Abstract
Background: After percutaneous coronary intervention (PCI), patients with acute ST-segment elevation myocardial infarction (STEMI) could have an inflammatory response, which may lead to the risk of no-reflow due to microvascular obstruction. However, the association between changes in the levels of inflammatory response-related factors and no-reflow after PCI in patients with acute STEMI is still controversial. Methods: In this study, a meta-analysis was conducted. Studies from the database established before April 2024 were retrieved in PubMed, Web of Science, and EMBASE. Case-control or cohort studies were included. Repetitive publications, studies without full access and successful data extraction, fragmentary information, animal experiments, summary, and systematic reviews were excluded, and Review Manager 5.3 software was used to process the data. Results: The meta-analysis showed that elevated levels of high-sensitivity C-reactive protein (Hs-CRP) (Z = 22.87, P < 0.001), platelet/lymphocyte ratio (PLR) (Z = 19.17, P < 0.001), leukocyte (Z = 9.98, P < 0.001), and neutrophil count (Z = 5.75, P < 0.001) were significantly related with the risk of no-reflow. In addition, the increase of red blood cell volume width (RDW) was also a risk factor for no-reflow. Conclusion: Refined results of Hs-CRP, PLR, RDW, leukocytes, and neutrophil can provide clinicians with effective tools to reduce the risk of no-reflow in patients with acute STEMI after PCI.
Keywords: Inflammatory response, acute STEMI, PCI, no-reflow, meta-analysis
Introduction
Cardiovascular disease is a leading cause of death worldwide. In China alone, over 290 million people are at risk of developing cardiovascular diseases, and the mortality rate from acute myocardial infarction (AMI) is rising annually [1]. ST-segment elevation myocardial infarction (STEMI) accounts for approximately 80% of AMI cases and is one of the primary causes of death and disability globally. STEMI is characterized by acute, irreversible myocardial injury [2] and presents with a sudden onset and severe condition. For patients experiencing acute STEMI (aSTEMI), it is crucial to restore effective coronary blood flow and perfusion as quickly as possible, typically through thrombolysis or percutaneous coronary intervention (PCI). PCI, first developed by GrÃntzig and his colleagues in Switzerland, can promptly open the infarct-related artery, quickly restore coronary blood flow, and reduce the extent of myocardial infarction. With its minimal invasiveness and rapid recovery time, PCI has become the preferred treatment for STEMI [3]. Study by Goff et al. also demonstrated that, compared to thrombolytic therapy, PCI is more effective in restoring thrombolysis in myocardial infarction (TIMI) blood flow, thereby reducing mortality [4]. However, recent studies have indicated that some STEMI patients undergoing primary PCI often experience a poor prognosis, such as the no-reflow phenomenon [5].
Scholars such as Chan and Tonomura have adopted Kloner’s perspective and considered the no-reflow phenomenon as characterized by inadequate myocardial perfusion despite the reopening of the epicardial coronary arteries, which occurs when blood flow to ischemic myocardial tissues does not return to normal after the temporary closure of these arteries has been alleviated or resolved [6,7]. Various national and international reports indicate that the incidence of no-reflow in patients treated with PCI ranges from approximately 2% to 44%, with the associated mortality rate ranging from 7.4% to 30.3% [8,9]. As modern medicine increasingly recognizes the adverse impact of slow blood flow and no-reflow on the prognosis of patients with aSTEMI, most studies have concluded that no-reflow is an independent predictor of negative outcomes in aSTEMI patients post-PCI. However, many studies have yet to identify a mechanism or correlate that to fully explain the role of no-reflow in influencing the prognosis of aSTEMI patients undergoing PCI.
The etiology and pathogenesis of no-reflow after PCI in patients with aSTEMI are complex, involving factors such as distal atherosclerotic thromboembolism, ischemic injury, reperfusion injury, and increased susceptibility to coronary microcirculatory injury [10]. Additionally, inflammatory mediators have been shown to induce the expression of adhesion molecules on endothelial cells, promoting leukocyte adhesion and infiltration. This process can lead to microvascular occlusion and impaired blood flow. The inflammatory response also triggers endothelial cell activation, resulting in capillary endothelium swelling, which increases capillary permeability and leads to microvascular obstruction, thereby heightening the risk of no-reflow [11]. Thus, the inflammatory response may be a major contributing factor to the occurrence of no-reflow after PCI in patients with aSTEMI. However, the predictive value of inflammatory response markers for the no-reflow phenomenon remains controversial. For example, Li et al. suggested that indicators of the inflammatory response, such as high-sensitivity C-reactive protein (Hs-CRP), were risk factors for the occurrence of no-reflow after PCI in aSTEMI patients [12]. In contrast, Kuliczkowski et al. found no statistically significant difference in inflammatory markers, such as Hs-CRP, interleukin (IL)-6, and IL-10, between patients with no-reflow and those with normal blood flow [13]. Therefore, the purpose of this study was to perform a meta-analysis to explore the relationship between inflammatory markers and the risk of developing no-reflow after PCI in aSTEMI patients, with the goal of providing valuable insights for early clinical prediction of no-reflow.
Data and methods
Search strategy
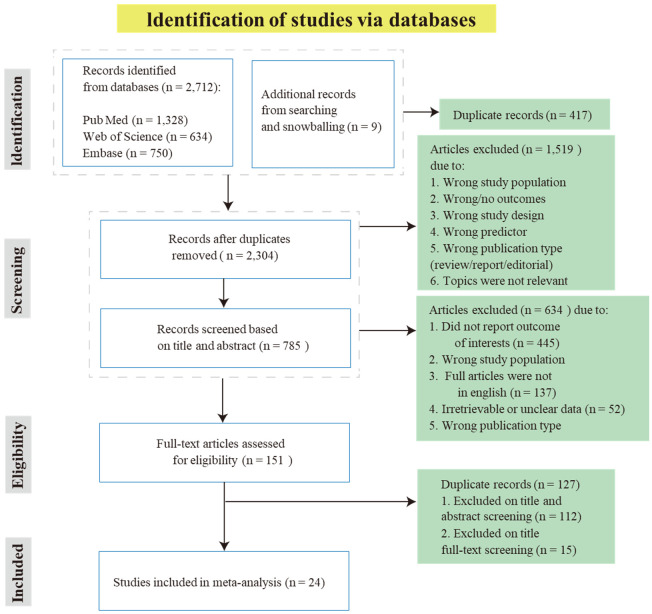
This study has been registered with PROSPERO (CRD42024571822). The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed for this meta-analysis, and all pooled data were obtained from published studies [14]. The PRISMA flowchart is shown in Figure 1. Randomized controlled trials, prospective studies, cohort studies, and case-control studies from inception to April 2024 were searched in PubMed, Web of Science, and EMBASE. The search terms included “inflammatory factor”, “STEMI”, “aSTEMI patients”, “PCI”, and “no-reflow”. Additionally, we manually screened the reference lists of the identified articles for additional studies not found during the electronic search.
