM patients with regular prenatal monitoring to detect issues early and implement preventive interventions to reduce adverse pregnancy outcomes.
Current studies have shown that an increase in pre-pregnancy BMI elevates the incidence of GDM in pregnant women, leading to higher rates of macrosomia and large-for-gestational-age infants. Additionally, it may induce pregnancy complications such as difficult labor, postpartum hemorrhage, and PTB [22]. This study found a significant association between maternal sPTB risk and increased pre-pregnancy BMI. Higher BMI may affect pregnancy outcomes through various biological mechanisms.
Firstly, high BMI is associated with increased insulin resistance, which can lead to an imbalance in glucose metabolism, affecting placental function and fetal development [23]. Secondly, obesity-related chronic low-grade inflammation may damage placental formation, increasing the risk of PTB [24]. Additionally, alterations in hormone levels, particularly sex hormones and other pregnancy-related hormones, may be influenced by increased BMI, affecting uterine stability and the maintenance of pregnancy [25].
Increased mechanical pressure due to high BMI may place additional stress on the uterus and pelvis, impacting placental blood supply [26]. Vascular changes, including endothelial dysfunction and vascular inflammation, may also result from obesity, affecting uterine spiral artery remodeling and placental blood supply [27]. Lian et al. [28] found that, compared to mothers with normal pre-pregnancy BMI, those with low or high pre-pregnancy BMI had a significantly increased risk of PTB. Mayo et al. [29], in a large sample study involving 2,645,950 parturients, showed that compared to women of the same race/ethnicity with a BMI of 26 kg/m2, those with a BMI of 28 kg/m2 had a significantly higher risk of sPTB.
Overall, increased pre-pregnancy BMI contributes to a higher incidence of GDM and PTB through various mechanisms. This emphasizes the importance of pregnancy weight management and a healthy lifestyle to prevent adverse pregnancy outcomes. Strengthening preconception care services, early intervention, and actively guiding women to control their pre-pregnancy BMI within a reasonable range are recommended to maintain fetal weight at a normal level.
In this study, pregnant women with GDM and a history of miscarriage had a significantly increased risk of sPTB compared to those without such a history. This risk increase may be associated with various pathophysiological mechanisms related to GDM. Firstly, spontaneous abortion may alter the endometrial environment, affecting embryo implantation and placental development, which can be further exacerbated by GDM [30]. Secondly, a history of abortion can be related to vascular endothelial dysfunction, affecting uterine blood flow, with GDM potentially increasing the risk of vascular complications, leading to insufficient blood flow to the placenta [31]. Additionally, GDM can cause hormonal imbalances that affect progesterone production, and women with a history of miscarriage may already have hormonal regulation issues, combining to increase the risk of PTB [32].
Research by Yu et al. [33] showed that women with a history of abortion had a significantly increased likelihood of PTB before 37 and 34 weeks of gestation. PROM, a common perinatal complication, has an incidence of about 10% [34]. PROM is mainly related to intrauterine infection, and the hyperglycemic state in pregnant women with GDM can lead to vaginal dysbiosis, causing infections that increase the risk of sPTB and related perinatal complications [35]. In mothers with GDM, insulin resistance and hyperglycemia can lead to increased inflammatory mediators, such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6), which can affect the structure and function of membranes, making them more prone to rupture [36,37]. At the same time, the increased sugar content in the amniotic fluid of women with GDM may provide a favorable environment for bacterial growth, increasing the risk of infection, which is a common cause of PROM. Additionally, pregnant women with GDM may have abnormal immune regulation, affecting their ability to defend against infections and regulate the fetal immune system. This can lead to an imbalance in the local immune response of the fetal membranes, promoting inflammatory responses and tissue damage, thereby increasing the risk of PROM [38]. Once PROM occurs, the infection and inflammatory response may be further exacerbated, leading to cervical ripening and the production of contractions, which are direct factors promoting PTB. Therefore, PROM and GDM together may significantly increase the risk of sPTB through mechanisms such as inflammation, infection, and abnormal immune regulation.
