Abstract
Objective: In South Asia, Curcuma longa and Allium sativum are extensively used as household remedies for wound care for veterinary and human infectious diseases. However, little pharmacologic data is present to support this folklore. A series of in vitro and in vivo experiments were conducted to validate the folkloric practice of these herbs. Methods: In vitro experiments, phytochemistry, polyphenolic content, acute dose dermal toxicity, antimicrobial activity, and antioxidant assays were conducted. For in vivo experiments, the decoction was prepared and tested for a wound cure against an experimentally induced excision wound on the dorsal region of rats under ketamine anesthesia. Rats were divided into five groups (5 rats in each). Group 1 was treated with standard Povidone-Iodine, Group 2 was treated with distilled water, group 3 received topical application of the decoction, Group 4 received topical as well as 1 mL oral decoction, and Group 5 received topical as well as oral 1 mL water. Histopathology, leukocyte count and acute oral dose toxicity were estimated. Result: After the ninth post-wounding day, the wound contractions recorded in each group were group-1 (83.11%), group-2 (19.21%), group-3 (91.01%), group-4 (100%), and group-5 (16.55%) similarly less cytoarchitectural damage and more promising cellular repair were observed in both decoctions treated groups as compared to standard and control. A less exaggerated WBC profile was recorded in decoction-treated groups compared to standard and control, while decoction showed significant antibacterial potential even against the resistance strains of standard antibiotics. Decoction showed no dermal and oral toxicity in the animals tested. Conclusion: Decoction of A. sativum and C. longa possesses excellent wound healing potential because of the variety of phytoconstituents linked to the antibacterial, antioxidant, and immunomodulator spectrum and can be used as an effective household remedy for wound healing with no notable toxicity.
Keywords: Povidone-iodine, Allium sativum, Curcuma longa, wound, antimicrobial
Introduction
A wound is an injury or lesion in which the skin is usually broken or punctured. For a histopathologic definition, a wound is a tissue insult that harms the skin’s dermis [1].
Wound healing is a sequential process based on four programmed and overlapping phases. All the phases occur in a complex biochemical and pathobiologic pathway. The first phase starts instantly after the tissue injury, with vascular restraint and fibrin clot formation. As a result, pro-inflammatory cytokines and growth factors such as β platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), transforming growth factor (TGF), and the epidermal growth factor (EGF) are released by this clot and the neighboring injured tissues. In restricted bleeding, pro-inflammatory cells travel into the lesion and trigger the inflammatory phase, attributed to the sequential penetration of lymphocytes such as macrophages and neutrophils [2]. The role of neutrophils is vital in the clearance of pervading microorganisms and cellular detritus in the lesion, and it also generates substances like reactive oxygen species (ROS), proteases, and other mediators, which accentuate the effect [3]. Infectious bacteria frequently develop resistance to publicly traded antibiotics for the treatment of bacterial illnesses as a result of their widespread and uncontrolled usage.
Herbal medicine uses many natural herbs and ingredients, and these treatments have shown profound results. The healing capability of many natural plants has been observed since ancient times. Garlic and curcumin share a vital importance among those plants. The World Health Organization (WHO) [4] states that most people in developing nations may benefit from primary health care provided by herbal medicines, the most effective means of treating illness. Almost three-quarters of all new medications authorized by the Food and Drug Administration (FDA) between 1983 and 1994 were herbal remedies or pharmaceuticals with a botanical component [5].
Curcumin is the yellow pigment polyphenol of turmeric (Curcuma longa), which was well-known more than a century ago. Curcuma longa plant belongs to the ginger family. It is used in Southeast Asian cultures, especially Ayurvedic medicine [3]. For centuries, it has been known that turmeric has anti-inflammatory potential. However, pharmacologic and biochemical research completed within the past decades has resulted in evidence that this effect of turmeric is because of curcumin diferuloylmethane. Curcumin has been shown to modulate numerous transcription factors, redox status, protein kinases, adhesion molecules, cytokines, and enzymes associated with inflammation. The inflammation process has significantly affected most autoimmune and neoplastic diseases, including neurodegenerative, pulmonary, metabolic, and cardiovascular disorders [6].
In India’s traditional system of Ayurveda, turmeric has a long history of being used to treat respiratory conditions such as sinusitis, cough, asthma, a few liver diseases, and diabetic wounds [7,8].