Figure 1.
Flow chart of research steps for the meta-analysis.
Study selection, data extraction and inclusion criteria
Research selection and data extraction
In this meta-analysis, Le Yu and Juming Chen independently conducted the study selection and data collection process. Baseline data collected from each study included the following: authors, year of publication, country, sample size of different groups, age and sex of subjects, history of diabetes mellitus and hypertension, and levels of inflammatory markers. Any disagreements during the study selection process were resolved by Jing Zhang, who acted as an evaluator in consultation with Le Yu and Juming Chen. Studies were initially screened by title and abstract before proceeding to a full-text review.
Inclusion criteria
(1) Study subjects were patients with aSTEMI who underwent PCI surgery; (2) Studies that included patient subgroups based on post-PCI perfusion status (no-reflow vs. reflow); (3) Studies where the level of inflammatory markers was identified as a key factor affecting post-PCI perfusion; (4) Research studies in the form of case-control, prospective cohort, or retrospective cohort design.
In the included studies, the TIMI flow grading, assessed 2 hours after the initial PCI, was used clinically for coronary reperfusion evaluation. The TIMI grades are categorized as 0, 1, 2, or 3, with reperfusion impairment after PCI defined as TIMI grade ≤ 2 (no-reflow) [12,15].
Exclusion criteria
(1) Case reports, systematic reviews, and studies lacking human data; (2) Studies with incomplete data; (3) Studies in which outcome indicators were unclear or could not be translated into the desired effect size indicators; (4) Studies with significant design flaws, such as the absence of a control group or improper randomization.
Quality assessment of included studies
The quality of each included study was independently assessed by two authors using the Newcastle-Ottawa Scale (NOS) system [16]. (1) For case-control studies: The quality was evaluated based on the selection of cases and controls, comparability of cases and controls, and exposure. A maximum of 9 stars could be awarded across 8 items, with up to 2 stars for the comparability of cases and controls and one star for each of the remaining 7 items. (2) For cohort studies: The quality was assessed based on the selection of cohorts, comparability of cohorts, and outcomes. There were 8 items in total, with a maximum of 1 star per item, allowing for a maximum of 8 stars.
Statistical analysis
Statistical analyses were performed using Review Manager 5.3 software. The weighted mean difference (MD) was used as the statistical measure, and its 95% confidence interval (CI) was calculated. The chi-square test was applied to assess between-study heterogeneity, and the I2 statistic was calculated. When heterogeneity was acceptable (P > 0.1, I2 < 50%), a fixed-effects model (Mantel-Haenszel method) was used. If heterogeneity persisted (P < 0.1, I2 > 50%), a sensitivity analysis was performed by systematically excluding each study one at a time to identify the source of heterogeneity, and a random-effects model (DerSimonian-Laird method) was applied if significant heterogeneity remained. Additionally, potential publication bias was evaluated using a funnel plot approach. A p-value of < 0.05 (two-tailed) was considered statistically significant.
Results
Literature search results
In this study, we initially identified 2,753 records from the database searches (1,348 in PubMed, 646 in Web of Science, 750 in Embase, 9 in others). After removing 417 duplicates, 2,336 remained. Following a review of titles and abstracts, and subsequent full-text assessments, 2,185 records were excluded. We then conducted a detailed review of 151 full-text articles and excluded 127 following the preset criteria. This process resulted in the inclusion of 24 eligible studies (Figure 1).
Research features and data extraction
The characteristics of the 24 studies included in this research are detailed in Table 1. The studies comprised case-control studies, retrospective cohort studies, and prospective cohort studies, with a total sample size of 10,381 patients, of whom 2,105 experienced no-reflow. Publication years ranged from 2009 to 2022. Among the studies, 11 involved patients from Turkey, 12 from China, 1 from the United States, and 1 from Egypt.
Table 1.
Characteristics of the included literatures [no-reflow/reflow, n (%)]
| Author | Year | Country | Sample | Age | Males | Diabetes | Hypertension | Inflammatory factor |
|---|---|---|---|---|---|---|---|---|
| Celik [18] | 2016 | Turkey | 198 | 62±11 | 144 (72.7) | 72 (36.4) | 92 (46.5) | RDW/Neutrophil |
| 382 | 58±12 | 307 (80.4) | 89 (23.3) | 155 (40.6) | ||||
| Isik [19] | 2016 | Turkey | 30 | 64.5±12.4 | 23 (76.7) | 4 (13.3) | 13 (43.3) | |
| 66 | 58.9±12.3 | 51 (77.3) | 12 (18.2) | 20 (30.3) | ||||
| Wang [20] | 2016 | China | 43 | 65.3±12.7 | 31 (72.1) | 16 (37.2) | 27 (62.8) | Leukocyte/Neutrophil |
| 193 | 61.0±13.1 | 161 (83.4) | 59 (30.6) | 118 (61.1) | ||||
| Kurtul [21] | 2017 | Turkey | 194 | 67.1±13.4 | 122 (62.9) | 75 (38.7) | 84 (43.3) | |
| 1012 | 57.1±12.4 | 786 (77.7) | 275 (27.2) | 338 (33.4) | ||||
| Karabağ [22] | 2018 | Turkey | 343 | 59.0±12.8 | 272 (79.3) | 101 (29.4) | 155 (45.2) | |
| 874 | 56.0±11.6 | 720 (82.4) | 179 (20.5) | 336 (38.4) | ||||
| Tian [23] | 2017 | China | 56 | 56.1±11.7 | 50 (89.3) | 14 (25.0) | 30 (53.6) | |
| 305 | 55.0±11.8 | 269 (88.2) | 80 (26.2) | 165 (54.1) | ||||
| Sevket [24] | 2016 | Turkey | 199 | 62±12 | 145 (72.9) | 73 (36.7) | 92 (46.2) | |
| 401 | 58±12 | 325 (81.0) | 92 (22.9) | 158 (39.4) | ||||