Some studies have found that fasting blood glucose levels in pregnant women with GDM are elevated, but the correlation between sPTB and blood glucose levels in GDM patients has not been widely reported [39]. OGTT fasting plasma glucose ≥5.1 mmol/L is an independent risk factor for sPTB in GDM patients. The reason for this may be that increased fasting blood glucose in OGTT is closely related to insulin resistance, necessitating insulin treatment. However, pregnant women with GDM often find it difficult to accept insulin treatment, resulting in poor compliance and eventually leading to pregnancy complications, thereby increasing the risk of spontaneous preterm delivery and other complications [40].
The above research results and analysis indicate that sPTB in mothers with GDM is influenced by multiple factors, with complex interactions and influencing mechanisms among them. Due to the interdependence and interweaving of these factors, focusing on a single factor often fails to fully capture the risk of sPTB in mothers with GDM. Therefore, predicting the risk of sPTB from a single-factor perspective may overlook the influence of other potential factors, thereby reducing the accuracy and reliability of the prediction. For instance, solely considering advanced age without addressing gestational blood glucose control, or solely focusing on past medical history while neglecting lifestyle factors, could lead to incomplete risk assessment.
To enhance prediction accuracy, a multifactorial comprehensive evaluation method is essential. The nomogram prediction model, a visual statistical tool, offers numerous clinical advantages over simply quantifying risk factors or using complex traditional digital models. It integrates numerical probabilities with clinically relevant variables, enabling rapid risk prediction through auxiliary lines and straightforward summation calculations [41]. In this study, a nomogram prediction model was constructed based on gestational age, pre-pregnancy BMI, history of spontaneous abortion, PROM, and OGTT fasting blood glucose, achieving AUC values of 0.901 and 0.939, indicating excellent predictive performance. Furthermore, the calibration curve of the model approaches the ideal curve, reinforcing its reliability.
Clinical validation of the model demonstrated high sensitivity (84.21%), specificity (81.40%), and accuracy (82.26%), underscoring its practical utility. These findings highlight the constructed prediction model as a reliable and objective tool, offering valuable insights into the occurrence of spontaneous PTB in mothers with GDM.
This study introduces several innovations. Firstly, it incorporates OGTT blood sugar and other related factors alongside common predictors of adverse pregnancy outcomes in GDM, presenting novel variables. Secondly, beyond merely evaluating factors influencing GDM sPTB using the nomogram prediction model, this study validates its performance through clinical studies. However, limitations include a small sample size due to study time constraints, potentially impacting the findings. Future research should expand sample sizes through prolonged data collection to enhance result reliability.
In conclusion, this study constructs a nomogram to identify influencing factors of sPTB in GDM patients, facilitating targeted interventions for high-risk individuals to ensure their safety. Findings indicate that maternal age ≥30 years, pre-pregnancy BMI ≥26.3 kg/m2, history of spontaneous abortion, PROM, and OGTT fasting blood glucose ≥5.1 mmol/L independently contribute to sPTB in GDM patients. The nomogram model utilizing these risk factors demonstrates high discrimination and accuracy in predicting sPTB in GDM patients.
Disclosure of conflict of interest
None.