Curcumin (diferuloylmethane), the major curcuminoid available in turmeric, gives it a yellow color. Curcumin possesses significant anti-inflammatory, anti-carcinogenic, anti-infective, anti-mutagenic, antioxidant and anti-coagulant effects. Curcumin has also revealed significant effects on ulcer healing potential. It modulates various stages of the natural ulcer healing process to rush healing [6]. The recent literature on the ulcer healing potential of curcumin also provides evidence for its ability to improve granulation tissue formation, tissue remodeling, collagen deposition and ulcer contraction. Curcumin has maximum therapeutic effects on skin ulcers [8].
Garlic (Allium sativum) has been used as an ethnobotanical remedy for thousands of years. It has a rich pharmacological profile. The British used garlic to treat injured soldiers in World War I. It can be used raw, powdered, and in other appropriate dosage forms. This bulb effectively boosts the body’s natural defenses to restrict the growth of infection in a lesion. Traditional healers commonly use garlic to improve immunity and treat infections at the wound site [9].
Garlic has been commonly used for treating various skin ailments for centuries [9]. A. sativum belongs to the family Liliaceae. Biochemically, it contains various vital elements such as enzymes (for example, alliinase), compounds enzymatically produced compounds from alliin (for example, allicin) and sulfur-containing compounds such as alliin [10]. Other constituents, such as flavonoids, oligosaccharides, selenium and arginine, are also present in garlic [10]. Garlic is a polar compound of phenolic and steroidal nature which shows many pharmacologic properties [11]. Garlic extracts arouse immune functions such as cytokine release, proliferation of lymphocytes, and phagocytosis [12].
Various compounds of garlic have been reported for antiviral effects by retarding the proliferation of virally affected cells [12]. It has been reported effective for wound healing in the chicken wound model by increasing re-epithelialization [13].
S-allylmercaptocysteine (SAMC) and S-allylcysteine (SAC) are the main organo-sulfur compounds in garlic extract, which are essential in preventing oxidant damage [14]. They apply the antioxidant action of hunting reactive oxygen species (ROS), boosting cellular antioxidant enzymes such as glutathione, superoxide, peroxidase, catalase, and dismutase. They are reported to protect DNA against UV-induced damage and UV-induced immune suppression [15].
Our study is the first time that decoction of both herbs has been used, considering their efficacy in Phase 1 (cleaning) and Phase 2 (angiogenesis) of the wound healing process, and it is also the first time that the oral and topical dosing patterns are studied simultaneously.
Materials and methods
Drugs and chemicals
Pharmaceutical-grade drugs are utilized in the experiment. Lignocaine gel andpovidone-iodine solution (10% w/v) were purchased from Abbott Laboratories. Ketamine was obtained from Indus Pharma Pvt. (Ltd.). All the reagents and chemicals were used in the experiments were analytical grade.
Plant material
A. sativum (Bulb) and C. longa (Rhizome) were purchased from a local spice store of Nasheman Colony, Bosan Road, Multan, Pakistan. The plant material was authenticated by Professor of Botany Dr. Zaheer Rana at the Government College of Sciences, Multan, Pakistan (FTM-2115-PP 035/21).
Preparation of extracts
A. sativum and C. longa (40 g each) were homogenized in 200 mL of water with a blender for 2 minutes. Pasted plant material (17 g) was subjected to fresh hot water extraction by adding 300 mL of hot water. The plant material was first passed through a herbal wire filter, then through the Whattman-1 Filter paper. The extract obtained was stored at -4°C in air-tight jars in the lab refrigerator [15].
Animals
Rats of either sex (body weight 250 to 350 g) were purchased from the animal house of the Bahauddin Zakariya University, Multan. Rats were housed under standard laboratory conditions at 27°C room temperature with 12-hour light and dark cycles. All animal procedures were performed according to the guidelines of National Institute of Health (NIH) animal use for experimental purposes [16]. “Animal Ethical Committee” with a reference number AEC/Pharma/12/2021 was dated 7th December, 2021.
Grouping of animals
Rats were alienated into 5 groups, 5 rats/each as follows: Group 1: Treated with standard Povidone-Iodine Solution (1 mL topically); Group 2: Treated with distilled water (1 mL topically); Group 3: Treated with decoction (1 mL topically); Group 4: Treated with decoction topically and oral (1 mL/kg); Group 5: Treated with distilled water topically and orally (1 mL/kg).
In vivo experiments
Wound healing activity
Rats were anaesthetized with ketamine (i.p) before and during the wound creation, as described by [17]. Hairs (dorsal thoracic region) of rats were shaved, and local anesthetic (lignocaine) was applied. With the help of a scalpel (No. 10), a full-thickness circular wound of 200 mm2 was created. The wound was left open. The percent wound contraction was measured on day 0, 3rd, 6th, and 9th post-wounding days. Healing of the injured area was calculated in Cm with the help of scale and considered as the initial area of wound healing. Decoction was applied once daily to all groups at 10 Am.