| Orhan [25] | 2009 | Turkey | 137 | 60±12 | 103 (75) | 37 (27) | 55 (40) | |
| 206 | 57±11 | 165 (80) | 45 (22) | 76 (37) | ||||
| Huang [26] | 2016 | China | 28 | 56.8±13.0 | 21 (75) | 2 (7.1) | 7 (25.0) | |
| 115 | 58.0±11.0 | 92 (80) | 11 (9.6) | 31 (27.0) | ||||
| Ren [27] | 2016 | China | 19 | 63±10 | 12 (63.2) | 12 (63.2) | 14 (73.7) | |
| 64 | 57±10 | 46 (71.8) | 23 (35.9) | 42 (65.6) | ||||
| Sheng [28] | 2016 | China | 130 | 66.6±5.2 | 106 (81.5) | 38 (29.6) | 65 (50.0) | |
| 32 | 65.4±5.0 | 26 (81.3) | 10 (31.3) | 16 (50.0) | ||||
| Li [12] | 2018 | China | 38 | 65.6±11.2 | 20 (52.6) | 10 (26.3) | 19 (50.0) | Hs-CRP |
| 165 | 61.2±10.1 | 90 (54.5) | 40 (24.2) | 63 (38.2) | ||||
| Kuliczkowki [13] | 2015 | America | 27 | 61.0±7.2 | 18 (66.7) | -- | 20 (74) | |
| 33 | 62.8±5.4 | 21 (63.6) | -- | 25 (75) | ||||
| Hu [17] | 2022 | China | 32 | 63.0±11.7 | 23 (71.9) | 17 (53.1) | 14 (43.8) | |
| 44 | 54.9±9.00 | 33 (75.0) | 14 (43.8) | 14 (31.82) | ||||
| Su [29] | 2018 | China | 41 | 65.0±10.2 | 27 (68.3) | 18 (43.9) | 17 (41.5) | |
| 214 | 57.3±9.5 | 156 (72.9) | 67 (31.3) | 78 (36.4) | ||||
| Dogdu [30] | 2020 | Turkey | 35 | 64.0±11.8 | 28 (80.0) | 17 (48.6) | 14 (40.0) | |
| 45 | 66.8±11.5 | 32 (71.1) | 11 (24.4) | 16 (35.6) | ||||
| Zhao [31] | 2019 | China | 98 | 64.1±11.5 | 67 (68.4) | 37 (37.8) | 24 (24.5) | |
| 412 | 60.4±11.0 | 325 (78.9) | 113 (27.4) | 78 (18.9) | ||||
| Dong [38] | 2014 | China | 23 | 64.2±14.7 | -- | 11 (47.8) | 33 (42.9) | |
| 77 | 62.9±9.4 | -- | 30 (39.0) | 10 (43.5) | ||||
| ŞENÖZ [32] | 2021 | Turkey | 43 | 61.7±12.5 | 27 (62.8) | 20 (46.5) | 30 (69.7) | PLR/Neutrophil |
| 204 | 57.3±13.1 | 152 (74.5) | 53 (25.9) | 128 (62.7) | ||||
| Esenboga [33] | 2021 | Turkey | 110 | 62.1±12.7 | 77 (70.0) | 91 (82.7) | 57 (51.8) | |
| 400 | 62.0±11.5 | 314 (78.5) | 151 (37.8) | 237 (59.3) | ||||
| Kurtul [34] | 2015 | Turkey | 120 | 68.0±13.0 | 79 (65.8) | 43 (35.8) | 52 (43.3) | |
| 737 | 57.0±12.0 | 569 (77.2) | 198 (26.9) | 269 (36.5) | ||||
| Özmen [35] | 2021 | Turkey | 60 | 72.5±7.1 | 32 (53.3) | 19 (31.7) | 34 (56.7) | |
| 66 | 68.2±7.6 | 39 (59.1) | 32 (48.5) | 40 (60.7) | ||||
| Badran [36] | 2020 | Egypt | 58 | 52.9±11.1 | 49 (84.5) | 26 (44.8) | 31 (53.4) | |
| 142 | 111 (78.2) | 62 (43.7) | 71 (50.0) | |||||
| Wang [37] | 2018 | China | 43 | 65.3±12.7 | 31 (72.1) | 16 (37.2) | 27 (62.8) | Neutrophil |
| 193 | 61.0±13.1 | 161 (83.4) | 59 (30.6) | 118 (61.1) |
As for inflammatory parameters associated with patients’ perfusion status, Hs-CRP was included in 7 studies, platelet-lymphocyte ratio (PLR) in 5 studies, red blood cell distribution width (RDW) in 2 studies, and leukocyte count in 9 studies. The age of the patients ranged from 52.9 to 72.5 years. The proportion of male patients varied from 52.4% to 83.6%. The prevalence of diabetes mellitus ranged from 9.6% to 82.7%, and the prevalence of hypertension ranged from 18.9% to 75%.
Quality assessment of included studies
In this study, we used the NOS to evaluate the quality of the 24 selected studies, applicable to both case-control and cohort studies. As shown in Tables 2 and 3, all studies received scores ranging from 6 to 9 points. Notably, the study by Li received no stars because it only reported that 38 patients had no-reflow in the abstract, and the selection criteria for the control group were unclear. Similarly, Kuliczkowski’s study received a score of 6 due to an unclear case definition and a lack of specification regarding whether the STEMI patients were acute or non-acute. Overall, 2 studies received 6 stars, 15 studies received 7 stars, 5 studies received 8 stars, and 1 study received 9 stars. These results indicate that most of the studies included in this meta-analysis were of relatively high quality.
Table 2.
Quality of the included case-control studies
| Study | Selection of case and controls | Comparability of cases and controls | Exposure | Total | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Is the case definition adequate | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of Non-ascertainment for Response cases and controls Rate | ||
| Hu 2022 [17] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Celik 2016 [18] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Isik 2016 [19] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 |
| Wang 2016 [20] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Kurtul 2017 [21] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Karabağ 2018 [22] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 |
| Tian 2017 [23] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Sevket 2016 [24] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Orhan 2009 [25] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 |
| Huang 2016 [26] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Ren 2016 [27] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 |
| Sheng 2016 [28] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Li 2018 [12] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |
| Su 2018 [29] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Kuliczkowki 2015 [13] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |
| Dogdu 2020 [30] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Zhao 2019 [31] | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | |
| ŞENÖZ 2021 [32] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Esenboga 2021 [33] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 |
| Kurtul 2015 [34] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Özmen 2021 [35] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Badran 2020 [36] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Wang 2018 [37] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
Table 3.