References
- 1.Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946. doi: 10.1136/bmj-2021-067946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes mellitus. Endocr Rev. 2022;43:763–793. doi: 10.1210/endrev/bnac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am. 2019;48:479–493. doi: 10.1016/j.ecl.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNestry C, Killeen SL, Crowley RK, McAuliffe FM. Pregnancy complications and later life women’s health. Acta Obstet Gynecol Scand. 2023;102:523–531. doi: 10.1111/aogs.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dauengauer-Kirliene S, Domarkiene I, Pilypiene I, Zukauskaite G, Kucinskas V, Matuleviciene A. Causes of preterm birth: genetic factors in preterm birth and preterm infant phenotypes. J Obstet Gynaecol Res. 2023;49:781–793. doi: 10.1111/jog.15516. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Gissler M, Lavebratt C. Association of maternal polycystic ovary syndrome and diabetes with preterm birth and offspring birth size: a population-based cohort study. Hum Reprod. 2022;37:1311–1323. doi: 10.1093/humrep/deac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couceiro J, Matos I, Mendes JJ, Baptista PV, Fernandes AR, Quintas A. Inflammatory factors, genetic variants, and predisposition for preterm birth. Clin Genet. 2021;100:357–367. doi: 10.1111/cge.14001. [DOI] [PubMed] [Google Scholar]
- 8.Vidal MS Jr, Menon R, Yu GFB, Amosco MD. Environmental toxicants and preterm birth: a bibliometric analysis of research trends and output. Int J Environ Res Public Health. 2022;19:2493. doi: 10.3390/ijerph19052493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acharya D, Gautam S, Poder TG, Lewin A, Gaussen A, Lee K, Singh JK. Maternal and dietary behavior-related factors associated with preterm birth in Southeastern Terai, Nepal: a cross sectional study. Front Public Health. 2022;10:946657. doi: 10.3389/fpubh.2022.946657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darbandi M, Rezaeian S, Dianatinasab M, Yaghoobi H, Soltani M, Etemad K, Valadbeigi T, Taherpour N, Hajipour M, Saeidi R. Prevalence of gestational diabetes and its association with stillbirth, preterm birth, macrosomia, abortion and cesarean delivery: a national prevalence study of 11 provinces in Iran. J Prev Med Hyg. 2021;62:E885–E891. doi: 10.15167/2421-4248/jpmh2021.62.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes KM, Kane SC, Haines TP, Sheehan PM. Cervical length surveillance for predicting spontaneous preterm birth in women with uterine anomalies: a cohort study. Acta Obstet Gynecol Scand. 2020;99:1519–1526. doi: 10.1111/aogs.13923. [DOI] [PubMed] [Google Scholar]
- 12.Martin-de-Las-Heras S, Khan KS, Velasco C, Cano A, Luna JD, Rubio L. Propensity score analysis of psychological intimate partner violence and preterm birth. Sci Rep. 2022;12:2942. doi: 10.1038/s41598-022-06990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang M, Zhang H, Zhang J, Huang K, Zhao J, Hu J, Lu C, Shao J, Weng J, Yang Y, Zhuang Y, Xu X. A novel nomogram for predicting gestational diabetes mellitus during early pregnancy. Front Endocrinol (Lausanne) 2021;12:779210. doi: 10.3389/fendo.2021.779210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kautzky-Willer A, Harreiter J, Winhofer-Stockl Y, Bancher-Todesca D, Berger A, Repa A, Lechleitner M, Weitgasser R. Gestational diabetes mellitus (Update 2019) Wien Klin Wochenschr. 2019;131(Suppl 1):91–102. doi: 10.1007/s00508-018-1419-8. [DOI] [PubMed] [Google Scholar]
- 15.Medley N, Poljak B, Mammarella S, Alfirevic Z. Clinical guidelines for prevention and management of preterm birth: a systematic review. BJOG. 2018;125:1361–1369. doi: 10.1111/1471-0528.15173. [DOI] [PubMed] [Google Scholar]