Additionally, 1 mL oral extract through oral gavages was given to Group 4-, and 1-mL distilled water was given to Group 5. On the 9th post-wounding day, the percentage of wound closure was calculated using the following formula.
Histopathology of wounds
Excision biopsies were taken from the wounded skin from each group on 3rd, 6th and 9th day post wound infliction. Samples were put in formaldehyde solution (10%), and then subjected to various steps of tissue processing. Slides from each sample were stained with Hematoxylin and Eosin (H&E). Pictures were taken from all slides using a microscope equipped with camera and results were interpreted by the histopathologist [17].
Leukocyte count estimation
Blood specimens were collected from each group on the 1st, 5th and 9th day of the experiment. Specimens of blood were collected from the rat from the lateral vein located in the tail [18]. The sample was transferred to EDTA tube and subsequently evaluated. An examination of the blood components, including the number of leukocytes, lymphocytes, neutrophils, and monocytes, was carried out by a hematology analyzer.
Acute oral dose toxicity test
Rats were introduced orally through oral gavages of 5 mL decoction to observe acute oral dose toxicity [19].
In-vitro experiments
Phytochemical analysis
Preliminary phytochemical tests were performed for the possible screening of phytochemical constituents such as tannins, saponins, phlobatannins, flavonoids, glycosides, and terpenoids by using the standard procedure [20].
Estimation of total phenol content
Total phenolic content was calculated using [20] protocol. The decoction (0.5 g/20 mL) was prepared in distilled water and then added 4 mL Folin-Ciocalteu’s reagent (Sigma, USA). The solution was allowed to settle for 7 minutes, and then 5 mL of 20% sodium carbonate was added. The solutions were incubated in darkness for 2 hours at room temperature. A spectrophotometer (UV-VIS Spectrophotometer 3000, India) was used to measure the absorbance at 740 nm. Gallic acid (standard) was used at a dose of 5, 10, 25, 50, 75 and 100 mg L-1 for plotting the calibration curve. Quantification of TPC was expressed in terms of Gallic acid equivalent (GAE) mg g-1 of dried fraction. All the samples were analyzed in triplicate.
Acute dermal dose toxicity study
Rats were shaved, and 3 mL decoction was topically applied to the entire body of the rats to observe acute dermal dose toxicity [21].
Antioxidant activity
DPPH assay was performed to quantify the antioxidant activity of decoction. In short, the methanol-diluted sample was combined with decoction to get a final volume of 5 mL for the DPPH test. Subsequently, the conjunction was kept in darkness for 40 minutes. The absorbance of the given solution at a wavelength of 517 nm was determined using a spectrophotometer [21]. A mathematical calculation was employed to calculate the percent of DPPH scavenging capacity.
Antimicrobial activity
Two different kinds of discs were utilized in the disc diffusion method [22] to determine the antibacterial activity. Discs containing decoction served as sample discs and contained conventional antibiotics for the positive control. The discs with a diameter of 6 mm were prepared from the Whatman-1 filter using a punch machine. All of the glassware was sterilized through the use of dry heat. Media made of nutritional agar and dextrose agar were sterilized in an autoclave set at 121°C for 30 minutes after being produced in distilled water. After pouring the mixture into individual Petri dishes, it was left to cool and solidify into a gel. The ideal thickness for a gel layer is two to three millimeters. After incubating the test Petri dishes at 37°C overnight, only those that showed no growth were chosen for subsequent experiments. Using the streaking method, the bacterial and fungal cultures were transferred from inoculums to Petri dishes using sterile aluminum wire loops. Using a horizontal laminar flow cabinet, the entire treatment was performed under stringent aseptic conditions.
The cultures of bacteria were kept in an incubator at 37°C for 24 hours, while the cultures of fungi were left at ambient temperature for 48 hours. In order to compare the extracts with the positive and negative controls, we measured their zone of inhibition (mm) at the conclusion of the incubation time [23]. All tests were conducted three times, as indicated in the table, to ensure the accuracy of the results.
Statistical analysis
SPSS software (Spss Inc., Schicago, USA) were used to analyze the data and results were expressed as mean ± SEM. The variables first being tested for normality through the Shapiro-Wilk test, while the difference between experimental groups was analyzed using one-way ANOVA followed by the Bonferroni test. Data were considered significant at p-value ≤ 0.05.
Results
Preliminary phytochemical evaluation
Phytochemical evaluation of A. sativum detected the presence of tannins, terpenoids, saponin, steroids, flavonoids, coumarins and cardiac glycoside, while C. longa was revealed inphytoconstituents flavonoid, terpenoid, and saponin (Table 1).