Quality of the included cohort studies
| Study | Selection of cohorts | Comparability of cohorts | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Dong 2014 [38] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
Meta-analysis results
Hs-CRP
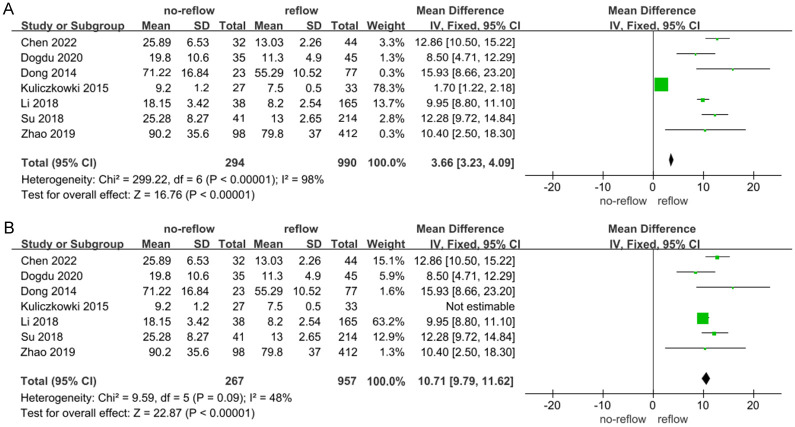
Data from 7 studies [12,13,17,29-31,38] examining the relationship between Hs-CRP levels and no-reflow risk were analyzed using a meta-analysis with a random-effects model. This analysis revealed substantial heterogeneity among the studies (I2 = 98%, indicating high variability) (Figure 2A). Sensitivity analyses, involving the exclusion of individual studies, demonstrated that excluding Kuliczkowski’s study reduced the heterogeneity among the remaining 6 studies (I2 = 48%) (Figure 2B). The pooled analysis of these 6 studies, which exhibited no significant heterogeneity, found that elevated Hs-CRP levels were significantly associated with an increased risk of no-reflow occurrence (Z = 22.87, P < 0.01) (Figure 2B). Additionally, an evaluation for publication bias using a funnel plot (Figure 3) indicated that the 6 studies fell within the confidence intervals, suggesting no evidence of publication bias and aligning with the results from the forest plot.
Figure 2.
Correlation between C-reactive protein (Hs-CRP) and no-reflow after percutaneous coronary intervention (PCI) in patients with acute STEMI. A: I2 = 98%; B: I2 = 48%. CI: confidence interval.
Figure 3.
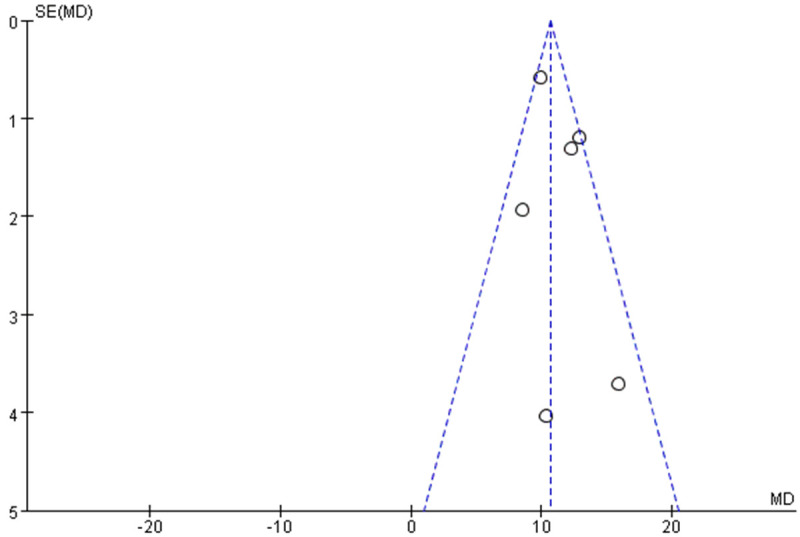
Funnel plot of hazard of publication bias. I2 = 48%.
PLR
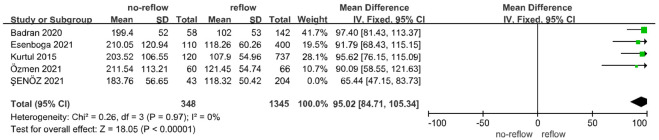
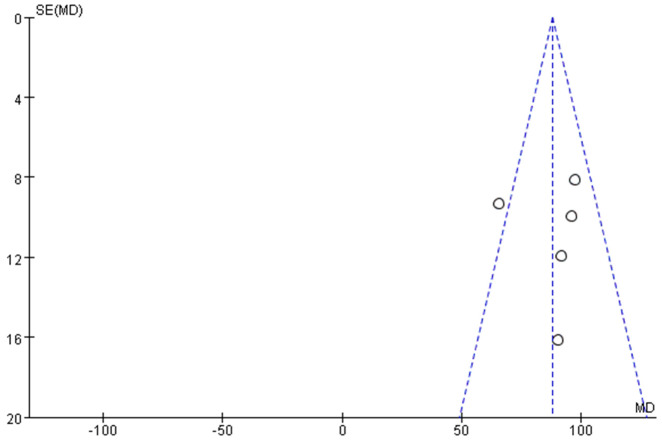
Data from 5 studies [32-36] investigated the relationship between PLR and no-reflow risk, including a total of 391 patients with no-reflow. A meta-analysis using a fixed-effect model indicated no significant heterogeneity among the 5 studies (I2 = 49%, below the 50% threshold) (Figure 4). The analysis revealed that elevated PLR levels were significantly associated with an increased risk of no-reflow occurrence (Z = 19.17, P < 0.01) (Figure 4). Examination of publication bias using a funnel plot (Figure 5) showed that most studies fell within the confidence intervals, suggesting no evidence of publication bias and consistent with the findings from the forest plot.
Figure 4.
Correlation between platelet-lymphocyte ratio (PLR) and no-reflow after PCI in patients with acute STEMI. CI: confidence interval.
Figure 5.
Funnel plot of hazard of publication bias. I2 = 49%.