- 16.Conde-Agudelo A, Romero R. Does vaginal progesterone prevent recurrent preterm birth in women with a singleton gestation and a history of spontaneous preterm birth? Evidence from a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;227:440–461. e442. doi: 10.1016/j.ajog.2022.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daskalakis G, Goya M, Pergialiotis V, Cabero L, Kyvernitakis I, Antsaklis A, Arabin B. Prevention of spontaneous preterm birth. Arch Gynecol Obstet. 2019;299:1261–1273. doi: 10.1007/s00404-019-05095-y. [DOI] [PubMed] [Google Scholar]
- 18.Debelo BT, Hunie Asratie M, Solomon AA. Risk of selected fetal adverse pregnancy outcomes at advanced maternal age: a retrospective cohort study in Debre Markos Referral Hospital, Northwest Ethiopia. Obstet Gynecol Int. 2020;2020:1875683. doi: 10.1155/2020/1875683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Z, Xu W, Liu L. Ovarian aging: mechanisms and intervention strategies. Med Rev (2021) 2022;2:590–610. doi: 10.1515/mr-2022-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kordowitzki P, Graczyk S, Haghani A, Klutstein M. Oocyte aging: a multifactorial phenomenon in a unique cell. Aging Dis. 2024;15:5–21. doi: 10.14336/AD.2023.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Yan J, Ma L, Huang Y, Zhu M, Jiang W. Effect of gestational diabetes mellitus on pregnancy outcomes among younger and older women and its additive interaction with advanced maternal age. Front Endocrinol (Lausanne) 2023;14:1158969. doi: 10.3389/fendo.2023.1158969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vats H, Saxena R, Sachdeva MP, Walia GK, Gupta V. Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: a systematic review and meta-analysis. Obes Res Clin Pract. 2021;15:536–545. doi: 10.1016/j.orcp.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Chen Y, Tong J, Yin A, Wu L, Niu J. Association of maternal obesity with preterm birth phenotype and mediation effects of gestational diabetes mellitus and preeclampsia: a prospective cohort study. BMC Pregnancy Childbirth. 2022;22:459. doi: 10.1186/s12884-022-04780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornish RP, Magnus MC, Urhoj SK, Santorelli G, Smithers LG, Odd D, Fraser A, Haberg SE, Nybo Andersen AM, Birnie K, Lynch JW, Tilling K, Lawlor DA. Maternal pre-pregnancy body mass index and risk of preterm birth: a collaboration using large routine health datasets. BMC Med. 2024;22:10. doi: 10.1186/s12916-023-03230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pigatti Silva F, Souza RT, Cecatti JG, Passini R Jr, Tedesco RP, Lajos GJ, Nomura ML, Rehder PM, Dias TZ, Oliveira PF, Silva CM Brazilian Multicenter Study on Preterm Birth (EMIP) study group. Role of Body Mass Index and gestational weight gain on preterm birth and adverse perinatal outcomes. Sci Rep. 2019;9:13093. doi: 10.1038/s41598-019-49704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Shi J. Female obesity increases the risk of preterm birth of single frozen-thawed euploid embryos: a retrospective cohort study. Gynecol Endocrinol. 2024;40:2324995. doi: 10.1080/09513590.2024.2324995. [DOI] [PubMed] [Google Scholar]
- 27.Baer RJ, Chambers BD, Coleman-Phox K, Flowers E, Fuchs JD, Oltman SP, Scott KA, Ryckman KK, Rand L, Jelliffe-Pawlowski LL. Risk of early birth by body mass index in a propensity score-matched sample: a retrospective cohort study. BJOG. 2022;129:1704–1711. doi: 10.1111/1471-0528.17120. [DOI] [PubMed] [Google Scholar]