Table 1.
Phytochemical evaluation of A. sativum and C. longa extract
| Plant sample | Terpenoid | Steroids | Tannins | Saponin | C. glycoside | Caumarins | Flavonoid | Phlobatanins |
|---|---|---|---|---|---|---|---|---|
| Allium sativum L. | + | + | + | + | + | + | + | - |
| Curcuma longa L. | + | - | - | + | - | - | + | - |
Presence (+), absence (-). Each datum is the average of two independent determinations.
Estimation of total polyphenol content
The total polyphenol content was detected in both the extracts are shown in Table 2.
Table 2.
Polyphenol content of A. sativum and C. longa decoction
| Sample | Phenol Contents (GAE mg/g)* |
|---|---|
| Allium sativum L. | 54.25 ± 0.15 |
| Curcuma longa L. | 25.45 ± 0.48 |
Values are expressed as means ± standard deviation (n = 3).
Statistically significant.
Wound healing activity
Decoction showed almost complete wound closure in treated group 3 on 9th post-wounding day while on 7th day with group 4 (Table 3; Figure 1). This study revealed that all five groups depicted wound contraction day by day. Most interestingly, 19.21% wound contraction was recorded on 9th post-wounding day in group-2 animals (which could be due to the self-immunity of the rats), whereas group-1 treated animals expressed 83.11% healing. Group 3 showed 91.01% healing, 100% maximum percentage of healing in group 4, and 16.55% in group 5 (Table 3). Both the groups treated with A. sativum and C. longa decoction were found to be statistically significant with respect to their respective control. Figure 1 shows the percent wound contraction of different groups in rats.
Table 3.
Effect of decoction on the excision wound model in rat
| Post-wounding Days | Group-1 | Group-2 | Group-3 | Group-4 | Group-5 |
|---|---|---|---|---|---|
| 0 | 200.55 ± 1.2 | 200.85 ± 2.1 | 200.15 ± 1.1 | 201.13 ± 2.2 | 200.15 ± 2.1 |
| 3 | 148.03 ± 2.3 | 176.40 ± 1.4 | 162.22 ± 1.6 | 142.45 ± 1.3 | 180.10 ± 1.4 |
| 6 | 85.15 ± 0.7 | 162.11 ± 1.3 | 62.98 ± 0.87 | 14.54 ± 0.90 | 172.11 ± 1.3 |
| 9 | 34.11 ± 1.4 | 156.21 ± 0.9 | 18.36 ± 1.7 | 0.00 ± 0.0 | 164.51 ± 0.9 |
| p-value | 0.019 | 0.735 | 0.043 | 0.021 | 0.086 |
Value is mean ± SEM of five animals (n = 5) in each group. The number in parenthesis indicates percentage of wound contraction. All are significant at P < 0.05 as compared to group I (control).
Figure 1.
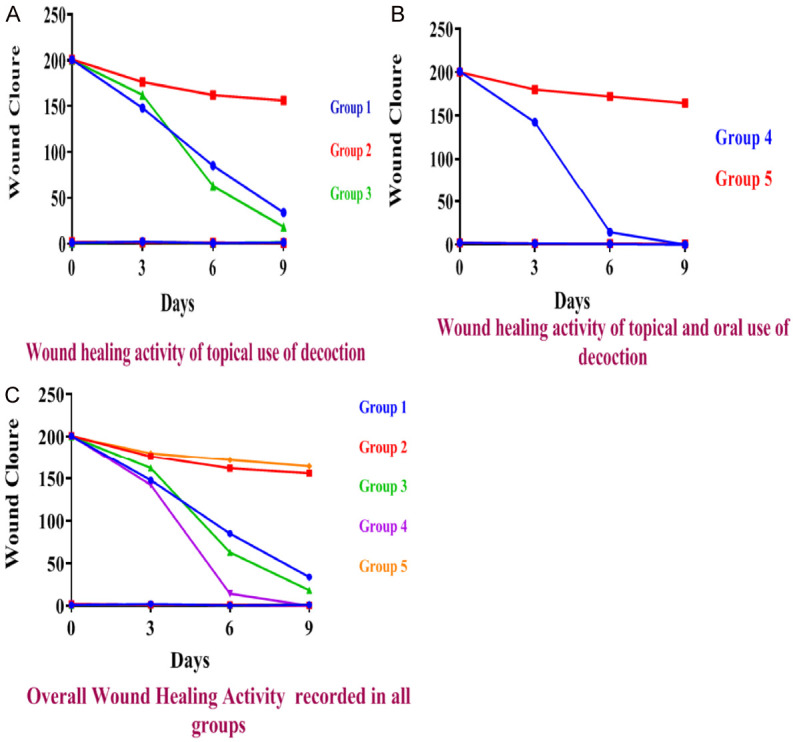
Wound healing activity of decoction, standard, and control recorded after topical application (A), wound healing activity of decoction and control recorded after oral and topical use (B), and overall wound healing activity (C) were recorded in all groups.