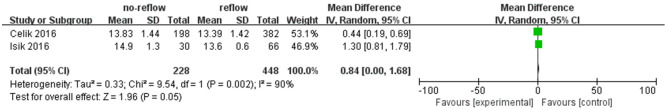
RDW
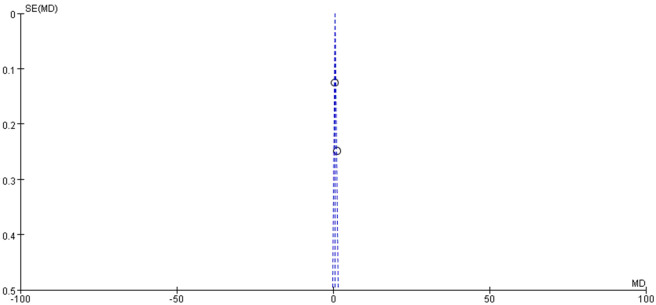
Data from 2 studies [18,19] examined the relationship between RDW and no-reflow risk, including a total of 228 patients with no-reflow phenomenon. In Celik’s study, RDW levels were statistically different between the no-reflow and reflow groups (13.83 ± 1.44% vs. 13.39 ± 1.42%, P < 0.05). Similarly, Isik’s study found significant differences in RDW levels between the no-reflow and reflow groups (14.9 ± 1.3% vs. 13.6 ± 0.6%, P < 0.05). However, the meta-analysis using a random-effects model did not reveal a significant association between elevated RDW and the occurrence of no-reflow (Z = 1.96, P = 0.05) (Figure 6). The results of the publication bias analysis are depicted in Figure 7.
Figure 6.
Correlation between red blood cell volume width (RDW) and no-reflow after PCI in patients with acute STEMI. CI: confidence interval.
Figure 7.
Funnel plot of hazard of publication bias. I2 = 90%.
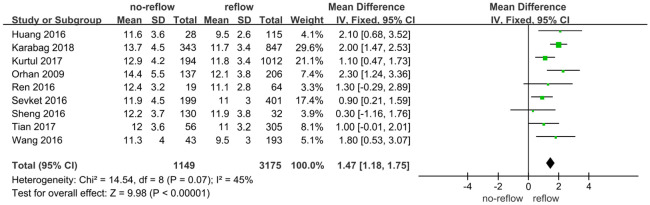
Leukocyte
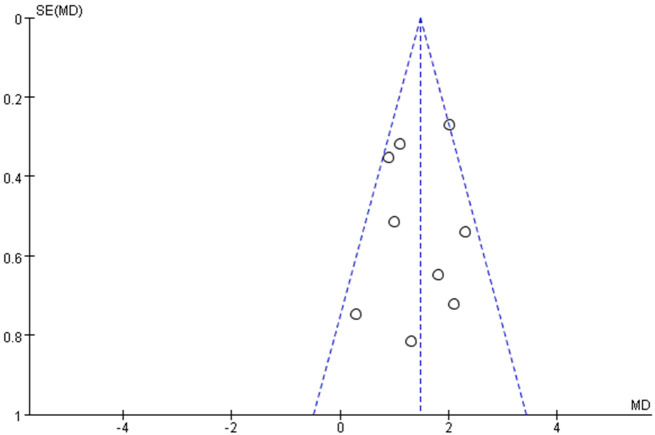
Data from 9 studies [20-28] investigated the relationship between leukocyte levels and no-reflow risk, including a total of 1,149 patients with no-reflow. A meta-analysis using a random-effects model revealed no significant heterogeneity among the studies (I2 = 45%, below the 50% threshold) (Figure 8). The analysis indicated that elevated leukocyte levels were significantly associated with an increased risk of no-reflow occurrence (Z = 9.98, P < 0.01) (Figure 4). Examination of publication bias using a funnel plot (Figure 9) showed that most studies fell within the confidence intervals, suggesting no evidence of publication bias and consistent with the forest plot results. This indicates that higher leukocyte levels are associated with an increased risk of no-reflow.
Figure 8.
Correlation between leukocyte and no-reflow after PCI in patients with acute STEMI.
Figure 9.
Funnel plot of hazard of publication bias. I2 = 45%.
Neutrophil
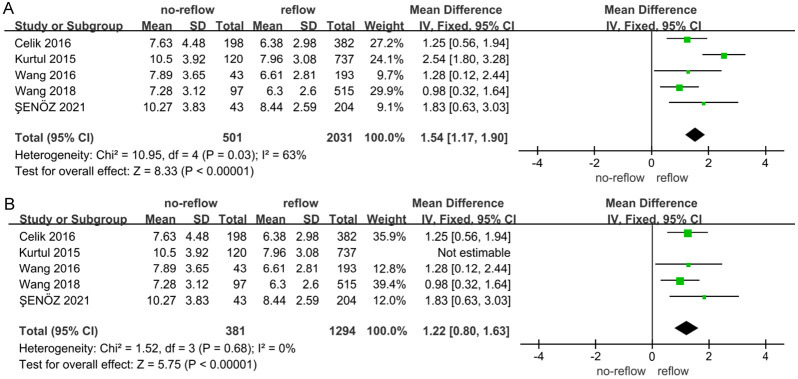
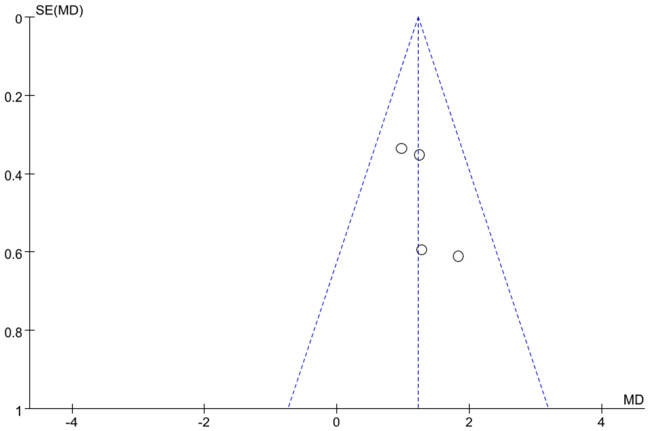
Data from 5 studies [18,29,32,34,37] exploring the association between neutrophil levels and no-reflow risk were analyzed using a meta-analysis with a random-effects model. This analysis revealed substantial heterogeneity among the studies (I2 = 63%, indicating high variability) (Figure 10A). Sensitivity analyses, which involved excluding individual studies, demonstrated that removing Kurtul’s study resulted in no heterogeneity among the remaining 4 studies (I2 = 0%) (Figure 10B). The pooled analysis of these 4 studies, which exhibited no significant heterogeneity, found that elevated neutrophil levels were significantly associated with an increased risk of no-reflow (Z = 5.75, P < 0.001). An assessment for publication bias using a funnel plot (Figure 11) showed that the 4 studies fell within the confidence intervals and were evenly distributed around the center line, indicating no evidence of publication bias and confirming the results from the forest plot.