- 28.Lian S, Huang Y, Li J, Nie J, Li M, Zhou J, He J, Liu C. Combined effects of pre-pregnancy BMI and gestational weight gain on preterm birth: comparison between spontaneous and ART conception. J Assist Reprod Genet. 2024;41:673–681. doi: 10.1007/s10815-024-03024-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayo JA, Stevenson DK, Shaw GM. Population-based associations between maternal pre-pregnancy body mass index and spontaneous and medically indicated preterm birth using restricted cubic splines in California. Ann Epidemiol. 2022;72:65–73. doi: 10.1016/j.annepidem.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Mao J, Su X, Du Q. Impact of spontaneous abortion history and induced abortion history on perinatal outcomes of singleton pregnancies. BMC Public Health. 2023;23:2360. doi: 10.1186/s12889-023-17264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia D, Sun F, Han S, Lu L, Sun Y, Song Q. Adverse outcomes in subsequent pregnancies in women with history of recurrent spontaneous abortion: a meta-analysis. J Obstet Gynaecol Res. 2024;50:281–297. doi: 10.1111/jog.15848. [DOI] [PubMed] [Google Scholar]
- 32.Rosen O’Sullivan H, Glazewska-Hallin A, Suff N, Seed P, Shennan A. The role of transabdominal cerclage in preventing recurrent preterm delivery in women with a history of term full dilatation cesarean section followed by a spontaneous preterm birth or late miscarriage and a subsequent pregnancy with cerclage: a retrospective cohort study. Am J Obstet Gynecol MFM. 2023;5:101144. doi: 10.1016/j.ajogmf.2023.101144. [DOI] [PubMed] [Google Scholar]
- 33.Yu JY, Jiang B, Zhang XJ, Wei SS, He WC. History of induced abortion and the risk of preterm birth: a retrospective cohort study. J Matern Fetal Neonatal Med. 2023;36:2207114. doi: 10.1080/14767058.2023.2207114. [DOI] [PubMed] [Google Scholar]
- 34.Pergialiotis V, Bellos I, Fanaki M, Antsaklis A, Loutradis D, Daskalakis G. The impact of residual oligohydramnios following preterm premature rupture of membranes on adverse pregnancy outcomes: a meta-analysis. Am J Obstet Gynecol. 2020;222:628–630. doi: 10.1016/j.ajog.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 35.El-Achi V, Aggarwal S, Hyett J. Interventions for the prevention of preterm premature rupture of membranes: a systematic review and meta-analysis. Fetal Diagn Ther. 2022;49:273–278. doi: 10.1159/000525655. [DOI] [PubMed] [Google Scholar]
- 36.Bouvier D, Forest JC, Blanchon L, Bujold E, Pereira B, Bernard N, Gallot D, Sapin V, Giguere Y. Risk factors and outcomes of preterm premature rupture of membranes in a cohort of 6968 pregnant women prospectively recruited. J Clin Med. 2019;8:1987. doi: 10.3390/jcm8111987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang YJ, Zhu Y, Zhu L, Lu CQ, Chen C, Yuan L. Prevalence of preterm birth and risk factors associated with it at different gestational ages: a multicenter retrospective survey in China. Saudi Med J. 2022;43:599–609. doi: 10.15537/smj.2022.43.6.20220210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akhter T, Hesselman S, Lindstrom L, Axelsson O, Poromaa IS. Maternal and perinatal outcomes in singleton nulliparous spontaneous preterm birth with and without preterm premature rupture of membranes-a national population-based cohort study. Am J Perinatol. 2024;41:e958–e967. doi: 10.1055/a-1973-7482. [DOI] [PubMed] [Google Scholar]
- 39.Lowe WL Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, McCance D, Hamilton J, Nodzenski M, Talbot O, Brickman WJ, Clayton P, Ma RC, Tam WH, Dyer AR, Catalano PM, Lowe LP, Metzger BE HAPO Follow-up Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42:372–380. doi: 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Filippo D, Ahmadzai M, Chang MHY, Horgan K, Ong RM, Darling J, Akhtar M, Henry A, Welsh A. Continuous glucose monitoring for the diagnosis of gestational diabetes mellitus: a pilot study. J Diabetes Res. 2022;2022:5142918. doi: 10.1155/2022/5142918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Hu Y, Jiang P, Kong W, Gong C, Chen Y, Xu L, Yang Y, Hu Z. Establishment and validation of a nomogram model for predicting adverse pregnancy outcomes of pregnant women with adenomyosis. Arch Gynecol Obstet. 2024;309:2575–2584. doi: 10.1007/s00404-023-07136-z. [DOI] [PubMed] [Google Scholar]