Impact on the WBCs
A. sativeum and C. longa decoction showed promising results regarding the WBC count (leukocytes, lymphocytes, neutrophils and monocytes) in a duration-dependent manner in comparison to the control. The effect of the various WBCs is described in Table 4.
Table 4.
Impact of decoction on the WBC profile
| WBCs | Post-wounding Days | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|---|
| Leukocyte (cells/μL) | 1 | 9309 ± 5.6 | 8180 ± 1.4 | 10500 ± 9.9 | 10650 ± 3.5 | 8200 ± 1.4 |
| 5 | 9500 ± 3.5 | 8250 ± 3.5 | 10450 ± 4.9 | 10790 ± 4.2 | 8250 ± 3.5 | |
| 9 | 9750 ± 5.6 | 8006 ± 1.8 | 10590 ± 5.6 | 10820 ± 3.5 | 8000 ± 1.8 | |
| Lymphocyte (%) | 1 | 30.10 ± 0.7 | 40.00 ± 1.4 | 31.00 ± 1.4 | 32.80 ± 0.7 | 40.00 ± 1.4 |
| 5 | 33.80 ± 2.1 | 44.30 ± 0.7 | 30.50 ± 0.7 | 29.40 ± 0.0 | 45.50 ± 0.7 | |
| 9 | 29.60 ± 2.8 | 43.00 ± 1.4 | 30.00 ± 1.4 | 31.00 ± 0.0 | 43.00 ± 1.4 | |
| Neutrophil (%) | 1 | 26.80 ± 0.7 | 35.00 ± 0.7 | 28.30 ± 2.1 | 25.00 ± 0.7 | 35.50 ± 0.7 |
| 5 | 33.90 ± 0.7 | 38.70 ± 1.4 | 26.08 ± 1.4 | 24.80 ± 0.7 | 39.00 ± 1.4 | |
| 9 | 29.70 ± 1.4 | 37.10 ± 2.1 | 23.30 ± 1.4 | 24.10 ± 1.4 | 37.50 ± 2.1 | |
| Monocyte (%) | 1 | 3.10 ± 0.9 | 5.35 ± 0.7 | 2.60 ± 0.2 | 2.60 ± 0.4 | 5.55 ± 0.7 |
| 5 | 4.30 ± 0.7 | 6.10 ± 1.2 | 2.33 ± 0.7 | 2.25 ± 0.6 | 6.15 ± 1.2 | |
| 9 | 4.00 ± 0.0 | 6.25 ± 0.6 | 3.00 ± 1.3 | 2.50 ± 0.5 | 6.45 ± 0.6 |
Antioxidant activity
A. sativum and C. longa decoction have shown excellent antioxidant potential in DPPH assay. Decoction had a significant effect on oxidative stress, with a percent inhibition of 87% (Figure 2).
Figure 2.
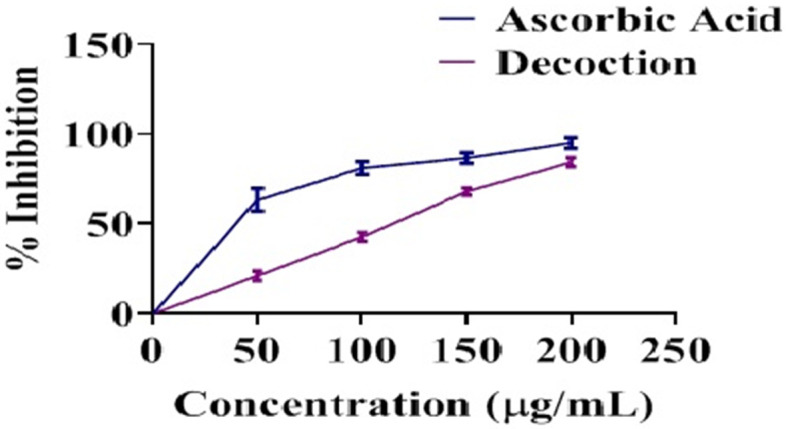
Antioxidant potential of decoction in comparison to ascorbic acid (n = 3).
Toxicity study
A. sativum and C. longa decoction have shown good tolerability without any vital sign of dermal allergy or discomfort in animal behavior in 24 h of in vitro acute dermal toxicity test; similarly, in vivo acute oral dose toxicity test of decoction has not caused lethargy, morbidity, or mortality in any animal.