Figure 10.
Correlation between neutrophil and no-reflow after PCI in patients with acute STEMI. A: I2 = 63%; B: I2 = 0%. CI: confidence interval.
Figure 11.
Funnel plot of hazard of publication bias. I2 = 0%.
Discussion
Inflammatory responses may influence the occurrence of no-reflow in patients with aSTEMI following PCI through several mechanisms: (1) Microvascular damage: The inflammatory response releases cytokines, oxygen free radicals, and other mediators that can damage the microvasculature. This damage is reflected in changes in biomarkers such as RDW, PLR, Hs-CRP, and neutrophil levels, increased microvascular permeability, extravasation of blood components, and the formation of microthrombi impede blood flow. Additionally, elevated Hs-CRP may exacerbate atherosclerotic thrombosis by increasing the expression and activity of major fibrinolysis inhibitors, which can further contribute to the development of no-reflow after PCI [12,13,17-38]. (2) Leukocyte aggregation: Inflammation within the body leads to the accumulation of leukocytes in the damaged myocardial tissue, which can obstruct microvessels and disrupt blood flow [20,21]. Additionally, advanced age, male sex, and a history of diabetes mellitus or hypertension have been identified as potential risk factors for no-reflow after PCI. However, these risk factors have been described in various ways, and the specific relationship between the inflammatory response and no-reflow in aSTEMI patients undergoing PCI remains unclear [39]. In our study, we conducted a meta-analysis to explore the predictive role of inflammation-related parameters in the occurrence of no-reflow, aiming to provide clinical guidance for managing this complication.
In the inflammatory response, elevated levels of Hs-CRP can reflect the degree of inflammation in the vessel wall and contribute to vascular endothelial dysfunction. This dysfunction increases monocyte adhesion and migration by upregulating adhesion molecules on vascular endothelial cells, which can lead to the occurrence of no-reflow [40]. Wu et al. also suggested that Hs-CRP levels could potentially predict no-reflow occurrence after PCI, indicating that high Hs-CRP levels may signal a greater risk of this complication [41]. Our meta-analysis found that patients in the no-reflow group had higher Hs-CRP levels compared to those in the normal group, aligning with previous studies. However, it is worth noting that when the included studies were 7, the I2 was 98%, and there was significant heterogeneity. Notably, excluding Kuliczkowski’s study resolved the heterogeneity, likely because Hs-CRP levels in that study were considerably lower than those in the other 6 studies.
Additionally, our meta-analysis revealed that PLR levels were higher in the no-reflow group compared to the normovolemic group across the 5 studies included. PLR, a novel index in modern medicine, has been suggested as a predictor of major adverse cardiovascular outcomes, and previous research has indicated that increased PLR may also be associated with the no-reflow phenomenon [42]. Inflammatory mediators can elevate platelet levels by stimulating megakaryocyte proliferation, potentially leading to atherosclerotic thrombus formation and disrupted blood flow. In inflammatory conditions, there is a positive correlation between inflammatory markers such as CRP, interleukin, and tumor necrosis factor alpha, and elevated platelet counts, alongside reduced lymphocyte counts, which are often poor prognostic indicators in chronic diseases [43]. The trends observed in PLR metrics across these studies align with our findings, demonstrating that PLR is an important and independent predictor of no-reflow in patients with aSTEMI undergoing PCI.
Elevated RDW levels are commonly observed in inflammatory responses and may be linked to subclinical inflammation as well as increased mortality in certain cardiovascular diseases. Isik et al. found that high RDW levels could elevate the risk of no-reflow in patients with aSTEMI post-PCI, suggesting that microvascular inflammation is a contributing factor to no-reflow [29]. However, since only 2 studies included RDW in this analysis, the results may not be robust. RDW has only recently been recognized as an inflammatory marker, and research on its association with no-reflow after PCI in aSTEMI patients remains limited. Furthermore, during inflammation, the total leukocyte count typically increases, along with enhanced functional activity and adhesion to vascular endothelial cells. This increased adhesion is a probable cause of no-reflow. Our meta-analysis corroborates this, showing that leukocyte levels were significantly higher in the no-reflow group compared to the normovolemic group across all 9 studies included.
During inflammation, neutrophils are among the first leukocytes to respond, playing a crucial role in the inflammatory process [18,32,37]. Inflammatory sites release various chemoattractants, such as leukotriene B4, platelet-activating factor, and IL-8, which guide neutrophils to the inflammation site by binding to specific receptors on their surface. Studies indicate that the total number of neutrophils increases with inflammation.
In our study, the meta-analysis initially showed substantial heterogeneity with 5 studies included. This heterogeneity was resolved when Kurtul’s study was excluded, leaving a heterogeneity of 0% among the remaining 4 studies. This discrepancy may be attributed to Kurtul’s study having notably higher neutrophil levels compared to the other studies. Despite this, our findings are consistent with previous research, showing that patients in the no-reflow group had higher neutrophil levels than those in the normovolemic group. This increase may be due to the inflammatory response accelerating neutrophil production in the bone marrow (myeloproliferation) and enhancing their release into the peripheral blood [29,34].
Conclusion
Including Hs-CRP, PLR, RDW, leukocyte, and neutrophil levels in hazard assessments and diagnostic criteria can help clinicians more accurately identify the risk of no-reflow in aSTEMI patients after PCI and facilitate the development of personalized treatment plans. This approach underscores the critical role of inflammation in treatment strategies and suggests new avenues for future research and therapeutic interventions.
However, there are several limitations to this meta-analysis: (1) Publication bias: The analysis was based solely on published studies obtained through electronic searches, excluding unpublished literature, which may have introduced bias. (2) Limited data on RDW: Only 2 studies included RDW, likely because this index has only recently gained attention. Its role in predicting no-reflow after PCI in aSTEMI patients needs further investigation. (3) Other inflammatory markers: The inflammatory response also affects other markers (e.g., monocytes, erythrocyte sedimentation rate). Further research is needed to determine whether these markers are significant in predicting no-reflow in aSTEMI patients post-PCI.
Disclosure of conflict of interest
None.