Antibacterial activity
Decoction has shown promising results against both strains of selected bacterial Spp. The outcome of decoction against bacteria with respect to standards are tabled in Table 5.
Table 5.
Antibacterial activity of A. sativum and C. longa decoction
| Bacteria | Zone of inhibition by decoction (mm) | Standard Zone of inhibition (mm) | |
|---|---|---|---|
| Bacillus pumilus | 20.44 | Gentamycin | 16.21 |
| Staphylococcus aureus | 22.31 | Amoxicillin | R |
| Escherichia coli | 23.98 | Ceftriaxone | R |
| Klebsiella pneumonia | 18.44 | Levofloxacin | 19.20 |
Values are expressed as means of triplicate inhibition (n = 3), R = resistance.
Histopathology of wounds
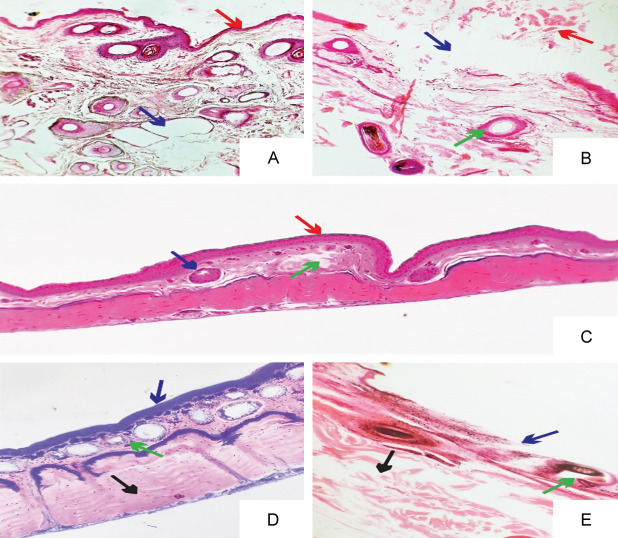
Photomicrograph showing histopathologic variations of wound Group 1 to group 5 animals as shown in Figure 3A-E and Supplementary Figure 1.
Figure 3.
Photomicrograph showing histopathologic variations of wound Group 1 animals showed (A) the intact epidermis with a thin layer of keratin above epidermis (red arrow). However, in the dermis there were vacuoles representing degenerative changes in collagen and elastic fibers (blue arrow). The rats of group 2 showed (B) the sloughing of epidermis (blue arrow) with necrotic debris accumulated above the epidermis (red arrow). The dermis also had intact hair follicles representing pathologic modifications (green arrow). Group 3 treated rats showed (C) no damage to epithelium (red arrow) with normal hair follicles in the dermis (blue arrow). However, there were some atrophied connective tissues in dermis (green arrow). The epithelial layers in dermis were normal with intact stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale (blue arrow) in Group 4 treated rats (D). The dermis had normal hair follicles and connective tissue (green arrow). Also, smooth muscles under dermis did not show any pathologic changes (black arrow). Group 5 treated rats (E) had sloughed off epithelium under light microscope (blue arrow). The hair follicles and connective tissues in dermis also showed coagulative changes (green arrow). Moreover, atrophied smooth muscles were also observed in muscular layer under dermis layer (black arrow), Magnification: ×100.
Discussion
Various phytoconstituents of the plant have been documented to be fruitful in wound contraction. Plant products, chosen for their lower profile of side effects, non-toxicity, efficacy, and widespread availability, offer a reassuring option for wound therapy [24].
Antioxidant characteristics may promote wound healing [24]. Numerous studies have shown that antioxidants may play an essential role in wound contraction and may be a vital causative factor in the biochemical process of healing [17,20]. Both plants have shown excellent antioxidant activity [25]. In the DPPH assay, the antioxidant potential shown by the decoction of both medicinal plants is exceptional, with an impressive 87% inhibition (Figure 2).