References
- 1.Lin YC, Chen JC, Lin JM, Hsu CH, Wu CF, Kao SH. Differential serum proteomic signatures between acute aortic dissection and acute myocardial infarction. Biomedicines. 2023;11:161. doi: 10.3390/biomedicines11010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Huang S, Zhou Q, Dou L, Lin D. The predictive value of laboratory parameters for no-reflow phenomenon in patients with ST-elevation myocardial infarction following primary percutaneous coronary intervention: a meta-analysis. Clin Cardiol. 2024;42:e24238. doi: 10.1002/clc.24238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goff SL, Mazor KM, Ting HH, Kleppel R, Rothberg MB. How cardiologists present the benefits of percutaneous coronary interventions to patients with stable angina: a qualitative analysis. JAMA Intern Med. 2014;174:1614–1621. doi: 10.1001/jamainternmed.2014.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katayama Y, Taruya A, Kashiwagi M, Ozaki Y, Shiono Y, Tanimoto T, Yoshikawa T, Kondo T, Tanaka A. No-reflow phenomenon and in vivo cholesterol crystals combined with lipid core in acute myocardial infarction. Int J Cardiol Heart Vasc. 2022;38:100953. doi: 10.1016/j.ijcha.2022.100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sia CH, Tan SH, Chan SP, Marchesseau S, Sim HW, Carvalho L, Chen R, Amin NHM, Fong AY, Richards AM, Yip C, Chan MY. Enhanced thrombin generation is associated with worse left ventricular scarring after ST-segment elevation myocardial infarction: a cohort study. Pharmaceuticals (Basel) 2022;15:718. doi: 10.3390/ph15060718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonomura D, Shimada Y, Yamanaka Y, Terashita K, Suzuki T, Nishiura S, Yoshida M, Tsuchida T, Fukumoto H. Laser vaporization of atherothrombotic burden before drug-coated balloon application in ST-segment elevation myocardial infarction: two-year outcomes of the laser-DCB trial. Catheter Cardiovasc Interv. 2022;99:1758–1765. doi: 10.1002/ccd.30149. [DOI] [PubMed] [Google Scholar]
- 8.Sakakura K, Funayama H, Taniguchi Y, Tsurumaki Y, Yamamoto K, Matsumoto M, Wada H, Momomura SI, Fujita H. The incidence of slow flow after rotational atherectomy of calcified coronary arteries: a randomized study of low speed versus high speed. Catheter Cardiovasc Interv. 2017;89:832–840. doi: 10.1002/ccd.26698. [DOI] [PubMed] [Google Scholar]
- 9.Prati F, Romagnoli E, Limbruno U, Pawlowski T, Fedele S, Gatto L, Di Vito L, Pappalardo A, Ramazzotti V, Picchi A, Trivisonno A, Materia L, Pfiatkosky P, Paoletti G, Marco V, Tavazzi L, Versaci F, Ston GW. Randomized evaluation of intralesion versus intracoronary abciximab and aspiration thrombectomy in patients with ST-elevation myocardial infarction: the COCTAIL II trial. Am Heart J. 2015;170:1116–1123. doi: 10.1016/j.ahj.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Harrison RW, Aggarwal A, Ou FS, Klein LW, Rumsfeld JS, Roe MT, Wang TY American College of Cardiology National Cardiovascular Data Registry. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol. 2013;111:178–184. doi: 10.1016/j.amjcard.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Wong DT, Puri R, Richardson JD, Worthley MI, Worthley SG. Myocardial ‘no-reflow’-diagnosis, pathophysiology and treatment. Int J Cardiol. 2013;167:1798–1806. doi: 10.1016/j.ijcard.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Fu DG, Liu FY, Zhou H, Li XM. Evaluation of related factors, prediction and treatment drugs of no-reflow phenomenon in patients with acute ST-segment elevation myocardial infarction after direct PCI. Exp Ther Med. 2018;15:3940–3946. doi: 10.3892/etm.2018.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuliczkowski W, Gasior M, Pres D, Kaczmarski J, Laszowska A, Szewczyk M, Hawranek M, Tajstra M, Zeglen S, Polonski L, Serebruany VL. Aspirin ‘resistance’: impact on no-reflow, platelet and inflammatory biomarkers in diabetics after ST-segment elevation myocardial infarction. Cardiology. 2015;131:41–50. doi: 10.1159/000371793. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. 2017;10:215–223. doi: 10.1016/j.jcin.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 16.Song R, Lin H, Chen Y, Zhang X, Feng W. Effects of methimazole and propylthiouracil exposure during pregnancy on the risk of neonatal congenital malformations: a meta-analysis. PLoS One. 2017;12:e0180108. doi: 10.1371/journal.pone.0180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu CK, Cai RP, He L, He SR, Liao JY, Su Q. A nomogram model for predicting the occurrence of no-reflow phenomenon after percutaneous coronary intervention using the lncRNA TUG1/miR-30e/NPPB biomarkers. J Thorac Dis. 2022;14:2158–2168. doi: 10.21037/jtd-22-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celik T, Balta S, Demir M, Yildirim AO, Kaya MG, Ozturk C, Demirkol S, Unlu M, Kilic S, Aydin İ, Iyisoy A. Predictive value of admission red cell distribution width-platelet ratio for no-reflow phenomenon in acute ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiol J. 2016;23:84–92. doi: 10.5603/CJ.a2015.0070. [DOI] [PubMed] [Google Scholar]
- 19.Isik T, Kurt M, Tanboga IH, Ayhan E, Gunaydin ZY, Kaya A, Uyarel H. The impact of admission red cell distribution width on long-term cardiovascular events after primary percutaneous intervention: a four-year prospective study. Cardiol J. 2016;23:281–288. doi: 10.5603/CJ.a2015.0080. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Ren L, Liu N, Lei L, Ye H, Peng J. Association of monocyte count on admission with angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Kardiol Pol. 2016;74:1160–1166. doi: 10.5603/KP.a2016.0065. [DOI] [PubMed] [Google Scholar]
- 21.Kurtul A, Acikgoz SK. Usefulness of mean platelet volume-to-lymphocyte ratio for predicting angiographic no-reflow and short-term prognosis after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2017;120:534–541. doi: 10.1016/j.amjcard.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Karabağ Y, Çağdaş M, Rencuzogullari I, Karakoyun S, Artaç İ, İliş D, Yesin M, Çağdaş ÖS, Altintaş B, Burak C, Tanboğa HI. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Invest. 2018;48:e12928. doi: 10.1111/eci.12928. [DOI] [PubMed] [Google Scholar]
- 23.Tian J, Liu Y, Liu Y, Song X, Zhang M, Xu F, Yuan F, Lyu S. Prognostic association of circulating neutrophil count with no-reflow in patients with ST-segment elevation myocardial infarction following successful primary percutaneous intervention. Dis Markers. 2017;2017:8458492. doi: 10.1155/2017/8458492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balta S, Celik T, Ozturk C, Kaya MG, Aparci M, Yildirim AO, Demir M, Kilic S, Aydin İ, Iyisoy A. The relation between monocyte to HDL ratio and no-reflow phenomenon in the patients with acute ST-segment elevation myocardial infarction. Am J Emerg Med. 2016;34:1542–7. doi: 10.1016/j.ajem.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Maden O, Kacmaz F, Selcuk H, Selcuk MT, Aksu T, Tufekcioglu O, Senen EK, Balbay Y, Ilkay E. Relationship of admission hematological indexes with myocardial reperfusion abnormalities in acute ST segment elevation myocardial infarction patients treated with primary percutaneous coronary interventions. Can J Cardiol. 2009;25:e164–8. doi: 10.1016/s0828-282x(09)70090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang GY, Yang LJ, Wang XH, Wang YL, Xue YZ, Yang WB. Relationship between platelet-leukocyte aggregation and myocardial perfusion in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Heart Lung. 2016;45:429–33. doi: 10.1016/j.hrtlng.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Ren F, Mu N, Zhang X, Tan J, Li L, Zhang C, Dong M. Increased platelet-leukocyte aggregates are associated with myocardial no-reflow in patients with ST elevation myocardial infarction. Am J Med Sci. 2016;352:261–6. doi: 10.1016/j.amjms.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 28.Sheng F, Chen B, He M, Zhang M, Shen G. Neutrophil to lymphocyte ratio is related to electrocardiographic sign of spontaneous reperfusion in patients with ST-segment elevation myocardial infarction. Arch Med Res. 2016;47:180–5. doi: 10.1016/j.arcmed.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Su Q, Ye Z, Sun Y, Yang H, Li L. Relationship between circulating miRNA-30e and no-reflow phenomenon in STEMI patients undergoing primary coronary intervention. Scand J Clin Lab Invest. 2018;78:318–324. doi: 10.1080/00365513.2018.1467571. [DOI] [PubMed] [Google Scholar]
- 30.Dogdu O, Kucukukur MK, Necip NII, Kobat MA, Karasu MKM, Ozkan OKK, Mehmet MBB, Ilgin IKK. Assessment of growth differentiation factor 15 levels on coronary flow in patients with STEMI undergoing primary PCI. Diseases. 2017;38:987–987. doi: 10.3390/diseases8020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Yang J, Ji Y, Wang S, Wang T, Wang F, Tang J. Usefulness of fbrinogen-to-albumin ratio to predict no-refow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels. 2019;34:1600–1607. doi: 10.1007/s00380-019-01399-w. [DOI] [PubMed] [Google Scholar]
- 32.Şenöz O, Emren SV, Erseçgin A, Yapan Emren Z, Gül İ. Platelet-lymphocyte ratio is a predictor for the development of no-reflow phenomenon in patients with ST-segment elevation myocardial infarction after thrombus aspiration. J Clin Lab Anal. 2021;35:e23795. doi: 10.1002/jcla.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esenboga K, Kurtul A, Yamanturk YY, Tan TS, Tutar DE. Systemic immune-inflammation index predicts no-reflow phenomenon after primary percutaneous coronary intervention. Acta Cardiol. 2022;77:59–65. doi: 10.1080/00015385.2021.1884786. [DOI] [PubMed] [Google Scholar]
- 34.Kurtul A, Yarlioglues M, Celik IE, Duran M, Elcik D, Kilic A, Oksuz F, Murat SN. Association of lymphocyte-to-monocyte ratio with the no-reflow phenomenon in patients who underwent a primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis. 2015;26:706–712. doi: 10.1097/MCA.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 35.Özmen Ç, Akray A, Iltas A, Yildiz PÖ, Yildiz I, Aktas H. Relationship between platelet/lymphocyte ratio and no-reflow formation in patients with ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention. Cukurova Med J. 2021;46:1441–1448. [Google Scholar]
- 36.Badran HM, Fatah AA, Soltan G. Platelet/lymphocyte ratio for prediction of no-reflow phenomenon in ST-elevation myocardial infarction managed with primary percutaneous coronary intervention. J Clin Transl Res. 2020;6:20–26. doi: 10.18053/jctres.06.202001.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Ren L, Liu N, Peng J. Utility of hematological parameters in predicting no-reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2018;24:1177–1183. doi: 10.1177/1076029618761005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong M, Mu N, Ren F, Sun X, Li F, Zhang C, Yang J. Prospective study of effects of endogenous estrogens on myocardial no-reflow risk in postmenopausal women with acute myocardial infarction. J Interv Cardiol. 2014;27:437–443. doi: 10.1111/joic.12137. [DOI] [PubMed] [Google Scholar]
- 39.Bolayır HA, Güneş H, Kıvrak T, Şahin Ö, Akaslan D, Kurt R, Bolayır A, İmadoğlu O. The role of SCUBE1 in the pathogenesis of no-reflow phenomenon presenting with ST segment elevation myocardial infarction. Anatol J Cardiol. 2017;18:122–127. doi: 10.14744/AnatolJCardiol.2017.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sulicka-Grodzicka J, Szczepaniak P, Jozefczuk E, Urbanski K, Siedlinski M, Niewiara Ł, Guzik B, Filip G, Kapelak B, Wierzbicki K, Korkosz M, Guzik TJ, Mikolajczyk TP. Systemic and local vascular inflammation and arterial reactive oxygen species generation in patients with advanced cardiovascular diseases. Front Cardiovasc Med. 2023;10:1230051. doi: 10.3389/fcvm.2023.1230051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X, Ji G, Wang Q, Chen J, Cai XY, Song J, Yan Y, Huang H. Assessment of vasa vasorum on coronary plaques in patients with acute coronary syndromes using intravascular ultrasound and iMap analysis: a retrospective cohort study. Medicine (Baltimore) 2023;102:e34458. doi: 10.1097/MD.0000000000034458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balta S, Demırkol S, Kucuk U. The platelet lymphocyte ratio maybe useful inflammatory indicator in clinical practice. Hemodial Int. 2013;17:668–669. doi: 10.1111/hdi.12058. [DOI] [PubMed] [Google Scholar]
- 43.Kurtul A, Yarlioglues M, Murat SN, Ergun G, Duran M, Kasapkara HA, Demircelik MB, Cetin M, Ocek AH. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am J Cardiol. 2014;114:342–347. doi: 10.1016/j.amjcard.2014.04.045. [DOI] [PubMed] [Google Scholar]