Microbial control at the wound site is crucial for better wound healing (phase 1) [26]. Both plants reported antimicrobial effects [27], which may further support their folkloric claims. The decoction of both has shown significant antibacterial activity against both strains (Table 5). Antibacterial resistance is one of the major concerns for global health. In this study, decoction was effective against both strains, in which bacteria have developed resistance against standard drugs (amoxicillin and ceftriaxone). The antibacterial potential of decoction showed more profound inhibition than standard drugs as both plants shared different target pathways, which may be responsible for the synergistic effectr of the decoction. Preparing and developing new remedies to control bacterial growth in post-operative patients is necessary. Phenolic compounds can be responsible for the anti-inflammatory activity of A. sativum and C. longa [21], which helps the healing process [6]. Tannins and flavonoids due to their antimicrobial and astringent potential, are known to enhance wound healing, which is responsible for better wound closure and epithelization [8] and also triggers the formation of human skin fibroblast and collagen [12]. Chromatographic findings show the presence of both the phytoconstitutents [28]. White blood cells’ principal function is to eliminate infection-causing microbes from the wound. Blood cells remove diseased and dead tissue from wounds and activate growth factors that guide the transformation of fibrin clots into healthy, vascularized tissue [29]. The decoction of A. sativum and C. longa has shown promising time-dependent results in WBCs compared to the control (Table 4). Acute dose toxicity studies of decoction (dermal and oral) showed no sign of toxicity, allergy, rash, lethargy, morbidity, or mortality, complimenting the 2009 guidelines put out by the European Medicines Agency. A. sativum was determined to be safe in doses of up to 30 ml/kg in Wistar rats [30] and C. longa was determined to be safe in doses of up to 1.8 mg/kg orally in a 90-day toxicological study.
Conclusion
The wound-healing activity of Curcuma longa and Allium sativum may be due to the variety of their phytoconstituents. Their rapid effect on wound healing may be because of their cumulative and complementary effects in (phase 1 and phase 2) wound healing, antioxidant, and antibacterial properties. Oral and topical applications produced were most effective among the two methods tested and had quick responses affecting multiple pathways involved in wound healing.
Acknowledgements
The authors extend their appreciation to Taif University, Saudia Arabia, for supporting this work through project number (TU-DSPP-2024-138).
Disclosure of conflict of interest
None.
Abbreviations
- PDGF
Platelet-derived growth factor
- FGF
fibroblast growth factor
- TGF
transforming growth factor
- EGF
epidermal growth factor
- WHO
World Health Organization
- FDA
Food and Drug Administration
- C. longa
Curcuma longa
- A. sativum
Allium sativum
- SAMC
S-allylmercaptocysteine
- SAC
S-allylcysteine
- H&E
Hematoxylin and Eosin
- GAE
Gallic acid equivalent
- TPC
Total phenolic content
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- I.p
Intraperitoneal
- OCED
Organization for Economic Cooperation and Development
Supporting Information
References
- 1.Gosain A, Luisa A. Aging and wound healing. World J Surg. 2004;28:321–326. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 2.Akinpelu DA, Onakoya TM. Antimicrobial activities of medicinal plants used in folklore remedies in South-Western. Afr J Biotechnol. 2006;5:1078–1081. [Google Scholar]
- 3.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal MO, Yahya EB. In vivo assessment of reversing aminoglycoside antibiotics nephrotoxicity using Jatropha mollissima crude extract. Tissue Cell. 2021;72:101525. doi: 10.1016/j.tice.2021.101525. [DOI] [PubMed] [Google Scholar]
- 5.Ain QU, Iqbal MO, Khan IA, Bano N, Naeem M, Jamaludin MI, Devaraj S. Phytochemical, antioxidant, antipyretic and anti-inflammatory activities of aqueous-methanolic leaf extract of Mangifera indica . Am J Transl Res. 2023;15:4533–4543. [PMC free article] [PubMed] [Google Scholar]
- 6.Akbik D, Ghadiri M, Chrzanowski W, Ramin R. Curcumin as a wound healing agent. Life Sci. 2014;121:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Saeed M, Muhammad N, Khan H, Khan SA. Analysis of toxic heavy metals in branded Pakistani herbal products. J Chem Soc Pak. 2010;32:471. [Google Scholar]
- 8.Jagetia GC, Venkatesha VA. Effect of mangiferin on radiation-induced micronucleus formation in cultured human peripheral blood lymphocytes. Environ Mol Mutagen. 2005;46:12–21. doi: 10.1002/em.20124. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay D, Arunachalam G, Ghosh L, Rajendran K, Mandal AB, Bhattacharya SK. Antipyretic activity of Alstonia macrophylla wall ex A. DC: an ethnomedicine of Andaman Islands. J Pharm Pharm Sci. 2005;8:558–64. [PubMed] [Google Scholar]
- 10.Akinpelu DA, Onakoya TM. Antimicrobial activities of medicinal plants used in folklore remedies in South-Western. Afr J Biotechnol. 2006;5:1078–1081. [Google Scholar]
- 11.Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1121:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Bojs G, Svensson A. Contact allergy to garlic used for wound healing. Contact Dermatitis. 1988;3:179–181. doi: 10.1111/j.1600-0536.1988.tb04515.x. [DOI] [PubMed] [Google Scholar]
- 13.Ejaz S, Chekarova I, Cho JW, Lee SY, Ashraf S, Lim CW. Effect of aged garlic extract on wound healing: a new frontier in wound management. Drug Chem Toxicol. 2009;32:191–203. doi: 10.1080/01480540902862236. [DOI] [PubMed] [Google Scholar]
- 14.Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131:1010S–1015S. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal MO, Ahmed MM, Arshad S, Javaid U, Khan IA, Manzoor M, Andleeb S, Riaz R, Munawar SH, Manzoor Z, Mumtaz A. Nephroprotective effects of Alhagi camelorum against cisplatin-induced nephrotoxicity in albino wistar rats. Molecules. 2022;27:941. doi: 10.3390/molecules27030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Committee on Care, and Use of Laboratory Animals. Guide for the care and use of laboratory animals. No. 86. US Department of Health and Human Services, Public Health Service, National Institutes of Health; 1986. [Google Scholar]
- 17.Khan IA, Aziz A, Sattar M, Munawar SH, Manzoor Z, Raza MA, Fatima G, Hannan A. Evaluation of wound healing potential of Rumex vesicarius L. leaf extract and fractions in rabbit. Afr J Tradit Complement Altern Med. 2015;12:60–64. [Google Scholar]
- 18.Khan IA, Aziz A, Bashir S, Raza MA, Fatima G. Dermatological evaluation of counter irritant effect of methanol leaf extract of Rumex vesicarius Linn. In rabbits. J Pak Med Assoc. 2016;66:49–52. [PubMed] [Google Scholar]
- 19.Iqbal MO, Manzoor M, Mumtaz A, Riaz R, Arshad S, Khan IA, Javaid U, Manzoor Z, Munawar SH, Andleeb S, Ahmed MM, Aslam A. Evaluation of the hepatoprotective activity of hydroalcoholic extract of Alhagi camelorum against valproic acid-induced hepatotoxicity in rats. Biomed Pharmacother. 2022;150:112953. doi: 10.1016/j.biopha.2022.112953. [DOI] [PubMed] [Google Scholar]
- 20.Lakhanpal P, Rai DK. Quercetin: a versatile flavonoid. Internet J Med Updat. 2007;2:22–37. [Google Scholar]
- 21.Khan IA, Lodhi AH, Munawar SH, Manzoor A, Manzoor Z, Raza MA. Formulation and evaluation of rutin-allicin gel against diabetic foot ulcer. Lat Am J Pharm. 2020;39:725–729. [Google Scholar]
- 22.Khan IA, Aziz A, Manzoor Z, Munawar SH, Sarwar HS, Afzal A, Raza MA. Study on antipyretic activity of Rumex vesicarius leaves extract in albino rabbits. Vet World. 2014;7:44–48. [Google Scholar]
- 23.Iqbal MO, Naeem M, Mumtaz A, Ahmed MM, Ahmad A, Riaz R, Mesbah Z, Munawar N. Biochemical evaluation and medicinal ability of Jatropha mollissima in hepatic disorders. Am J Transl Res. 2022;14:7178–7188. [PMC free article] [PubMed] [Google Scholar]
- 24.Khan IA, Hussain M, Munawar SH, Iqbal MO, Arshad S, Manzoor A, Shah MA, Abbas K, Shakeel W, Syed SK. Jasminum sambac: a potential candidate for drug development to cure cardiovascular ailments. Molecules. 2021;26:5664. doi: 10.3390/molecules26185664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan IA, Aziz A, Manzoor Z, Munawar SH, Sarwar HS, Afzal A, Raza MA. Study on antipyretic activity of Rumex vesicarius leaves extract in albino rabbits. Vet World. 2014;7:44–48. [Google Scholar]
- 26.Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373:545–9. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez AM, DeOcesano-Pereira C, Teixeira C, Moreira V. IL-1β and TNF-α modulation of proliferated and committed myoblasts: IL-6 and COX-2-derived prostaglandins as key actors in the mechanisms involved. Cells. 2020;9:2005. doi: 10.3390/cells9092005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumari M, Mona S, Keshri P. Wound healing effect of Azadirachta indica and Curcuma longa in guinea pigs. Scholars Bulletin. 2015;1:271–275. [Google Scholar]
- 29.Tsuchiya H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanaka T, Iinuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus . J Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Liang X, Wang B, Lin Z, Ye M, Ma R, Zheng M, Xiang H, Xu P. Six herbs essential oils suppressing inflammatory responses via inhibiting COX-2/TNF-α/IL-6/NF-κB activation. Microchem J. 2020;156:104769. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.